ABSTRACT
Aloe, eugenol, and thymol were combined in package for improving the quality and bioactive components of tomato during storage. Packaged tomatoes with these compounds were stored at 10 ± 1oC and 85 ± 5% relative humidity for 16 days and lycopene, phenolics, ascorbic acid, antioxidant activity, microbial spoilage (counts), and total soluble solids of tomatoes were evaluated. Higher levels of lycopene, phenolics, and ascorbic acid were observed for aloe-, thymol-, or eugenol-incorporated to modified atmosphere packages, from which the aloe gel showed the highest efficiency. High and significant correlation was found between antioxidant activity and total phenolics content (R2 = 0.72**, p < 0.01) of stored tomatoes. Beside this, aloe gel was the most effective treatment in reducing the microbial counts of tomato inside the package followed by thymol and eugenol during storage.
Introduction
Tomato (Solanum lycopersicum Mill) has been known as a nutritious fruit for a long time but its antioxidant properties that demonstrate health benefits including alleviation of chronic and cardiovascular diseases are more pronounced in last decades.[Citation1–Citation3] The fruit contains numerous bioactive compounds with antioxidant properties, which attributed to carotenoids,[Citation2,Citation4] total phenolics (TP), and ascorbic acid.[Citation5,Citation6] It also has been shown that the antioxidant potential of tomato varies among pulp, skin, and seed of fruits[Citation5] and its ripening stages.[Citation7,Citation8]
Maintaining fruit quality in terms of appearance, flavor, and nutritive value is crucial. Modified atmosphere packaging (MAP) has shown as an effective way in quality maintenance of fresh produce by decreasing weight loss and delayed-respiration, but it seems that traditional (passive) MAP is not able to ensure the quality and safety issues to fulfill consumer demand.[Citation9,Citation10] Passive MAP increases the humidity inside the package, and, therefore, enhances the risk of microbial growth,[Citation11] while addition of antioxidants to gelatin-calcium carbonate composite films (active MAP) facilitated the conformational changes of the secondary structure and functional group of gelatin in films which improved radical scavenging capacities of composite films.[Citation12]
Alternaria alternata, Rhizopus stolonifera, and Botrytis cinerea are considered the most important pathogens of deterioration in tomato.[Citation13] Different treatments such as ozone cyclic exposure,[Citation14] ultraviolet-B (UV-B) irradiation,[Citation15] and incorporation of methyl jasmonate with MAP[Citation16] were used to maintain postharvest quality and enhancing antioxidant capacity in tomato; however, due to global concern about environment and human health, more convenient treatments need to be developed. Recently, it has been shown that the edible coating of cherry tomatoes, hydroxypropyl methylcellulose-beeswax formulated with antifungal food additives, reduced the Alternaria black spot and maintained post-harvest quality during cold storage.[Citation17]
Incorporation of natural compounds such as aloe gel, essential oils (EOs) into package with natural-base compound to protect packaged fruits from microbial spoilage, and, therefore, extending the shelf life of produce has overcome the extensive uses of chemicals and irradiation.[Citation18] The efficiency of eugenol, thymol, and menthol in combination with MAP was reported in controlling microbial spoilage of table grape berry[Citation19] and sweet cherry.[Citation20] Besides reducing the occurrence of decay, the overall fruit quality of table grape and sweet cherry were preserved during post-harvest period in those fruit that treated by EOs. Also many EOs of medicinal and aromatic plants have been confirmed to possess the antioxidant potential, which may play an important role in neutralizing free radicals, quenching singlet oxygen, or decomposing hazardous peroxides.[Citation21]
The antimicrobial effect of aloe gel as an edible coating has recommended in many studies in fruits. Also it has been found that microbial populations were significantly reduced in aloe-treated sweet cherries than untreated fruits.[Citation22] Therefore, the purpose of this study was to determine the incorporation of eugenol, thymol and aloe gel into MAP in retaining bioactive components and reducing decay potential of fruit during storage under modified atmosphere condition. Also, as part of present study the possible correlation of proposed antioxidant potential of component was discussed.
Materials and methods
Plant material and treatments
Tomato fruits cv. Assale were obtained from a commercial greenhouse near Rafsanjan (30o30ʹN, 56o05ʹE). Fruits were harvested at the mature red stage (surface color of the fruit was red) and transferred to laboratory immediately. Tomatoes free of visual defects and uniform in color and size were selected, randomized, and divided into 32 experimental units. Each treatment has four replications and contains eight individual fruits. Another four experimental units were used to analyze the characteristics of the fruits at harvest. The following four treatments were considered: (1) immersion in a solution of aloe vera gel (pharmaceutical quality, 100% purity) manufactured by Roig Farma S.A. (Tarrasa, Barcelona, Spain) which was diluted 1:3 with distilled water for 8 min;[Citation22] (2) and (3) treatments with eugenol or thymol (99.5% purity and purchased from Sigma), were performed by placing 75 μL of these EOs on sterile gauze inside the package, avoiding the contact with the fruit, and hermetically sealed;[Citation20] and (4) immersion in distilled water served as control. All treatments were packed in a container (60 × 165 × 245 mm) and sealed with low-density polyethylene film (LDPE), which its permeability to carbon dioxide and oxygen is 7700–77,000 and 3900–13,000 cm3/m2/mil/day/atm, respectively,[Citation23] then stored at 10 ± 1oC and 85 ± 5% relative humidity (RH) in permanent darkness.
Microbiological and total soluble solids (TSS) analysis
For all microbiological counts, 10 g of sample were weighed under sterilized condition and transferred into 90 mL 1% peptone water and homogenized using a stomacher (Bag Mixer 400, InterScience, France). A dilution series of each sample was prepared from 10−1 to 10−6, medium used was plate count agar for yeast and mold counts (PetrifilmTM Aerobic Count Plate, Laboratories 3MTM Sante, France). After incubation for 3 days at 25oC, colonies on the plates corresponding to dilutions showing between 30 and 300 and colony forming units (CFUs) were considered.[Citation20] The results were expressed as Log CFU g−1. All tests were run in duplicate. TSS was measured using a pocket digital refractometer (Atago Co., Ltd., Tokyo, Japan) with a range of 0–32%, by placing 1–2 drops of clear fruit juice.
Ascorbic acid and lycopene determination
Ascorbic acid content was determined by titrating a known weight of sample against 2,6-dichlorophenol-indophenol dye using 3% metaphosphoric acid as extracting medium. L-ascorbic acid was used as standard and the results were expressed as mg 100 g−1 fresh weight (FW).[Citation24] Lycopene extraction was based on the method described previously.[Citation25] In brief, tomato fruits were finely ground using mortar and pestle. Ground tissues were kept on ice and out of light after preparation and until assayed. Approximately 0.5 g duplicate samples were weighed from each sample into two vials that contained 5 mL of 0.5% (w/v) butylated hydroxytoluene (BHT) in acetone, 5 mL of 95% ethanol, and 10 mL of hexane. After shaking, 3 mL of deionized water were added to each vial and the samples were shaken for an additional 5 min on ice. The vials were then left at room temperature for 5 min to allow for phase separation. The absorbance of the upper, hexane layer was measured in a 1 cm path length quartz cuvette at 503 nm blanked with hexane. The lycopene content of each sample was then estimated using the absorbance at 503 nm. The amounts of lycopene in tissues were then estimated by the following formula:
A503 the absorbance at 503 nm and 3.12 is the extinction coefficient.[Citation25]
Measurements of antioxidant activity (AA) and TP
AA and TP were determined by the method previously described,[Citation26] with slight modification. For each sample, 5 g of fruit pulp were homogenized in 10 mL of 50 mM phosphate buffer pH = 7.8 and then centrifuged at 13,000 rpm for 15 min at 4°C. The supernatant as extract was used for AA and TP quantification in duplicate. The radical-scavenging capacity of extracts of fresh samples was measured using the enzymatic system composed of the ABTS (2, 20 azinobis 3-ethylbenzothiazoline-6-sulfonic acid diammonium salt, Sigma–Aldrich) the horse radish peroxidase enzyme, and its oxidant substrate (hydrogen peroxide), in which ABTS•+ radicals are generated and monitored at 730 nm with a ultraviolet-visible (UV-Vis), recording spectrophotometer (Helios Zeta UVVIS, Thermo Fisher Scientific, UK), at exactly 1 min after initial mixing. The decrease in absorbance after adding the extract was proportional to AA of the sample. Ascorbic acid was used to prepare the standard curve and activity was reported as mg ascorbic acid equivalent 100 g−1.
TP were determined using the Folin–Ciocalteau reagents. In brief, the extracts were appropriately diluted and then oxidized with 2.5 mL of freshly diluted (1:10) 2 N Folin–Ciocalteau reagent. This reaction was neutralized by adding 2.0 mL of 7.5% w/v sodium carbonate, and the samples were shacked by vortex for 20 s. The samples were then incubated at 50oC for 5 min and the absorbance was measured at 760 nm. Gallic acid was used as standard, and results were expressed as milligram gallic acid equivalents 100 g−1.[Citation26]
Statistical analysis
Data from measured parameters were subjected to analysis of variance (ANOVA). Mean comparisons were performed using Tukey test to examine if differences between treatments and storage time were significant at p < 0.05. Correlations were performed between TP and lycopene with AA taking into account all sampling dates.
Results and discussion
Lycopene and ascorbic acid
As shown in , after 8 days of storage the lycopene content was higher in EOs treatments while there was no significant difference between aloe and control treatments. However, at the end of experiment, it was higher in aloe-treated fruit followed by eugenol, control, and thymol treatments. After 8 days of storage, no significant differences was observed in ascorbic content of tomato fruit in different treatments (), but it was higher in EOs and aloe-treated tomato in compare to control at the end of storage.
Figure 1. A: Changes in lycopene; and B: Ascorbic acid content of control. Aloe, eugenol, and thymol in tomatoes (cv. Assale) during storage at 10oC (values are mean ± standard errors).
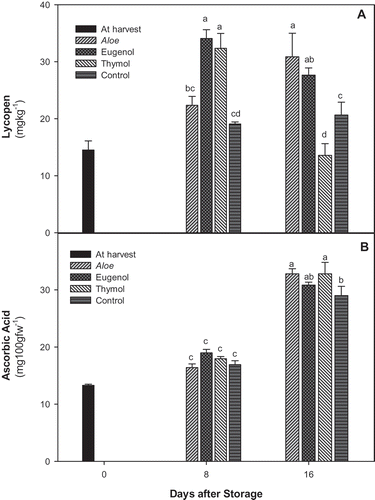
Lycopene content increased from maturity to ripening stages and then decreased toward senescence during storage.[Citation27] However, the pattern of lycopene accumulation may be influenced by different treatments and storage condition.[Citation27] A. vera gel treatments as edible coatings had efficacy on modification of the internal atmosphere (increase in CO2 and decrease in O2). It was shown that coating of peaches and plum fruit with either aloe vera or aloe arborescens gels significantly delayed ethylene production eventually leads to preserving the quality of climacteric fruit.[Citation28] Delayed senescence in tomato fruits may results from lower microbial activity leads to maintenance of overall quality of fruits.
The ascorbic acid contents of tomatoes showed significant increase during storage, regardless of applied treatments. This effect has also been observed earlier in tomato and was attributed to ripening process.[Citation5] Tomatoes are a rich source of ascorbic acid;[Citation6] however, conventional handling has a very detrimental effect on their ascorbic acid content.[Citation29] This method of marketing based on active packaging allows the retention of ascorbic acid content, particularly suitable for retail market and valuable in regards of nutritional fact.
AA and phenolic compound
The level of AA was 35.82 ± 3.92, 42.70 ± 3.95, and 36.35 ± 1.72 in control, eugenol, and thymol, respectively, after 8 days of storage, which decreased and reached to 24.22 ± 0.70, 25.01 ± 0.93, and 27.92 ± 3.26 at the end of storage. However, the aloe-treated tomatoes maintain the AA throughout the storage with a value about 35.5 mg equivalent ascorbic acid 100 g−1 ().
Figure 2. Changes in total A: antioxidant activity; and B: phenolics of control. Aloe, eugenol, and thymol in tomatoes (cv. Assale) during storage at 10oC (values are mean ± standard errors).
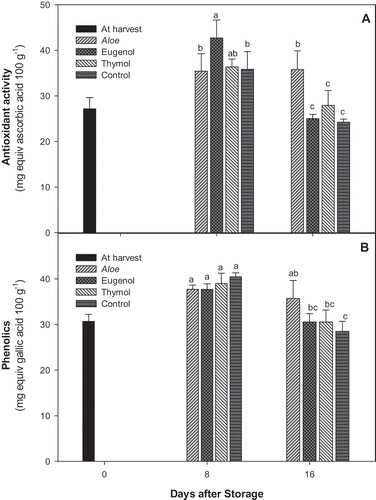
The levels of phenolics content declined over the 16-day period of storage in EOs and control treatments, but aloe-treated fruit has shown slight modification and keep the content nearly constant during storage, therefore, its level was higher in aloe-treated tomato compared to eugenol, thymol, and control treatments at the end of experiments ().
The antioxidant capacity of aloe extract was reported in many studies.[Citation30,Citation31] Individual phytochemicals identified in aloe gel included various phenolic acids, polyphenols, phytosterols, fatty acids, indoles, alkanes, pyrimidines, alkaloids, organic acids, aldehydes, dicarboxylic acids, ketones, and alcohols. Due to the presence of the antioxidant polyphenols, indoles, and alkaloids, the aloe gel shows antioxidant capacity as confirmed by different analyses.[Citation31] It was shown that coating of table grape based on aloe gel significantly delayed color changes and accelerated softening and ripening.[Citation32] Moreover, the sensory analyses revealed beneficial effects in terms of delaying rachis browning and dehydration and maintenance of the visual aspect of the berry without any detrimental effect on taste, aroma, or flavors.
It was reported that EOs components such as thymol or eugenol have an ability to increase phenolics levels in avocado fruit.[Citation33] They suggested that thyme oil has the ability to act as a signaling compound that triggers a signal similar to a mild stress condition on the fruit. Aloe gel treatment is considered as an edible coating, but at the same time contains different antioxidants, protecting fruit from accelerated ripening process. Caffeic acid, p-coumaric acid, and ferulic acid are phenolics which identified in tomato fruit and caffeic acid was the predominant phenolic acid in “Oregan Soring” and “Red Sun” cultivars of tomato fruit.[Citation34]
Correlation analysis
Antioxidant potential of tomato fruit depends on carotenoids and phenolic compounds.[Citation4,Citation5] In the present study, significant correlation was found between AA and the TP content (R2 = 0.72**, p < 0.001), but it was not significant (R = 0.26, NS) for AA and lycopene. Thus, it might be possible that phenolic compounds in tomato fruits have affected the AA more than carotenoids. It has been found that antioxidant characteristics of tomato fruit showed significant linear correlation with polyphenol and hydroxymethylfurfural content.[Citation4] Similar results from many studies showed that there is a positive correlation between phenolics compounds and AA in Alperujo varieties[Citation35] and Citrus bergamia Risso.[Citation36] Potential beneficial health effects of the phenolics in food[Citation3] and different parts of plant[Citation37] were studied and revealed that phenolic compound present in food may lower the risk of health disorder because of their AA. Therefore, the dipping of tomato in aloe gel may also be helpful in improving the efficacy of tomato consumption.
Microbial activity and TSS
As shown in , aloe markedly inhibited the mould and yeast growth during storage with the greater inhibitory effect at the 8th day of storage in comparison to other treatments. Moreover, thymol- and eugenol-treated tomatoes showed lower counts of mold and yeast during storage at 10oC as compared to the control. Therefore, the rate of mold and yeast increased over storage, but the application of EOs and aloe gel led to significantly lower counts at the end of storage.
Figure 3. Changes in A: mold and yeast counts; and B: TSS of control. Aloe, eugenol, and thymol in tomatoes (cv. Assale) during storage at 10oC (values are mean ± standard errors).
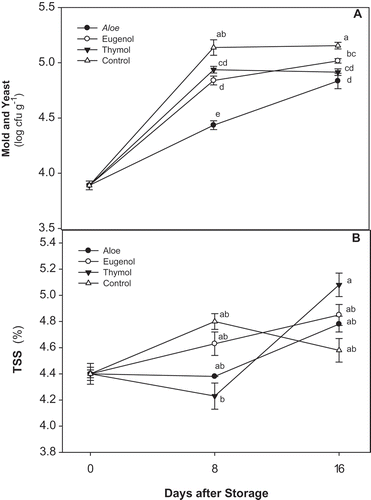
The antimicrobial properties of EOs have been recognized in nature[Citation38,Citation39] and in combination with MAP in the table grape[Citation19] and sweet cherry.[Citation20] Aloe vera extracts were also reported to have antimicrobial functions and had the potential to significantly reduce the mesophilic bacteria, yeast, and molds in modified atmosphere packed fruit.[Citation22] The antimicrobial activity of aloe gel was reported against plant pathogenic fungi, i.e., Penicillium digitatum, P. expansum, Botrytis cinerea, and Altenaria alternate.[Citation40]
With increased awareness by the environmental and health agencies and consumers about the harmful chemical residues in food, there is a growing demand in changing the legislation from chemical-based germicide to natural and safe compounds. Aloe and EOs are safe in nature and synthesized in higher plant and in compare to chemicals could be a promising means for the inhibition of microbial growth.
A gradual increase in TSS content of tomato fruits was observed during the complete storage period (), but in control fruit, it increased until the 8th day of storage, and then decreased into the end of the storage time with a value of 4.5%. The level was highest in thymol-treated tomato with a degree of 5.08%. At the end of storage, the TSS content was higher in EO-treated fruits followed by fruit coated with aloe gel.
Conclusion
From the current results, it may be assumed that the pre-treatment of tomato with aloe gel dipping or inclusion of thymol or eugenol into the MAP may have the potential to enhance bioactive component of fruit, including phenolics and lycopene, which results in higher AA. Furthermore, it was found that these combined treatments reduce the incidence of microbial spoilage inside the package. These benefits will provide higher nutritional value and healthy facts for the consumer and at the same time for growers to possess the possibility of extending shelf life, which is important for tomatoes marketability. Also, a good correlation was found between TP and AA, revealing that the phenolic compounds would determine the antioxidant potential of the tomato fruits.
Funding
This work was supported by Vali-e-Asr university of Rafsanjan (Projects: Agr86HS309).
Additional information
Funding
References
- Jacob, K.; Periago, M.J.; Bohm V.; Berruezo, G.R. Influence of Lycopene and Vitamin C From Tomato Juice on Biomarkers of Oxidative Stress and Inflammation. British Journal of Nutrition 2008, 99, 137–146.
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Carotenoid Compositions of Coloured Tomato Cultivars and Contribution to Antioxidant Activities and Protection Against H2O2-Induced Cell Death in H9c2. Food Chemistry 2013, 136, 878–888.
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. Journal of Functional Foods 2015, 18, 820–897.
- Helyes, L.; Lugasi, A. Formation of Certain Compounds Having Technological and Nutritional Importance in Tomato Fruits During Maturation. Acta Alimentaria 2006, 35, 183–193.
- Toor, R.K.; Savage, G.P. Antioxidant Activity in Different Fractions of Tomatoes. Food Research International 2005, 38, 487–494.
- El Airaj, H.; Gest, N.; Truffault, V.; Garchery, C.; Riqueau, G.; Gouble, B.; Page, D.; Stevens, R. Decreased Monodehydroascorbate Reductase Activity Reduces Tolerance to Cold Storage in Tomato and Affects Fruit Antioxidant Levels. Postharvest Biology and Technology 2013, 86, 502–510.
- Giudice, R.D.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant Bioactive Compounds in Tomato Fruits at Different Ripening Stages and Their Effects on Normal and Cancer Cells. Journal of Functional Foods 2015, 18, 83–94.
- Kaur, D.; Sharma, K.; Wani, A.A.; Gill, B.S.; Sogi, D.S. Physicochemical Changes in Seven Tomato (Lycopersicon Esculentum) Cultivars During Ripening. International Journal of Food Properties 2006, 9, 747–757.
- Colgecen, I.; Aday, M.S. The Efficacy of the Combined Use of Chlorine Dioxide and Passive Modified Atmosphere Packaging on Sweet Cherry Quality. Postharvest Biology and Technology 2015, 109, 10–19.
- Mangaraj, S.; Sadawarti, M.J.; Prasad, S. Assessment of Quality of Pears Stored in Laminated Modified Atmosphere Packages. International Journal of Food Properties 2011, 14, 1110–1123.
- Ayala-Zavala, J.F.; Del-Toro-Sanchez, L.; Alvarez-Parrilla, E.; Gonzalez-Aquilar, G.A. High Relative Humidity in-Package of Fresh-Cut Fruits and Vegetables: Advantage Or Disadvantage Considering Microbiological Problems and Antimicrobial Delivering Systems? Journal of Food Science 2008, 73, 41–47.
- Wang, Y.; Liu, A.; Ye, R.; Li, X.; Han, Y.; Liu, C. The Production of Gelatin-Calcium Carbonate Composite Films with Different Antioxidants. International Journal of Food Properties 2015, 18, 2442–2456.
- Sommer, N.F.; Fortlage, R.J.; Edwa, D.C. Postharvest Diseases of Selected Commodities. In Postharvest Technology of Horticultural Crops; Kader, A.; Ed.; University of California: Oakland, CA: 1992; 117–160.
- Aguayo, E.; Escalona, V.H.; Artes, F. Effect of Cyclic Exposure to Ozone Gas on Physiochemical, Sensorial and Microbial Quality of Whole and Sliced Tomatoes. Postharvest Biology and Technology 2006, 39, 169–177.
- Liu, C.; Hana, X.; Caia, L.; Lua, X.; Yinga, T.; Jianga, Z. Postharvest UV-B Irradiation Maintains Sensory Qualities and Enhances Antioxidant Capacity in Tomato Fruit During Storage. Postharvest Biology and Technology 2011, 59, 232–237.
- Siripatrawan, U.; Assatarakul, K. Methyl Jasmonate Coupled with Modified Atmosphere Packaging to Extend Shelf Life of Tomato (Lycopersicon Esculentum Mill.) During Cold Storage. International Journal of Food Science and Technology 2009, 4, 1065–1071.
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Perez-Gago, M.B. Hydroxypropyl Methylcellulose-Beeswax Edible Coatings Formulated with Antifungal Food Additives to Reduce Alternaria Black Spot and Maintain Postharvest Quality of Cold-Stored Cherry Tomatoes. Scientia Horticulturae 2015, 193, 249–257.
- Montero-Prado, P.; Rodriguez-Lafuente, A.; Nerin, C. Active Label-Based Packaging to Extend the Shelf-Life of “Calanda” Peach Fruit: Changes in Fruit Quality and Enzymatic Activity. Postharvest Biology and Technology 2011, 60, 211–219.
- Valero, D.; Valverde, J.M.; Martinez-Romero, D.; Guillen, F.; Castillo, S.; Serrano, M. The Combination of Modified Atmosphere Packaging with Eugenol Or Thymol to Maintain Quality, Safety and Functional Properties of Table Gtrapes. Postharvest Biology and Technology 2006, 41, 317–327.
- Serrano, M.; Martinez-Romero, D.; Castillo, S.; Guillen, F.; Valero, D. The Use of Natural Antifungal Compounds Improves the Beneficial Effect of MAP in Sweet Cherry Storage. Innovative Food Science and Emerging Technologies 2005, 6, 115–123.
- Dobravalskyte, D.; Venskutonis, P.R.; Talou, T. Antioxidant Properties and Essential Oil Composition of Calamintha Grandiflora L. Food Chemistry 2012, 135, 1539–1546.
- Martinez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillen, F.; Castillo, S.; Valero D.; Serrano, M. Postharvest Sweet Cherry Quality and Safety Maintenance by Aloe Vera Treatment: A New Edible Coating. Postharvest Biology and Technology 2006, 39, 93–100.
- Smith, J.P.; Ramaswamy, H.S.; Raghavan, G.S.V.; Ranganna, B. Packaging of Fruits and Vegetables. In Handbook of Postharvest Technology, Cereals, Fruits, Vegetables, Tea and Spices; Chakraverty, A.; Mujumdar, A.S.; Raghavan, G.S.V.; Ramaswamy, H.S.; Eds.; Marcel Dekker, Inc., Basel: New York, NY, 2003; 539–554.
- IFU. (3-17-58). International Federation of Fruit Juice Producers. Methods of Analysis and Microbiological Methods. http://www.ifu-fruitjuice.com/ifu-methods.
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. Journal of Food Composition and Analysis 2002, 15, 309–317.
- Serrano, M.; Guillen, F.; Martinez-Romero, D.; Castillo, S.; Valero, D. Chemical Constituents and Antioxidant Activity of Sweet Cherry at Different Ripening Stages. Journal of Agricultural and Food Chemistry 2005, 53, 2741–2745.
- Javanmardi, J.; Kubota, C. Variation of Lycopen, Antioxidant Activity, Total Soluble Solids and Weight Loss of Tomato During Postharvest Storage. Postharvest Biology and Technology 2006, 41, 151–155.
- Guillen, F.; Diaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martinez-Romero, D. Aloe Arborescens and Aloe Vera Gels As Coatings in Delaying Postharvest Ripening in Peach and Plum Fruit. Postharvest Biology and Technology 2013, 83, 54–57.
- Kader, A.; Morris, L.; Stevens, M.; Albright-Holton, M. Composition and Flavor Quality of Fresh Market Tomatoes As Influenced by Some Postharvest Handling Procedures. Journal of the American Society for Horticultural Science 1978, 103, 6–13.
- Hu, Y.; Xu, J.; Hu, Q. Evaluation of Antioxidant Potential of Aloe Vera (Aloe Barbadensis Miller) Extracts. Journal of Agricultural and Food Chemistry 2003, 51, 7788–7791.
- Nejatzadeh-Barandoz, F. Antibacterial Activities and Antioxidant Capacity of Aloe Vera. Organic and Medicinal Chemistry Letters 2013, 3, 5–8.
- Valverde, J.M.; Valero, D.; Martinez-Romero, D.; Guillen, F.; Castillo, S.; Serrano, M. Novel Edible Coating Based on Aloe Vera Gel to Maintain Table Grape Quality and Safety. Journal of Agricultural and Food Chemistry 2005, 53, 7807–7813.
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential Oil Vapours Suppress the Development of Anthracnose and Enhance Defence Related and Antioxidant Enzyme Activities in Avocado Fruit. Postharvest Biology and Technology 2013, 81, 66–72.
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of Total Phenolics and Phenolic Acids in Tomato (Lycopersicon Esculentum Mill) Fruits As Influenced by Cultivar and Solar UV Radiation. Journal of Food Composition and Analysis 2006, 19, 771–777.
- Zagmutt, S.; Guzman, L.; Orrego, R.; Wehinger, S.; Leiva, E. Phenolic Compound Identification and Antioxidant Capacity of Alperujo Extracts from Region del Maule, Chile. International Journal of Food Properties 2016, 19, 2016–2025.
- Sicari, V.; Loizzo, M.R.; Branca, V.; Pellicano, T.M. Bioactive and Antioxidant Activity from Citrus Bergamia Risso (Bergamot) Juice Collected in Different Area of Reggio Calabria Province, Italy. International Journal of Food Properties 2016, 19, 1962–1971.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16, 45–60.
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Combined Effects of Modified Atmosphere Packaging and Thymol for Prolonging the Shelf Life of Caprese Salad. Journal of Food Protection 2007, 3, 722–728.
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Alginate-Based Edible Coatings Enriched with Essential Oils Constituents on Arbutus Unedo L. Fresh Fruit Storage. Postharvest Biology and Technology 2015, 100, 226–233.
- Saks, Y.; Barkai, G.R. Aloe Vera Gel Activity Against Plant Pathogenic Fungi. Postharvest Biology and Technology 1995, 6, 159–165.
