ABSTRACT
Essential oils extracted by hydrodistillation from the aerial parts of Achillea millefolium was characterized by means of gas chromatography and nuclear magnetic resonance. A new compound, dihydro-a-cyclogeranyl hexanoate, along with 24 known were isolated from the essential oils A.millefolium. The new compound and borneol (known main component) were also subjected to antimicrobial, anti-inflammatory, and antioxidant activities. The new compound was particularly active against Escherichia coli, with the lowest minimum inhibitory concentration and minimum bactericidal/fungicidal concentration value. The new compound exhibited a higher activity in each antioxidant system with a special attention for β-carotene bleaching test, lipid peroxidation inhibition and reducing power. The new compound exhibited a potent nitric oxide scavenging effect and inhibited the expression of inducible nitric oxide synthase. These results indicated that dihydro-a-cyclogeranyl hexanoate might be applicable in natural medicine and healthy food.
Introduction
Aromatic plants are potential natural sources of novel antibiotics and particular interest has focused on their essential oils (EOs) as main sources of potent antimicrobial and antifungal compounds classified as terpenoids, flavonoids, and phenolics.[Citation1,Citation2] Biological activity of EOs depends on their chemical composition which is determined by the genotype and influenced by environmental and agronomic conditions. Aromatic and medicinal plants are known to produce certain bioactive molecules which react with other organisms in the environment, inhibiting bacterial or fungal growth.[Citation3] Indeed, natural crude extracts and biologically active compounds from plant species used in traditional medicine may represent valuable sources for such new preservatives.[Citation4] Application of plant materials as dietary regimens and preservatives is mainly due to their antioxidant, antimicrobial, and other biological potentials. Achillea, is one of the most important genera of the Compositae family. Achillea millefolium is one of the native species which grows wildly in different regions of Iran. This plant has been used as a medicinal herb for a long time and it now is an important drug used both in folk and official medicines.[Citation5] However, studies to support anti-inflammatory properties and lipid peroxidation inhibition of the volatile oil have not yet been reported. The objectives of this work were: To study the chemical composition and exploration of novel compounds from A.millefolium and to explore antimicrobial, anti-inflammatory, and antioxidant activity (AA).
Materials and methods
Plant materials and nano-Zn oxide treatments
The plant was identified by Mr. Esmaeili, and the voucher specimen was deposited at private herbarium of Dr, F. Esmaeili (voucher no. 158). Seeds of Achillea millefolium were sown in Jefe pot in experimental greenhouse, Iran (elevation 1339 m, latitude east 33.638, longitude north 46.431). Plants at two and four leave stage were sprayed with distilled water as a control and nano-Zn oxide at 2 and 4 mM. All sprays solution were sprayed to the point of run-off. The experiment was arranged in completely randomized block design with three replications for each treatment. The temperature conditions were 24 ± 5°C and 15 ± 4°C, during days and nights, respectively; with relative humidity of 70%. Aerial parts of A.millefolium were harvested and air-dried at ambient temperature in the shade.
General experimental procedures, isolation procedure of new compound
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a 400 (100)-MHz Varian spectrometer, d in parts per million (ppm), using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. High-performance liquid chromatography (HPLC) analysis was performed using a 250 mm × 20 mm, 5 µm, Kromasil 100-10-C18 column on an Agilent 1260 HPLC system. Dried and powdered aerial parts of A.millefolium were hydrodistilled in a Clevenger-type apparatus for 3 h. The oil of A.millefolium was subjected to silica gel column chromatography (silica gel 60, 180 g, 70–230 mesh) using a solvent mixture n-hexane-ethyl acetate (95:5, 90:10, 85:15, 80:20, 75:25) to isolate dihydro-a-cyclogeranyl hexanoate. The resulting fractions were concentrated under reduced pressure and examined by Thin-layer chromatography (TLC) to offer three main fractions. Its chemical structure was determined by mass spectrometry (MS), 1H NMR and 13C NMR spectroscopic methods and by comparing with previously reported spectral data[Citation6,Citation7] as hexyl ester of dihydro-a-cyclogeranic acid.
Oil isolation, isolate borneol, and identification of known compounds
The aerial parts of A.millefolium were ground and the resulting powder was subjected to hydrodistillation for 3 h in an all glass Clevenger-type apparatus according to the method recommended by the European Pharmacopoeia.[Citation8] The obtained EOs were dried over anhydrous sodium sulphate and after filtration, stored at +4ºC until tested and analyzed. The gas chromatography (GC)/MS analyses were executed on a Hewlett–Packard 5973N gas chromatograph equipped with a column HP-5MS (30 m length × 0.25 mm i.d., film thickness 0.25 lm) coupled with a Hewlett-Packard 5973N mass spectrometer. The column temperature was programmed at 50ºC as an initial temperature, holding for 6 min, with 3ºC increases per minute to the temperature of 240ºC, followed by a temperature enhancement of 15ºC per min up to 300ºC, holding at the mentioned temperature for 3 min. Injector port temperature was 290ºC and helium used as carrier gas at a flow rate 1.5 mL/min. Ionization voltage of mass spectrometer in the EI-mode was equal to 70 eV and ionization source temperature was 250ºC. Linear retention indices for all components were determined by coinjection of the samples with a solution containing homologous series of C8-C22 n-alkanes and comparing them and their mass spectra with those of authentic samples or with available library data of the GC/MS system (WILEY 2001 data software) and Adams libraries spectra.[Citation9] The oil of A.millefolium was subjected to silica gel column chromatography (silica gel 60, 180 g, 70–230 mesh) using a solvent mixture n-hexane-ethyl acetate (95:5, 90:10, 85:15, 80:20, 75:25) to isolate borneol.
Total phenolic determination
Total phenolic contents in aerial parts of A.millefolium were determined by Folin–Ciocalteu method.[Citation10] The total phenolic content was expressed as gallic acid equivalents (GAE; mg g_1).
Total flavonoid determination
Total flavonoid contents in aerial parts of A.millefolium were measured as previously described.[Citation11] The total flavonoid content was calculated as rutin equivalents (mg g−1).
Microorganisms
Gram-positive bacteria: Bacillus cereus (ATCC 10876), Enterococcus faecalis (ATCC 49452), Staphylococcus aureus (ATCC 25923). Gram-negative bacteria: Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Proteus mirabilis (ATCC 35659), Salmonella typhimurium (ATCC 13311), Citrobacter freundii (ATCC 8090). Fungal strains: Candida albicans (ATCC 10231) and Aspergillus fumigatus (ATCC 46645). The bacteria species were maintained in Mueller Hinton Agarand Tryptic Soy Agar. Strains of Candida spp. and Aspergillus spp. were maintained on Sabour and Dextrose Agar.
Antimicrobial activity
Minimum inhibitory (MIC) and minimum bactericidal/fungicidal (MBC/MFC) concentrations were determined by microdilution method in 96-well microtitre plates, described by Douk et al.,[Citation12] and EUCAST.[Citation13] Briefly, fresh overnight cultures of bacteria were adjusted with sterile saline to a concentration of 1.0 × 105 CFU per well, and 1.0 × 104 CFU per well for fungi. EOs were added in Tryptic Soy Broth (TSB) medium for bacteria, and Sabouraud dextrose broth (SDB) medium for fungi. The microplates were incubated for 24 h at 37°C for bacteria, and 48 h at 37°C for fungi. The MIC was defined as the lowest concentration of EO inhibiting the visible growth of the test strain. However, the MIC/MBC values for bacteria and fungi were detected following the addition of 40 µL of piodonitrotetrazoliumviolet (INT) 0.2 mg/mL and incubation at 37°C for 30 min.[Citation14] The MBCs/MFCs were determined by serial subcultivations of 10 µL into microtiter plates containing 100 µL of broth per well and further incubation for 24 h at 37°C. The lowest concentration with no visible growth was defined as the MFC, indicating 99.5% killing of the original inoculum. Following positive controls were used in both experiments: antibiotics (Streptomycin) and mycotic (Fluconazole). Each test was carried out in triplicates and repeated three times.
AA
The efficacy of the EOs to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was evaluated using a spectrophotometry method.[Citation15,Citation16] On the basis of bleaching of the bluish-red or purple color of DPPH solution as a reagent. Briefly, a 50 µL volume of various dilutions of each samples was mixed with 5 mL of 0.004% methanol solutions of DPPH followed by 30 min incubation at ambient temperature. Thereafter, the sample absorbance was recorded against control at 517 nm. The inhibition percentages were measured using Eq. (1). The antioxidants activity of the test samples in concentration providing 50% inhibition, were considered as IC50 (µg/mL).
Butylhydroxyanisole (BHA) and ascorbic acid were used as positive controls. All experiments were repeated three times, the results were averaged, and standard deviations were calculated.
Rapid screening for antioxidants
For screening of antioxidant compounds in aerial parts of A.millefolium EO, the TLC-bioautography method was carried out.[Citation17,Citation18] The diluted oil (1:20 in methanol) was spotted on silica gel sheets (silica gel 60 F254 TLC plates) and developed in n-hexane-ethyl acetate (9:1). Plates were sprayed with the methanolic solution of DPPH (0.2%). The active constituents were detected as yellow spots on a violet background. Only zones where their color turned from violet to yellow within the first 30 min (after spraying) were taken as positive results.
Activity guided fractionation of the EO for antioxidants
For the isolation and identification of the active compounds in the EO, TLC was performed using the conditions previously described.[Citation17] The regions showing DPPH scavenging activity were scrapped off then, they were eluted with chloroform. All resulting constituents were analyzed by GC/MS and also tested for their antioxidant activities.
β-carotene-linoleic acid model system (β-CLAMS)
The β-CLAMS method by the peroxides generated during the oxidation of linoleic acid at elevated temperature.[Citation19] The AA of the extracts was evaluated in term of β-carotene blanching using the following formula: AA (%) = [(A0 – A1)/A0] × 100. where A0 is the absorbance of the control at 0 min, and A1 is the absorbance of the sample at 120 min. The results are expressed as IC50 values (µg/mL). All samples were prepared and analyzed in triplicate.
Reducing power and lipid peroxidation inhibition
The ability of the extracts to reduce Fe3+ was assayed by the method of Oyaizu.[Citation20] One milliliter of aerial parts of A.millefolium EO and new component were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% K3Fe (CN) 6. After incubation at 50°C for 25 min, 2.5 mL of 10% trichloroacetic acid was added and the mixture was centrifuged at 650 g for 10 min. Finally, 2.5 mL of the upper layer was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% aqueous FeCl3. The absorbance was measured at 700 nm. The mean of absorbance values were plotted against concentration and a linear regression analysis was carried out. Increase absorbance of the reaction mixture indicated increased reducing power. EC50 value (µg/mL) is the effective concentration at which the absorbance was 0.5 for reducing power. Ascorbic acid was used as positive control. Lipid peroxidation inhibition was determined by Shirwaikar et al.[Citation21] Ascorbic acid and Trolox was used for comparison.
Anti-inflammatory activity
To evaluate the anti-inflammatory potential of the oils, NO production in lipopolysaccharide (LPS)-stimulated macrophages was used. Exponentially growing macrophages (RAW 264.7 cells) were plated in 24-well microplates at a density of 2 × 105 cells per well in 400 μL of culture medium and were allowed to adhere for 24 h at 37°C under 5% CO2. Cells were then treated with increasing concentrations of EO and pure compounds dissolved in Dimethyl sulfoxide (DMSO). The final concentration of solvent in the culture medium was maintained at 0.5% (v/v) to avoid solvent toxicity. Cells were then stimulated with 100 μg/mL LPS and incubated at 37°C under 5% CO2. After 24 h, cell-free supernatants were collected and NO was measured using the modified method of Green et al.[Citation22] Griess reagent (50 μL of 1% sulphanilamide and 50 μL of 0.1% N-1-naphtylethylenediamine dihydrochloride in 2.5% H3PO4) was added in equal volume (100 μL) to cell supernatant and incubated at room temperature for 30 min. N(G)-nitro-L-arginine methyl ester (L-NAME) was used as a positive control. Absorbance was measured using an ELISA automatic microplate reader at 550 nm and the nitrite concentration determined from a regression analysis prepared with serial dilutions of sodium nitrite.[Citation23]
Statistical analysis
The results are presented as mean±S.D and statistically analyzed by oneway analysis of variance (ANOVA) followed by Duncan test.
Results and discussion
Identification of new compounds
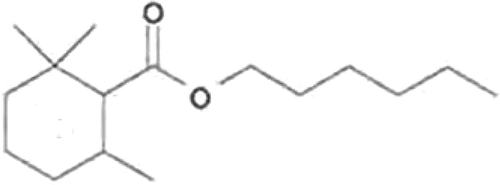
C1: Isolated compounds were identified by UV, MS, and NMR instruments. Identification of dihydro-a-cyclogeranyl hexanoate (hexyl 2,2,6-trimethylcyclohexylcarboxylate): molecular formula C16H30O2, MW 254. EIMS 70 eV, m/z (rel. int): 254 [M+] (0.8), 253 [M+- H] (5), 208 (8), 169 [C10H17O2+] (15), 168 [C10H16O2+] (72), 154 (26), 153 [C10H17O+] (100), 124 (C9H16] (22), 109 (18), 108 (12), 85 [C6H13+] (41), 57 [C4H9+] (43; ).
Identification of known compounds
The constituents of the obtained Eos of A.millefolium treated with nano Zn oxide are presented in . Twenty-four components were identified in nano Zn oxide-treated plants (). The differences were supposed to be the effects of nano Zn oxide on chemical composition of A.millefolium EO. α-Pinene, camphene, limonene, borneol, and carvacrol were increased with nano Zn oxide-treatment (). The yield of the A.millefolium oil was 1.54% v/w (2 mM) and 2.01% (4 mM). Nano Zn oxide significantly increased the yield of EO (). It seems that the use of nano-particles causes increasing in pod and dry leaf weight and finally will increase total yield.[Citation24] Lu et al.[Citation25] showed that application of nano fertilizers could increase the nitrate reductase enzyme in soybean (Glycine max L.), increase its abilities of absorbing and utilizing water and fertilizer, promote its antioxidant system, and, in fact, accelerate its germination and growth. A previous report by Afsharypour et al.[Citation26] indicated the major constituent of the EO of Achillea tenuifolia was caryophyllene oxide and in other studies, borneol was the second most abundant constituent of oil.[Citation27]
Table 1. New compounds and known from the aerial parts of A.millefolium.
Extraction yield, total phenolic contents, and total flavonoid contents
The AA of plant extracts has been correlated to their total phenolic content due to their property of scavenging free radicals.[Citation28] It is well-known that phenolic compounds contribute to quality and nutritional value in terms of modifying color, taste, aroma, and flavor and also in providing health-beneficial effects.[Citation29] They also serve in plant defence mechanisms to prevent damage by microorganisms, insects, and herbivores.[Citation29] Moreover, a few studies on the A.millefolium revealed that they are good dietary sources of antioxidants. Thus, we determined the total phenolic and flavonoid contents of the methanol extracts of A.millefolium wild vegetables. As shown in Table S2, the extraction yield of A.millefolium ranged from lowest 66.14 ± 24 mgg_1 (control) to highest 100.14 ± 55 mgg_1 (Nano Zn oxide [4 mM]). Among the three A.millefolium extracts, A.millefolium treated with nano Zn oxide at 4mM showed the highest total phenolic content (162.14 ± 57 mgg−1) and showed the highest total flavonoid content (121.47 ± 64 mg g−1). These results showed that the total phenolic and total flavonoid contents have an obvious variation in various concentrations.
Antimicrobial activity
The in vitro antimicrobial activities of new compound and borneol against the studied microorganisms were assessed by the MIC and MBC/MFC (). According to the results given in , new compound and borneol exhibited significant antimicrobial activity against all tested strains. Inhibition values were in the following range: MIC 2.5 ± 0.05 (Escherichia coli) to 25.0 ± 0.11µg/mL (Pseudomonas aeruginosa) and MBC 2.5 ± 0.08 µg/mL (E.coli) to 20.0 ± 0.65 µg/mL (Staphylococcus aureus) for bacteria, and MIC 3.0 ± 0.35 (Candida albicans) to 4.5 ± 0.65 µg/mL (Aspergillus fumigatus) and MFC 3.0 ± 0.21 (C.albicans) to 4.5 ± 0.84 µg/mL (A.fumigatus) for fungi. Results obtained from MIC and MBC/MFC indicated that the antimicrobial activity of the new isolated compound against E.coli and C. albicans was greater than those of borneol. Among the individual constituents of EOs, carvacrol, isoeugenol, nerol, citral, and sabinene exhibited potent anti-H. pylori effects.[Citation30] The major components of thyme and oregano EOs, thymol, and carvacrol, inhibited pathogenic bacterial strains, such as E. coli, Salmonella enteritidis, S.choleraesuis, and S. typhimurium.[Citation31] Eugenol, terpenen-4-ol, and carvacrol showed an inhibitory effect against the growth of four strains of E. coli O157:H7 and L. monocytogenes,[Citation32] but results obtained indicated that three isolated compounds was greater than those of monoterpenes (benzyl alchol, camphor, cinnamaldehyde, borneol, and cineole) but weaker than those of monoterpenes (thymol, carvacrol, carveol, and geraniol). Comparing the results of compounds C1 with that of standard, streptomycin, and fluconazole, it was concluded that the oils possess more potent anti-oral-pathogen activity. The compounds C1 expressed higher antibacterial activity than borneol and both antibiotics tested. In our study, most of the antimicrobial activity in EOs from A.millefolium appears to be associated with compounds C1 and borneol ().
Table 2. Effect of nano Zn oxide on extraction yields, total phenolic contents and total flavonoid contents of A.millefolium extracts.
Table 3. Antimicrobial activity of the new compound and borneol (µg/mL) from A.millefolium using minimum inhibitory (MIC) and minimum bactericidal/fungicidal (MBC/MFC) test.
AA
AA is a complex process usually occurring through several mechanisms. Due to its complexity, the evaluation of the AA for pure compounds or extracts should be carried out by more than one test method.[Citation33] The lower IC50 value indicates a stronger ability of the extract to act as a DPPH scavenger while the higher IC50 value indicates a lower scavenging activity of the scavengers as more scavengers were required to achieve 50% scavenging reaction. The results presented in revealed that compound C1 and borneol exhibited a remarkable activity. In particular, C1 exhibited clearly a higher activity (11.79 ± 0.21 µg/mL) followed by borneol (12.11 ± 0.60 µg/mL) and A.millefolium EO (12.97 ± 0.14 µg/mL; ).The positive controls BHT and ascorbic acid exhibited IC50 values equal to 13.87 ± 0.22 µg/ml and 12.97 ± 0.11 µg/mL, respectively. depicts the inhibition of β-carotene bleaching by the new compound and borneol. As shown in , the reducing power of new compound, expressed as CE50, was clearly more significant than that of the positive BHA and AA. Because of high antioxidant and free radical-scavenging activities of new compound, further investigation was carried out to identify its active constituents. Therefore, a preliminary screening was initially carried out using the dot-blot DPPH staining method on TLC. As the new compound and borneol presented a significant AA in the assays and bioautography test, it was subjected to the TLC for isolation of the active compounds. Components identified and their AA relative percentages have been shown in . The major compound found in the active band was dihydro-a-cyclogeranyl hexanoate (45.14%; ). Many aroma components of EOs, such as terpenes and terpenoids, were proposed to contribute to the AA of EOs; including thymol and eugenol, linalool and 1,8-cineole, results obtained indicated that two isolated compounds was greater than borneol. According to these results, there is a relationship between total phenolic contents and AA.
Table 4. Antioxidant activity new compound and borneol: scavenging activity (expressed as IC50 values: µg/mL), and b-carotene bleaching test. Reducing power was expressed as EC50 values (µg/mL). Butylhydroxyanisole (BHA) and ascorbic acid were used as positive controls.
Table 5. Components identified and their antioxidant activity relative percentages.
Lipid peroxidation inhibition
According to the results obtained, new compound and borneol significantly inhibited the formation of Thiobarbituric acid reactive substance (TBARS) in brain homogenates in a concentration-dependent manner (). The suppressive power on the lipid peroxidation of new compound were found to be the most potent (C1: 80.41 ± 0.24), followed by A.millefolium EO (78.54 ± 0.21 µg/mL). Ascorbic acid and Trolox showed significant suppressive power on lipid peroxidation in mice brain homogenate with IC50 value of 75.61 ± 0.21 and 72.18 ± 0.35 µg/mL (). Phenolic compounds exist in most plant tissues as secondary metabolites, i.e., they are not essential for growth, development, or reproduction but may play roles as antioxidants and in interactions between the plant and its biological environment. Phenolics are also important components of the human diet due to their potential AA, their capacity to diminish oxidative stress induced tissue damage resulted from chronic diseases and their potentially important properties such as anticancer activities.[Citation34] These results indicated that new compound displayed significant AA in inhibiting peroxidation of rat brain homogenate in vitro.
Table 6. Lipid peroxidation inhibition of new component and borneol (expressed as IC50 values: µg/ml). Trolox and ascorbic acid (AA) were used as positive controls.
Anti-inflammatory activity
The traditional use of EOs as anti-inflammatory agents suggests that they possess potent anti-inflammatory activity. The anti-inflammatory activity of new compound and borneol was evaluated on RAW 264.7 macrophages which were stimulated to induce an overproduction of NO. As presented in Supplementary , the new compound exhibited a strong inhibitory effect on LPS-induced NO secretion with C1: 88 ± 0.05 and inhibition observed at 45.0 μM. Comparatively, the L-NAME, used as positive control inhibited NO release by 73 ± 0.05% (). New compound was found to be the most active compound, inhibiting NO production by 88 ± 0.05% at 45.0 μM (). Therefore, this compound may be responsible for the anti-inflammatory activity of the oil. The anti-inflammatory potential of the new compound and borneol may be directly related to its scavenging ability and/or capacity to inhibit inducible nitric oxide (NO) synthase expression, the enzyme responsible for the release of high amounts of NO, during inflammatory conditions. Indeed, inflammatory mediators, such as NO have been reported to contribute to mutagenesis.[Citation35] This radical is an important regulator of physical homeostasis, whereas large amounts have been closely correlated with the pathophysiology of a variety of diseases and inflammations.[Citation35] EOs seem to be a good source of antioxidant and anti-inflammatory natural products.
Table 7. Effects of A.millefolium EO (45.0 μg/mL), new component and borneol (45.0 μM) on NO production in LPS-stimulated RAW-264.7 macrophages.
Conclusions
Our data indicate that the new compound extracted from A.millefolium exhibits potent biological activities, which support their use in traditional medicine. There was a good correlation between new compound and antimicrobial, anti-inflammatory, and antioxidant capacity of the extracts. In conclusion, A.millefolium extracts appear to contain new compound with antimicrobial, anti-inflammatory, and antioxidant activities.
References
- Rasooli, I.; Fakoor, M.A.; Yadegarinia, D.; Gachkar, L.; Allameh, A.; Rezaei, M.A. Antimycotoxigenic Characteristics of Rosmarinus Officinalis and Trachyspermum Copticum L. Essential Oils. International Journal of Food Microbiology 2008, 122, 135–139.
- Webster, D.; Taschereau, P.; Belland, R.J.; Sand, C.; Rennie, R.P. Antifungal Activity of Medicinal Plant Extracts; Preliminary Screening Studies. Journal of Ethnopharmacology 2008, 115, 140–146.
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercili, S. Total Phenolic Content, Antioxidant and Antimicrobial Activities of Some Medicinal Plants. Pakistan Journal of Pharmaceutical Sciences 2009, 22, 102–106.
- Al-Fatimi, M.; Wurster, M.; Schroder, G.; Lindequist, U. Antioxidant, Antimicrobial and Cytotoxic Activities of Selected Medicinal Plants from Yemen. Journal Ethnopharmacol 2007, 111, 657–666.
- Candan, A.M.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sokmen, A.; Askin, H.; Akpulat, H. Antioxidant and Antimicrobial Activity of the Essential Oil and Methanol Extracts of Achillea Millefolium Subsp. Millefolium Afan. (Asteraceae). Journal of Ethnopharmacology 2003, 87, 215–220.
- Fehr, C.; Galindo, J. Syntheses of the Enantiomers of g-Cyclogeranic Acid, g-Cyclocitral, and g-Damascone: Enantioselective Protonation of Enolates. Helvetica Chimica Acta 1995, 78, 539–552.
- Yamamoto, T.; Shimada, A.; Ohmoto, T.; Matsuda, H.; Ogura, M.; Kanisawa, T. Olfactory Study on Optically Active Citronellyl Derivatives. Flavour Fragrance Journal 2004, 19, 121–133.
- European Pharmacopoeia. 8th Ed; Maisonneuve, S.A.; Ed.; Sainte–Ruffine, 2013.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured: Carol Stream, IL, 2001; 469 pp.
- Jimoh, F.O.; Sofidiya, M.O.; Afolayan, A.J. Antioxidant Properties of the Methanol Extracts from the Leaves of Paullinia Pinnata. Journal of Medicinal Food 2007, 10, 707–711.
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.;Monaco, P. Antioxidant Properties of Sour Cherries (Prunus Cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruits. Journal of Agricultural and Food Chemistry 2008, 56, 1928–1935.
- Douk, K.D.; Dagher, M.S.; Sattout, J.E. Antifungal Activity of the Essential Oil of Origanum Syriacum L. Journal of Food Protection 1995, 58, 1147–1149.
- European Committee on Antibiotic Susceptibility. Method for Determination of Minimal Inhibitory Concentration (MIC) by Broth Dilution of Fermentative Yeasts. Discussion Document E. Dis. 7. 1. European Society of Clinical Microbiol-ogy and Infectious Diseases, Taufkirchen, Germany, 2002.
- Tsukatani, T.; Suenaga, H.; Shiga, M.; Noguchi, K.; Ishiyama, M.; Ezoe, T.; Matsumoto, K. Comparison of the WST-8 Colorimetric Method and the CLSI Brothmicrodilution Method for Susceptibility Testing Against Drug-Resistant Bacteria. Journal Microbiological Methods 2012, 90, 160–166.
- Cuendet, M.; Hostettmann, K.; Potterat, O. Iridoid Glucosides with Free Radicalm Scavenging Properties from Fagraea Blumei. Helvetica Chimica Acta 1997, 80, 1144–1152.
- Kirby, A.J; Schmidt, R.J. The Antioxidant Activity of Chinese Herbs for Eczema and of Placebo Herbs—I. Journal of Ethnopharmacology 1997, 56, 103–108.
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytotherapy Research 2000, 14, 323–328.
- Guleria, S.; Tiku, A.; Gupta, S.; Singh, G.; Koul, A.; Razdan, V. Chemical Composition, Antioxidant Activity and Inhibitory Effects of Essential Oil of Eucalyptus Teretecornis Grown in North-Western Himalaya Against Alternaria Alternata. Journal of Plant Biochemistry and Biotechnology 2012, 21, 44–50.
- Koleva, I.I.; Teris, A.B.; Jozef, P.H.; Linssen, A.G.; Lyuba, N.E. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochemistry Analysis 2002, 13, 8–17.
- Oyaizu, M. Studies on Products of the Browning Reaction Prepared from Glucose Amine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. In Vitro Antioxidant Studies on the Benzyl Tetra Isoquinoline Alkaloid Berberine. Biological & Pharmaceutical Bulletin 2006, 29, 1906–1910.
- Green, S.J.; Meltzer, M.S.; Hibbs, J.B.; Nacy, C.A. Activated Macrophages Destroy Intracellular Leishmania Major Amastigotes by An L-Arginine-Dependent Killing Mechanism. Journal of Immunology 1990, 144, 278–283.
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of Black Cumin Essential Oil and Its Main Terpenes from Tunisia. South African Journal of Botany 2010, 76, 210–216.
- Sheykhbaglou, R.; Sedghi, M.; Tajbakhsh Shishvan, M.; Seyed-Sharifi, R. Effect of Nano Iron Particles on Agronomic Traits of Soybean. Not Science Biological 2010, 2(2), 112–113.
- Lu, C.M.; Zhang, C.Y.W.; Tao, M.X. Research of the Effect of Nanometer on Germination and Growth Enhancement of Glycine Max L. and Its Mechanism. Soybean Science 2002, 21, 168–172.
- Afsharypour, S.; Asgary, S.; Lockwood, G.B. Constituents of the Essential Oil of Achillea Wilhelmsii from Iran. Planta Med 1996, 62, 77–78.
- Aghajani, Z.; Masoudi, S.H.; Rustaiyan, A. Composition of Essential Oil from Flowers of Achillea Tenuifolia Lam. Journal of Essential Oil Research 2000, 12, 723–724.
- Singleton, V.L.; Rosi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic–Phosphotungstic Acid Reagents. American Journal of Enolitic Viticum 1965, 16, 144–158.
- Doughari, J.H.; El-Mahmood, A.M.; Tyoyina, I. Antimicrobial Activity of Leaf Extracts of Senna Obtusifolia (L.). African Journal of Pharmacy and Pharmacology 2008, 2, 007–013.
- O’Gara, E.; Hill, D.; Maslin, D. Activities of Garlic Oil, Garlic Powder, and Their Diallyl Constituents Against Helicobacter Pylori. Applied and Environmental Microbiology 2000, 66, 2269–2273.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. International Journal of Food Microbiology 2004, 94, 223–253.
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibanez, E.; Senorans, F. J.; Reglero, G. Chemical Composition and Antimicrobial Activity of Rosmarinus Officinalis L. Essential Oil Obtained via Supercritical Fluid Extraction. Journal of Food Protection 2005, 68(4), 790–795.
- Aruoma, O.I. Methodological Considerations for Characterizing Potential Antioxidant Actions of Bioactive Components in Plant Foods. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis 2003, 523, 9–20.
- Khadem, S.; Marles, J. Monocyclic Phenolic Acids; Hydroxy and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005.
- Marletta, M.A. Nitric Oxide Synthase Structure and Mechanism. The Journal of Biological Chemistry 1993, 17, 12231–12234.