ABSTRACT
Anti-diabetic and anti-inflammatory activities of ethyl acetate-methanol extracts of cephalopods namely, Amphioctopus marginatus, Urothethis duvauceli, Sepia pharaonis, Sepiella inermis, and Cistopus indicus were evaluated. The ethyl acetate-methanol extracts of C. indicus exhibited significantly greater (p < 0.05) cyclooxygenase inhibition activities (IC90 ~ 1 mg/mL, respectively) compared to other cephalopod species. Likewise, C. indicus displayed greater 5-lipoxygenase inhibitory activity (IC90 1.69 mg/mL) compared to the other cephalopod species (IC90 > 2 mg/mL) considered in the present study. The solvent extracts derived from the members of the order Octopoda demonstrated fairly good α-amylase inhibitory activity (IC90 ≤ 2.5 mg/mL). Dipeptidyl peptidase-4 inhibitory activity of the ethyl acetate-methanol extract of C. indicus was found to be significantly greater (IC50 2.51 mg/mL) than other species of cephalopods (IC50 3.4–5.4 mg/mL; p < 0.05). The labeling of protons associated with different magnetic environments of the functional groups exhibited in the ethyl acetate-methanol extracts were analyzed by proton nuclear magnetic resonance spectroscopy that supported the in vitro anti-diabetic and anti-inflammatory results. The ethyl acetate-methanol extract of C. indicus and S. inermis displayed greater proton integrals (ΣH) of highly electronegative moieties appeared in the low-field region in the proton nuclear magnetic resonance spectroscopy spectra (C. indicus ΣHδ3.5–4.5 5.34, ΣHδ4.5–6.5 6.41; S. inermis ΣHδ3.5–4.5 6.52, and ΣHδ4.5–6.5 15.39) than other cephalopod species. A significant co-linearity was found between the electronegative groups present in the downfield position of nuclear magnetic resonance spectroscopy spectra vis-à-vis anti-diabetic and anti-inflammatory activities.
Introduction
Diabetes mellitus is a major global health challenge of current century afflicting over 366 million people world-wide, and by the year 2030, this endocrine disorder is predicted to affect over 552 million people, particularly from the middle/low wage countries.[Citation1] Type 1 diabetes was found to be due to the autoimmune destruction of insulin-producing islet β-cells of pancreas, while type 2 diabetes (T2D) involves metabolic disorder including insulin resistance and impaired control of hepatic glucose production.[Citation2] The incidence of T2D is rapidly expanding in recent days. Some of the main pathologies related to T2D are defective glucose metabolism, obesity, cardiovascular disease, and defective immune responses, leading to sepsis and death. A common tie connecting these apparently disparate complications is chronic inflammation,[Citation3] which consists of a tightly regulated cascade of immunological, physiological, and behavioral processes that are orchestrated by soluble immune signaling molecules called cytokines.[Citation4–Citation6] Cytokines can be generally classified as pro-inflammatory or anti-inflammatory and allow an organism to react rapidly to an immune challenge by coordinating an appropriate immune response.[Citation3]
A role for inflammatory mediators, such as cytokines in the destruction of β-cells in Type 1 diabetes has been clearly established in an earlier literature.[Citation7] Several studies have been carried out to develop the relationship between various inflammatory mediators and Type 2 diabetes mellitus (T2DM), and have found abnormally high levels of different cytokines, plasminogen activator inhibitor, chemokines, acute phase proteins, such as C-reactive protein (CRP) in T2D patients. This apparently indicated that high circulating levels of IL-6 and CRP are the primary predictive indicators for progression of T2DM.[Citation8,Citation9] Hyperglycemia refers to the constantly elevated levels of blood glucose that can be considered as key element causing the secretion of various inflammatory cytokines that imparts damaging effects on normal functioning of β-cells finally declining insulin secretion.[Citation7] Augmented levels of glucose in plasma are a primary motive for pathogenesis of T2DM. High levels of glucose were found to be toxic to the β-cells,[Citation10] thereby inducing the stimulation of various pro-inflammatory mediators, such as Tumor necrosis factor-α(TNF-α), IL-1β, IL-6, and various other IL-1 dependent cytokines and chemokines.[Citation11–Citation13] In T2D, the equilibrium between pro-inflammatory and anti-inflammatory cytokines is shifted toward pro-inflammation, potentially exacerbating the health hazards found in T2D.[Citation3] T2DM may be stated as a chronic form of autoinflammatory disease producing IL-1β from β-cells of pancreatic islets, which eradicates β-cells themselves[Citation11] leading to β-cell dysfunction. More recently, inflammatory mediators have become increasingly implicated in the development of T2D.[Citation12] Key observations in support of this concept have included the demonstration of the role for adipocyte-derived TNF-α in the development of insulin resistance. Since inflammation has been recognized as a key mechanism in the pathophysiology of T2DM, targeting inflammation is now receiving wider acceptance as an option for treating diabetes. There are ample evidences suggesting that anti-inflammatory treatment has improved glycemia and β-cell function in humans with T2DM.[Citation8]
Inhibition of enzymes involved in regulating glucose metabolism as a major strategy has been adopted for the treatment of T2DM. The main enzymes include α-glucosidase, α-amylase, and dipeptidyl peptidase 4 (DPP-4).[Citation14] α-Glucosidase and α-amylase are the major enzymes present in the intestinal lumen that hydrolyze disaccharides into monosaccharaides, thereby resulting in their absorption into the blood vascular system.[Citation15,Citation16] DPP-4, which was reported to lower the circulating level of incretin hormones is a known target for the treatment of T2DM.[Citation14] Cyclooxygenases (COX-1 and COX-2) and lipoxygenases are the major enzymes mediating inflammatory responses, and are the primary targets for non-steroidal anti-inflammatory drugs (NSAIDs).[Citation17]
Notably, the marine mollusks are considered as one of the significant sources to derive bioactive metabolites exhibiting antitumor, anti-inflammatory, and antioxidant activities.[Citation18] However, the species of mollusks belonging to the class Cephalopoda have not been explored in detail with regard to their anti-diabetic and anti-inflammatory activities. As a part of the ongoing research program carried out at our laboratory to explore novel sources of bioactivities from the marine organisms, five commonly available cephalopods namely, Amphioctopus marginatus, Urothethis duvauceli, Sepia pharaonis, Sepiella inermis, and Cistopus indicus were selected to evaluate their in vitro anti-diabetic and anti-inflammatory activities. The in vitro anti-diabetic activities of ethyl acetate-methanol (EtOAc-MeOH) extracts of the cephalopods were evaluated using α-glucosidase, α-amylase, and DPP-4 enzyme inhibition assays, while the anti-inflammatory potentials were assessed by the in vitro inhibition of COX-1 and COX-2 and 5-lipoxygenase (5-LOX) enzymes. The utilities of proton nuclear magnetic resonance spectroscopy (1H-NMR) to assess the abundance of the bioactive functional groups present in the EtOAc-MeOH extracts of the cephalopod species, and to illustrate the principles with regard to the presence of these functional groups vis-à-vis anti-inflammatory and anti-diabetic activities have been demonstrated. The present study revealed the utilities of the cephalopods as potential inhibitors of COX-1 and COX-2, lipoxygenase, and DPP-4, which are responsible to cause inflammatory diseases, and diabetes, for the first time. The results from the present study will be helpful to develop nutraceutical supplements and pharmacophore leads from these mollusk species in combating inflammation and T2D.
Materials and methods
Cephalopod material and preparation of solvent extracts
The cephalopod species were collected on board from the Arabian Sea, situated at Lat 8º48’ N; Long 78º9’ E and Lat 9º14’ N; Long 79º14’E, along the southwestern shoreline of the Indian subcontinent. The samples (10 kg of each species) were conveyed to the laboratory in insulated boxes loaded down with dry ice. They were thoroughly washed to remove mucus, debris, and other particles. The ink gland was precisely removed, and the tissues were grounded properly, before being stored at –80°C for further analysis. The ground cephalopod samples (1 kg) were freeze-dried by using a laboratory freeze-drier (Martin Christ, Germany) yielding the lyophilized powder (100 g, yield based on wet material 10%). The lyophilized powder of each group of cephalopod material was extracted with EtOAc-MeOH (1:1 v/v, 500 mL X 2), and was sonicated for 3 h under an inert atmosphere of N2. The samples were then filtered with Whatman filter paper (Whatman number 1) to acquire the clarified filtrates before being concentrated in vacuo (40°C) using a rotary evaporator (Heidolph Instruments GmbH & Co., Schwabach, Germany) to afford a dark brown oily viscous residue. The residue was further extracted with EtOAc-MeOH (1:1 v/v, 500 mL X 2) at an elevated temperature (40–45°C) for 3 h. The contents were filtered (Whatman number 1) and dried over anhydrous Na2SO4 (65–70 g), before being evaporated under reduced pressure using the rotary vacuum evaporator to yield the EtOAc-MeOH extracts of the cephalopods. The extracts were pooled with those obtained after sonication for further processing.
In vitro anti-diabetic activities of the EtOAc-MeOH extracts of cephalopods
The α-amylase inhibitory action was determined by mixing test samples (500 μL, 100–1000 mg/mL) and standard drug (α-carbose as positive control, 100–1000 mg/mL) separately, to the phosphate buffer (500 μL of 0.20 mM, pH 6.9) containing α-amylase (0.5 mg/mL) from porcine pancreas (Sigma-Aldrich Chemical Co. Inc., USA), before being incubated at 25°C for 10 min. Afterward, a starch solution (500 μL of a 1%) in sodium phosphate buffer (0.02 M, pH 6.9) was added to each tube. The reaction mixtures were, thereafter, incubated at 25°C for 10 min. The reaction was stopped with 3, 5-dinitrosalicylic acid (DNSA) color reagent (1.0 mL). The test tubes were then incubated in a boiling water bath for 5 min, and cooled at the room temperature. The reaction mixture was diluted with distilled water (10 mL), and the absorbance was recorded at 540 nm.[Citation19]
The inhibitory activity against α-glucosidase was determined by incubating a solution of starch substrate (2% w/v maltose or sucrose, 1 mL) with Tris buffer (0.2 M, pH 8) and various concentrations of EtOAc-MeOH extracts for 5 min at 37°C with minor modification of earlier methods.[Citation19] The reaction was initiated by adding α-glucosidase enzyme (1 mL of 1 U/mL yeast α-glucosidase, Sigma-Aldrich Chemical Co. Inc., USA) to the reaction mixture, followed by incubation for 10 min at 37°C. The reaction was terminated by heating the contents in a boiling water bath. DNSA (1 mL) was added with the product before being incubated for 5 min and added with distilled water (9 mL). The amount of liberated glucose was measured by glucose oxidase peroxidase method.[Citation19]
Another anti-diabetic assay using the method of DPP-4 inhibition was performed as described earlier.[Citation20] A pre-incubation volume 250 mL containing Tris HCl buffer (100 mM, pH 8.4), DPP-4 enzyme (7.5 mL of 0.2 U/mL) from porcine kidney (Sigma-Aldrich Chemical Co. Inc., USA) and various concentrations of test material/reference inhibitor were used. This reaction mixture was incubated at 37°C for 30 min, followed by addition of Gly-pro-pnitroanilide (10 mL of 1.4 mM substrate). The absorbance of the product was measured at 410 nm. Diprotein-A (Ile-Pro-Ile) was used as the standard drug. Percentage inhibition of DPP-4 (% I) was calculated by, % I = (Ac – As) /Ac × 100, where: Ac is the absorbance of the control and As is the absorbance of the sample. The results were expressed as IC90, the concentration at which it inhibits 90% of the enzyme activity.
In vitroanti-inflammatory assays
COX-1 and COX-2 inhibition assays were performed using 2, 7-dichlorofluorescein method with suitable modifications.[Citation21] In brief, leuco-2, 7-dichlorofluorescein diacetate (5 mg) was hydrolyzed at room temperature in 1 M NaOH (50 μL) for 10 min followed by the addition of 1 M HCl (30 μL) to neutralize the excess of NaOH before the resulting leuco-dichlorofluorescein (1-DCF) was diluted with 0.1 M Tris-buffer (pH 8). COX enzymes (COX-1 and COX-2) were diluted with 0.1 M Tris-buffer (pH 8), so that a known aliquot gave an absorbance change of 0.05/min in the test reactions. The test samples (or the equivalent volume of MeOH, 20 μL) were pre-incubated with the enzymes at room temperature for 5 min in the presence of hematin. Premixed phenol, 1-DCF and arachidonic acid were added to the enzyme mixture to initiate the reaction, and to give a final reaction mixture of arachidonic acid (50 μM), phenol (500 μM), 1-DCF (20 μM), and hematin (1 μM) in 1 mL final volume of 0.1 M Tris-buffer (pH 8). The reaction was recorded spectrophotometrically over 1 min at 502 nm. A blank reaction mixture (without enzyme) was analyzed in the spectrophotometer reference cell against each test reaction to account for any non-enzymatic activity attributed to the test sample.
5-LOX inhibition assay was carried out using the principle of 1-4 diene (linoleic acid) oxidations to 1-3-diene[Citation22] with suitable modifications. Briefly, an aliquot of the stock solution (50 μL, in dimethyl sulfoxide (DMSO) and tween 20 mixture; 29:1, w/w) of each test sample was placed in a 3 mL cuvette, followed by addition of pre-warmed 0.1 M potassium phosphate buffer (2.95 mL, pH 6.3) and linoleic acid solution (48 μL). Thereafter, ice-cold buffer (potassium phosphate; 12 μL) was added with 5-LOX (100 U) before being recorded spectrophotometrically at a wavelength of 234 nm. The control was prepared with DMSO: tween 20 mixture (no enzyme inhibition).
Spectroscopic analyses
The 1H-NMR spectral analysis of the the EtOAc-MeOH extracts of five cephalopod species were carried out on a Bruker AVANCE spectrometer (Bruker, DRX 600 MHz AV 600, Karlsruhe, Germany) in CDCl3 solvent. Tetramethylsilane (TMS, Cortec, Paris, France) was used as the internal standard (δ 0 ppm). Chemical shift (δ) values are expressed in parts per million (ppm), and referenced to the residual solvent signals of CDCl3. This study compared the characteristic functional groups present in each crude extracts. 1H-NMR spectra were integrated to get the aggregate number of protons in characteristic regions of the spectra (proton integral, ΣH, which included saturated hydrocarbons of primary, secondary, and tertiary origin (δ 0.5–2.0), functionalized hydride group of alkyl alkanoates/allylic/acetyl groups (δ 2.0–2.5), functionalized hydride of the substituted alkanol/methoxy (δ 2.5–3.5), protons of the parent hydride group of alkyl alkanoates/olefinic (δ 3.5–6.5), and aryl protons (δ 6.5–8.5).
Statistical analyses
Statistical evaluation was carried out by the SPSS software (SPSS Inc, Chicago, USA, ver. 13.0). Descriptive statistics were ascertained for all the contemplated attributes. Analyses were carried out in triplicate and the means of all parameters were examined for significance (p < 0.05) by analysis of variance (ANOVA). The Pearson correlation coefficient (r) was ascertained (p < 0.05) to assess the strength of the linear relationship between two variables.
Results and discussion
General
Marine organisms were shown to be rich sources of bioactive compounds, which have a positive influence on human health and may open a new perspective for pharmacological development.[Citation23–Citation26] Cephalopods are mollusk candidates, which constitute a major share of marine fauna, were reported to possess structurally diverse anti-stress metabolites with respect to bioactive properties.[Citation27] Even though many bioactive compounds have been isolated from marine animals, much of the potential compounds still remain unharnessed especially the organisms belonging to the class Cephalopoda of phylum Molluska. The present study is the first of its kind to report the bioactive properties of commonly available cephalopods namely, A. marginatus, U. duvauceli, S. pharaonis, S. inermis, and C. indicus with regard to their anti-diabetic and anti-inflammatory activities by various in vitro pharmacological models.
Recovery of the EtOAc-MeOH extracts of cephalopods
The yield of the EtOAc-MeOH extracts derived from A. marginatus, U. duvauceli, S. pharaonis, S. inermis, and C. indicus were found to be 4.3, 2.9, 4.1, 3.9, and 2.2%, respectively.
In vitroanti-diabetic activities of the EtOAc-MeOH extracts of cephalopods
The EtOAc-MeOH extract of S. inermis exhibited higher α-glucosidase inhibitory activity (IC90 2.42 mg/mL) compared to C. indicus and A. marginatus (IC90 2.8 mg/mL; p > 0.05). It is of note that the postprandial hyperglycemia is a key problem in the pathologies of T2DM. Ingestion of carbohydrate-rich diet causes elevation in blood glucose level by the rapid absorption of carbohydrates in the intestine aided by the action of glycoside hydrolases, which breaks dietary carbohydrates into absorbable monosaccharides.[Citation28] Thus, the use of glycosidase inhibitor, such as α-glucosidase and α-amylase inhibitors, would be a prospective therapeutic agent for the effective management of diabetes. The EtOAc-MeOH extracts of cephalopods assayed for α-glucosidase inhibition exhibited higher inhibitory effect (IC90 2.4–5.0 mg/mL; ) than those of other classes of mollusks.[Citation15] Although there have been no reports with regard to anti-diabetic and anti-inflammatory potential of cephalopod species, several authors reported bioactive properties of different groups of mollusks of marine origin.[Citation15,Citation29–Citation31] For example, Ravi et al.[Citation15] observed that the methanol extract of gastropod mollusk Hemifusus pugilinus exhibited greater anti-α-glucosidase activity (IC50 20.27 mg/mL) than the methanol extract of Natica didyma (IC50 56.44 mg/mL), although the anti-diabetic properties of this group of mollusks were significantly lesser than the EtOAc-MeOH extracts of the cephalopods considered in the present study (IC90 1.69–5.37 mg/mL; p < 0.05). The methanol extract of the marine gastropod mollusk Cerithidea obtusa extract was found to posses moderate anti-α-glucosidase inhibitory activity (IC50 36.40 mg/mL).[Citation30]
Table 1. Anti-diabetic and anti-inflammatory activities (IC90, mg/mL) of the EtOAc-MeOH extracts of the cephalopods.
The α-amylase inhibitory activities of C. indicus, S. inermis, and U. duvauceli were recorded to be significantly greater (IC90 ~ 1.7 mg/mL) when compared with other cephalopods in the present study (IC90 1.9 – 2.5 mg/mL; p < 0.05). A. marginatus displayed least α-amylase inhibitory activity (IC90 2.50 mg/mL) because of greater IC90 value than the other cephalopod species (IC90 lesser than 2 mg/mL). Notably, the pancreatic α-amylase is a key enzyme in the digestive system and catalyses the initial step in hydrolysis of starch to a mixture of smaller oligosaccharides consisting of maltose, maltotriose, and a number of small molecular weight α-(l–6) and α-(1–4) oligoglucans. These are then acted on α-glucosidases and further degraded to glucose, which on absorption enters the blood-stream.[Citation28] Degradation of this dietary starch proceeds rapidly and leads to elevated post-prandial hyperglycemia (PPHG). Retardation of starch digestion by inhibition of enzymes, such as α-amylase, plays a key role in the control of diabetes. Inhibitors of pancreatic α-amylase delay carbohydrate digestion causing a reduction in the rate of glucose absorption and lowering the post-prandial serum glucose levels.[Citation16] Therefore, the anti-diabetic potential of EtOAc-MeOH extract of cephalopods by α-amylase inhibition demonstrated its effectiveness as an anti-diabetic agent. The solvent extracts derived from the members of the order Octopoda demonstrated fairly good α-amylase inhibitory activity (IC90 ≤ 2.5 mg/mL), and in which, C. indicus displayed highest anti-α-amylase property (IC90 1.69 mg/mL). Anti α-amylase activities of the mollusks were supported by the earlier work of Sadhasivam et al.,[Citation16] which explained the α-amylase inhibitory properties of the methanolic extract of three marine mollusks, namely Aplysia sp, Bursatella leachii, and Kalinga ornata (93.0, 70.6, and 50.0%, respectively, 0.1 mg/mL). Abirami et al.[Citation32] also observed moderate α-amylase inhibitory activity by the purple fluid of the marine gastropod mollusk Dolabella auricularia. An α-amylase inhibition of 72% was observed by Ravi et al.[Citation15] for the methanolic extract of two marine mollusks Hemifusus pugilinus and Natica didyma. Reports of Tiwari et al.[Citation33] confirmed the anti-glycemic activities of the crude extracts of bivalve mollusks in animal model. It is significant to note that the cephalopod species, particularly C. indicus displayed potential anti-diabetic activities as determined by in vitro α-amylase/α-glucosidase inhibition assays. More importantly, the anti-diabetic activities of the mollusks belonging to the class Cephalopoda were found to be greater than other classes of mollusks (Gastropoda and Bivalvia) reported in the literature.[Citation32,Citation33]
DPP-4 inhibitory activities of the EtOAc-MeOH extracts of C. indicus (IC90 2.51 mg/mL), S. inermis (IC90 3.35 mg/mL), and A. marginatus (IC90 3.60 mg/mL) were found to be significantly greater than those displayed by the other cephalopod species. Notably, the IC90 of α-glucosidase and DPP-4 inhibitory activity of the EtOAc-MeOH extract of S. pharaonis was 5.37 mg/mL, which displayed its significantly lesser anti-diabetic properties compared to other species of cephalopods considered in the present study ().
The present study described the DPP-4 inhibitory activities of EtOAc-MeOH extracts from five different species of cephalopods. DPP-4 inhibitory activity of the EtOAc-MeOH extract of C. indicus was found to be significantly greater (IC90 2.51 mg/mL) than those derived from other titled cephalopod species (IC90 3.4-5.4 mg/mL; p < 0.05). However, there were no significant difference in DPP-4 inhibitory activities (IC90 3.4–3.6 mg/mL) of the EtOAc-MeOH extracts acquired from A. marginatus and S. inermis (). The potential anti-diabetic effect of the solvent extract from C. indicus, in particular, might, therefore, be attributed to the inhibitory effects against DPP-4 that retard the degradation of serine protease incretin hormones (glucagon like peptide and glucose-dependent insulinotropic polypeptide) to increase the production and release of insulin from the pancreatic β-cells.[Citation14] Thus, it is essential to arrest the activity of the enzyme DPP-4 in order to effectively maintain the serum glucose levels, and therefore, research has been focused on the strategy to inhibit the DPP-4 activity, and thereby preserving the activity of endogenous incretins.[Citation14,Citation34,Citation35] U. duvauceli and S. pharaonis displayed significantly lesser anti DPP-4 activities (IC90 > 4.5 mg/mL). It is significant to note that α-glucosidase and DPP-4 inhibitory activities (IC90 5.37 mg/mL) of the EtOAc-MeOH extracts from S. pharaonis was lowest among the cephalopod species considered in the present study (). The present study unequivocally proved that the two species belonging to Octopoda C. indicus and A. marginatus posses potential bioactivities capable of inhibiting the DPP-4 enzyme. Therefore, these species can be considered to be the potential candidate species for use against diabetes.
In vitroanti-inflammatory activities of the EtOAc-MeOH extracts of cephalopods
The in vitro anti-inflammatory activities of the cephalopods were studied using three assays, namely COX-1, COX-2 and 5-LOX inhibition assay. Inhibition of COX-1 and COX-2 and lipoxygenases has been a major strategy for alleviating the inflammation.[Citation25] The EtOAc-MeOH extracts of C. indicus exhibited significantly greater (p < 0.05) COX-1 and COX-2 inhibition activity (IC90 ~ 1 mg/mL) when compared to other cephalopod species. This was followed by A. marginatus and S. inermis, which demonstrated an IC90 value of 1.38 and 1.42 mg/mL, respectively, in descending order of anti COX-1 activity. There was no significant difference (p > 0.05) in the COX-2 inhibitiory activity of the EtOAc-MeOH extracts from S. inermis and A. marginatus (IC90 1.5 mg/mL). Likewise, C. indicus exhibited greater 5-LOX inhibitory activity (IC90 1.69 mg/mL) compared to the other cephalopod species considered in this study (IC90 > 2 mg/mL). EtOAc-MeOH extract of U. duvauceli recorded comparatively lesser anti-inflammatory activity with regard to inhibit COX-1 (IC90 2.20 mg/mL), COX-2 (IC90 2.08 mg/mL), and 5-LOX (IC90 2.42 mg/mL) than the other species of cephalopods (). Although no reports are available on the anti-inflammatory activities of cephalopods, there were reports on anti-inflammatory activities of mollusks from the previous literatures, mainly, the lipid rich extracts from the freeze-dried mussel powder (Lyprinol®),[Citation36] and methanol (MeOH) extracts of marine mollusks named Cypraea errones and Cypraea arabica.[Citation37] Chellaram and Edward reported the anti-inflammatory activities of the reef-associated mollusks, Trochus tentorium and Drupa margariticola.[Citation38,Citation39]
1H-NMR of the EtOAc-MeOH extracts of the cephalopod species provided a clear idea to compare the signal pattern and their intensities among distinctive functional group protons responsible for bioactive potentials. This gave an understanding into the various types of functional groups present in the EtOAc-MeOH extracts, and their relation with their anti-inflammatory and anti-diabetic activities. In particular, the labeling of protons associated with different magnetic environments of the functional groups exhibited in the EtOAc-MeOH extracts were analyzed by 1H-NMR spectroscopy ( and ), which corroborated the results obtained by the in vitro anti-diabetic and anti-inflammatory assays. Accordingly, the characteristic functional groups in specific regions with proton integral values were compared ( and ). The aliphatic hydrocarbons in the δH 0.1–2.0 region were found to be prominent in C. indicus, U. duvauceli, and S inermis compared to S. pharaonis and U. duvauceli. This region mainly accounted for –(CH2)n and –(CH3)n in straight chain, which probably linked to glycolipids, steroids, and other lipidic analogues.
Figure 1. Proton integral values in characteristic regions of 1H-NMR spectra acquired from the EtOAc-MeOH extracts of the five cephalopod species. The protons at the characteristic regions of the 1H-NMR spectra were integrated to get the proton integral (ΣH) in specific regions (δ0.5–2.0 saturated hydrocarbons; δ2.0–2.5 functionalized hydride group of alkyl alkanoates/allylic/acetyl groups; δ 2.5–3.5 functionalized hydride of the substituted alkanol/methoxy; δ 3.5–6.5 protons of the parent hydride group of alkyl alkanoates/olefinic; δ 6.5–8.5 aryl protons).
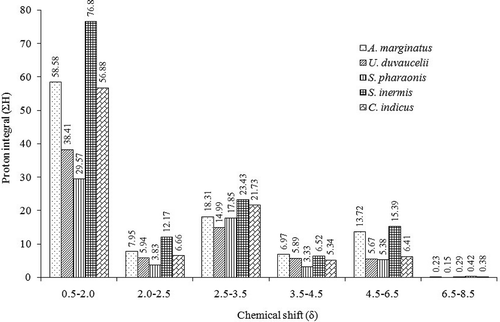
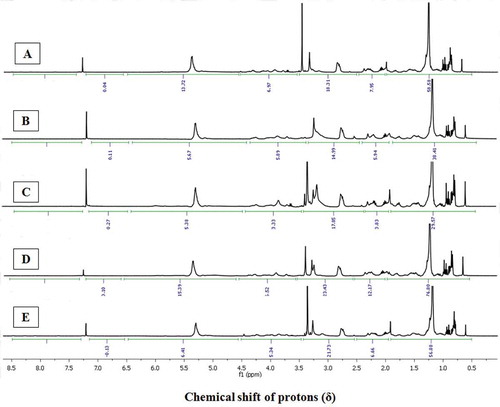
Figure 2. The 1H-NMR spectra of EtOAc-MeOH extracts of A: A. marginatu; B: U. duvauceli; C: S. pharaonis; D: S. inermis; and E: C. indicus. Chemical shift (δ) values were expressed in ppm, and were referenced to solvent signals of CDCl3.
Notably, the greater proton integral (ΣH) of the protons in 1H-NMR spectra due to saturated hydrocarbons (at δH 0.1–2) of the EtOAc-MeOH extracts derived from C. indicus, A. marginatus and S inermis (ΣH: 56.88, 58.58, and 76.80, respectively; ) were probably linked with long-chain fatty acids, phospholipids, sterols, or glycolipids. Greater logPOW values (1-octanol/water partition coefficients) of the lipidic compounds could possibly lead to the greater intermembrane permeability of the bioactive principles in the cell, thereby resulting in higher anti-inflammatory and anti-diabetic properties. The lipidic compounds belonging to phospholipids, sterols, or glycolipids might possess substituents of polar characteristics, particularly belonging to functionalized hydride group of alkyl alkanoates/allylic/acetyl groups (δH 2.0–2.5), functionalized hydride of the substituted alkanol/methoxy (δH 2.5–3.5), oxygenated functional moieties (δH 3.5–4.5), and alkenic groups (δH 4.5–6.5). This has been demonstrated by the greater proton integrals (ΣH) in these distinctive regions of 1H-NMR spectra of S. inermis (ΣHδ2.0–2.5 12.17, ΣHδ2.5–3.5 23.43, ΣHδ3.5–4.5 6.52, ΣHδ4.5–6.5 15.39), C. indicus (ΣHδ2.0–2.5 6.66, ΣHδ2.5–3.5 21.73, ΣHδ3.5–4.5 5.34, ΣHδ4.5–6.5 6.41), and A. marginatus (ΣHδ2.0–2.5 7.95, ΣHδ2.5–3.5 18.31, ΣHδ3.5–4.5 6.97, ΣHδ4.5–6.5 13.72; and ). It is of note that the DPP-4 scavengers, which possess electron withdrawing functionalities, can potentially react with the hydroxyl groups present in the active site amino acyl residues (particularly, tyrosyl, and seryl) of the DPP-4 enzyme. The greater proton integral of acyl or acetoxy groups in S. inermis and C. indicus might be the cause of greater anti-diabetic potential toward DPP-4, which was comparable with our earlier study on bivalve mollusks.[Citation40] The intense proton signals in the 1H-NMR spectra of the EtOAc-MeOH fractions of S. inermis, A. marginatus, and C. indicus might be due to the presence of RC(=O)CH3 or RCH2C(=O)OR1 groups, which prevent the development reactive oxygen species, thereby ceasing the free-radical induced inflammatory responses. Notably, the proton integrals in the downfield region of the 1H-NMR spectra at about δH 2–2.5 and 4.5–6.5 (olefinic protons) were frail for S. pharaonis (ΣH 3.83 and 5.38, respectively) and U. duvauceli (ΣH 5.94 and 5.67, respectively), which established that these functional groups were present in lesser intensities. On the contrary, the EtOAc-MeOH extract of C. indicus and S. inermis displayed greater proton integrals of highly electronegative moieties appeared in the low-field region in the 1H-NMR spectra. A significant co-linearity was found to exist between the electronegative groups present in the downfield position of 1H-NMR spectra vis-à-vis anti-diabetic and anti-inflammatory activities of EtOAc-MeOH fractions from the cephalopods C. indicus and S. inermis. Likewise, the greater anti-diabetic and anti-inflammatory potentials of C. indicus and S. inermis compared to other cephalopods were validated by 1H-NMR spectroscopic experiments.
Conclusions
As a part of the ongoing research program carried out at our laboratory to explore novel sources of bioactivities from the marine organisms, different species of deep sea cephalopods particularly belonging to Cystopus indicus, Sepiella inermis and Amphioctopus marginatus were identified as potential sources of bioactive leads with respect to anti-inflammatory and anti-diabetic properties. The utilities of 1H-NMR to assess the abundance of the bioactive functional groups present in the EtOAc-MeOH extracts of the cephalopod species and to illustrate the principles with regard to the presence of these functional groups vis-à-vis anti-inflammatory and anti-diabetic activities have been demonstrated. A significant co-linearity was found between the target bioactivities and the occurrence of the electronegative groups disposed in the downfield locale of 1H-NMR spectra. The present study provided a detailed anti-diabetic and anti-inflammatory profile of the commercially important cephalopod species, for the first time. In particular, Sepiella inermis, Amphioctopus marginatus, and Cystopus indicus can potentially be considered as new sources of important health foods, and can be used in formulating various nutraceuticals and functional food ingredients for biopharmaceutical industries in combating diabetes and inflammatory diseases.
Nomenclature
| COX-1 | = | cyclooxygenase-1 |
| COX-2 | = | cyclooxygenase-2 |
| 5-LOX | = | 5-lipoxygenase |
| DPP-4 | = | dipeptidyl peptidase-4 |
| 1H-NMR | = | proton nuclear magnetic resonance |
| ANOVA | = | analysis of variance |
| DMSO | = | dimethyl sulfoxide |
| EtOAc-MeOH | = | ethylacetate-methanol |
| 1-DCF | = | leuco-dichlorofluorescein |
| GLP-1 | = | glucagons like peptide |
| GIP | = | glucose-dependent insulinotropic polypeptide |
| T2DM | = | type 2 diabetes mellitus |
| DCF | = | leuco-dichlorofluorescein |
Declaration of conflicts
The authors declare that there is no conflict of interest
Acknowledgments
The authors thank the Director, Indian Council of Agricultural Research-Central Marine Fisheries Research Institute (ICAR-CMFRI), for his guidance and support. Thanks are due to the Head, Marine Biotechnology Division for facilitating the research works.
Funding
This work was supported by the funding under the project “Drugs from the sea” (grant number MoES-2/DS/6/2007 PC-IV) from Ministry of Earth Science (MoES), New Delhi, India. Minju Joy acknowledges MoES, for the award of a scholarship.
Additional information
Funding
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global Estimates of the Prevalence of Diabetes for 2011 and 2030. Diabetes Research and Clinical Practice 2011, 94(3), 311–321.
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The Pathogenesis and Pathophysiology of Type 1 and Type 2 Diabetes Mellitus. Journal of Physiology and Pathophysiology 2013, 4(4), 46–57.
- Guest, C.B.; Park, M.J.; Johnson, D.R.; Freund, G.G. The Implication of Proinflammatory Cytokines in Type 2 Diabetes. Frontiers in Bioscience 2008, 13, 5187–5194.
- Rowley, A.F. The Evolution of Inflammatory Mediators. Mediators of Inflammation 1996, 5, 3–13.
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435.
- Soehnlein, O.; Lindbon, L. Phagocyte Partnership During the Onset and Resolution Of Inflammation. Nature Reviews Immunology 2010, 10, 427–439.
- Greenfield, J.R.; Campbell, L.V. Relationship Between Inflammation, Insulin Resistance and Type 2 Diabetes: “Cause Or Effect?” Current Diabetes Reviews 2006, 2(2), 195–211.
- Spranger, J.; Kroke, A.; Mohlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M. Inflammatory Cytokines and the Risk to Develop Type 2 Diabetes: Results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817.
- Herder, C.; Brunner, E.J.; Rathmann, W.; Strassburger, K.; Tabak, A.G.; Schloot, D.R. Elevated Levels of the Anti-Inflammatory Interleukin-1 Receptor Antagonist Precede the Onset of Type 2 Diabetes: The Whitehall II Study. Diabetes Care 2009, 32, 421–423.
- Weir, G.C.; Weir, S.B. Five Stages of Evolving Beta-Cell Dysfunction During Progression to Diabetes. Diabetes 2004, 53(3), S16–S21.
- Dinarello, C.A. Anti-Inflammatory Agents: Present and Future. Cell 2010, 140(6), 935–950.
- Boni-Schnetzler, M.; Thorne, J.; Parnaud, G.; Marselli, L.; Ehses, J.A.; Kerr-Conte, J.; Pattou, F.; Halban, P.A.; Weir, G.C.; Donath, M.Y. Increased Interleukin (IL)-1β Messenger Ribonucleic Acid Expression in Beta-Cells of Individuals with Type 2 Diabetes and Regulation of IL-1β in Human Islets by Glucose and Auto-Stimulation. Journal of Clinical Endocrinology Metabolism 2008, 93, 4065–4074.
- Akash, M.S.; Shen, Q.; Rehman, K.; Chen, S. Interleukin-1 Receptor Antagonist: A New Therapy for Type 2 Diabetes Mellitus. Journal of Pharmaceutical Sciences 2012, 101, 1647–1658.
- Kim, H.J.; Kwak, W.Y.; Min, J.P.; Lee, J.Y.; Yoon, T.H.; Kim, H.D. Discovery of DA-1229: A Potent, Long Acting Dipeptidyl Peptidase-4 Inhibitor for the Treatment of Type 2 Diabetes. Bioorganic and Medicinal Chemistry Letters 2011, 21, 3809–3812.
- Ravi, C.; Karthiga, A.; Venkatesan, V. Isolation and Biomedical Screening of the Tissue Extracts of Two Marine Gastropods Hemifusus Pugilinus (Born, 1778) and Natica Didyma (Roding, 1798). Asian Fisheries Science 2012, 25, 158–169.
- Sadhasivam, G.; Muthuvel, A.; Vitthal, W.M.; Pachaiyappan, A.; Kumar, M.; Thangavel, B. In Vitro Antibacterial, Alpha-Amylase Inhibition Potential of Three Nudibranch Extracts from South East Coast of India. Journal of Coastal Life Medicine 2013, 1, 186–192.
- Anusree, M.; Chakraborty, K.; Makkar, F. Pharmacological Activities of Brown Seaweed Sargassum Wightii (Family Sargassaceae) Using Different in Vitro Models. International Journal of Food Properties 2016, DOI: 10.1080 ( published online).
- Anbuselvi, S.; Chellaram, C.; Jonesh, S.; Jayanthi, A.; Edward, J.K.P. Bioactive Potential of Coral Associated Gastropod, Trochus Tentorium of Gulf of Mannar, Southeastern India. Journal of Medical Sciences 2009, 9, 240–244.
- Hamdan, I.I.; Afifi, F.U. Studies on the in Vitro and in Vivo Hypoglycemic Activities of Some Medicinal Plants Used in Treatment of Diabetes in Jordanian Traditional Medicine. Journal of Ethnopharmacology 2004, 93, 117–121.
- Kojima, K.; Ham, T.; Kato, T. Rapid Chromatographic Purification of Dipeptidyl Peptidase-IV in Human Submaxiallry Gland. Journal of Chromatography 1980, 189, 233–240.
- Larsen, L.N.; Dahl, E.; Bremer, J. Peroxidative Oxidation of Leuco Dichloroluorescein by Prostaglandin H Synthase in Prostaglandin Biosynthesis from Polyunsaturated Fatty Acids. BBA-Lipid Metabolism 1996, 1299(1), 47–53.
- Baylac, S.; Racine, P. Inhibition of 5-Lipoxygenase by Essential Oils and Other Natural Fragment Extracts. International Journal of Aromatherapy 2003, 13(2–3), 138–142.
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Natural Product Reports 2005, 22, 15–61.
- Makkar, F.; Chakraborty, K. Antidiabetic and Anti-Inflammatory Potential of Sulphated Polygalactans from Red Seaweeds Kappaphycus Alvarezii and Gracilaria Opuntia. International Journal of Food Properties 2016, DOI:10.1080/10942912.2016.1209216
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant Activities and Phenolic Contents of Three Red Seaweeds (Division: Rhodophyta) Harvested from the Gulf of Mannar of Peninsular India. Journal of Food Science and Technology 2014, 52, 1189–2002.
- Chakraborty, K.; Joseph, D.; Joy, M.; Raola, V.K. Characterization of Substituted Aryl Meroterpenoids from Red Seaweed Hypnea Musciformis as Potential Antioxidants. Food Chemistry 2016, 212, 778–788.
- Chandran, B.; Kumar, G.R.; Ravichandran, S. Antimicrobial Activity from the Gill Extraction of Perna Viridis (Linnaeus 1758). Global Journal of Biotechnology and Biochemistry 2009, 4(2), 88–92.
- Berdanier, C.D.; Dwyer, J.T.; Feldman, E.B. Handbook of Nutrition and Food; 2nd Ed; CRC Press: Boca Raton, FL, 2007.
- Chakraborty, K.; Chakkalakal, S.J.; Joseph, D. Response of Pro-Inflammatory Prostaglandin Contents in Anti-Inflammatory Supplements from Green Mussel Perna Viridis L. in a Time-Dependent Accelerated Shelf-Life Study. Journal of Functional Food 2014, 7, 527–540.
- Cahyani, R.T.; Purwaningsih, S.; Fitria, A. Anti-Diabetic Potential and Secondary Metabolites Screening of Mangrove Gastropod Cerithidea Obtuse. Journal of Coastal Life Medicine 2015, 3(5), 356–360.
- Chakraborty, K.; Chakkalakal, S.J.; Joseph, D.; Asokan, P.K.; Vijayan, K.K. Nutritional and Antioxidative Attributes of Green Mussel (Perna Viridis L.) from the Southwestern Coast of India. Journal of Aquatic Food Product Technology 2016, DOI:10.1080/10498850.2015.1004498
- Abirami, P.; Arumugam, M.; Ajithkumar, T.T.; Balasubramaniam, T. Isolation and Characterization of 37 Kda Heparinise from the Purple Fluid of Dolabella Auricularia. Indian Journal of Marine Sciences 2011, 40, 112–116.
- Tiwari, P.; Rahuja, N.; Kumar, R.; Lakshmi, V.; Srivastava, M.N.; Agarwal, S.C. Search for Anti-Hyperglycemic Activity in Few Marine Flora and Fauna. Indian Journal of Science and Technology 2008, 1, 1–5.
- Mulakayala, N.; Reddy, C.H.U.; Iqbal, S.; Pal, J.M. Synthesis of Dipeptidyl Peptidase-4 Inhibitors: A Brief Overview. Tetrahedron 2010, 66, 4919–4938.
- Green, B.D.; Gault, V.A.; Flatt, P.R. Comparative Effects of GLP-1 and GIP on cAMP Production, Insulin Secretion, and in-Vivo Anti-Diabetic Actions Following Substitution of Ala8/Ala2 with 2-Aminobutyric Acid. Archives Biochemistry and Biophysics 2004, 428, 136–143.
- Halpem, G.M. Anti-Inflammatory Effects of a Stabilized Lipid Extract of Perna Canaliculus (Lyprinol). Allergy Immunology 2000, 32, 272–278.
- Kumar, S.S. Studies on the cowries (Mollusca: Gastropoda: Cypraeidea) of Gulf of Mannar, South east coast of India. Ph.D. Thesis submitted to Manonmaniam Sundaranar University, Tirunelveli, 2003; pp. 1–186.
- Chellaram, C.; Edward, J.K.P. Anti-Inflammatory Potential of Coral Reef Associated Gastropod, Drupa Margariticola. Indian Journal of Science and Technology 2009a, 2, 75–77.
- Chellaram, C.; Edward, J.K.P. In Vivo Anti-Inflammatory Bustle of Reef Associated Mollusc, Trochus Tentorium. Advanced Biotechnology 2009b, 9, 32–34.
- Joy, M.; Chakraborty, K.; Pananghat, V. Comparative Bioactive Properties of Bivalve Clams Against Different Disease Molecular Targets. Journal of Food Biochemistry 2016, DOI:10.1111/jfbc.12256
