ABSTRACT
In the current study, rheological and colorimetric methods were compared for determination of α-amylase activity of grain malt, used for Kyrgyz traditional fermented beverage Bozo. The highest α-amylase activity determined by colorimetric method was found in barley malt (32.83 A), followed by wheat (32.09 A), and was lowest in millet malt (10.82 A) and maize malt (10.76 A). Rheological parameters of millet porridge without and with malt were studied. The liquefaction effect of millet porridge after addition of malt was determined with rotational and oscillatory measurements. Results from statistical analysis (partial least squares regression) revealed that oscillatory rheological parameters (yield stress τ, deformation γ, and storage modulus G′) could be an effective indicator to predict the α-amylase activity of the investigated malt with R2 = 0.9572. Consequently, rheological oscillatory measurements were recommended as an alternative tool to determine α-amylase activity of grain malt.
Introduction
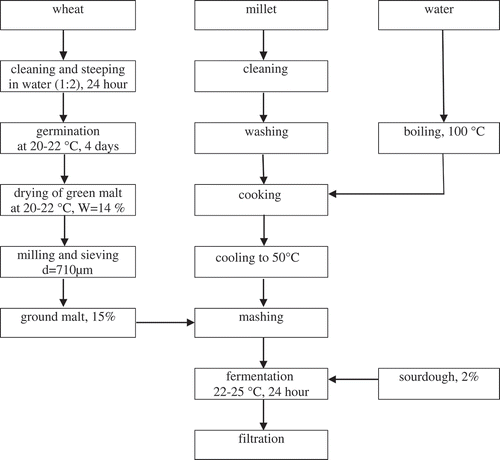
Bozo is a low-alcohol Kyrgyz traditional beverage made from cereals using both lactic acid bacteria and yeast fermentation. Generally it is characterized as a thick, sweet, slightly sour, beige, colloidal suspension. Due to its high nutritional values, Bozo is consumed daily in Bulgaria, Albania, Turkey, Romania, other Balkan countries and in Central Asia.[Citation1–Citation3] Bozo is produced from various types of cereals such as maize, wheat, and millet, but the beverage of the best quality and taste is usually made from millet.[Citation4] In the traditional Turkish method of Bozo production, cereal flour is boiled with five times its weight in water (w/v) for 1 h under continuous stirring. After cooling, it is diluted with 2.5 times the initial volume with water, followed by a sugar addition of approximately 20% g/L. As a starter culture, 2 % (w/v) of sourdough or a previous Bozo batch is used. Fermentation is usually carried out at 30°C for 24 h.[Citation5] One of the key features in preparation of Kyrgyz traditional Bozo is the malting process (). By malting grain, it becomes modified and enzymes are developed that are required for the degradation of the grain’s starch into sugars, including glucose, maltose, maltotriose, and higher molecular sugars—maltodextrines. Malt also contains small amounts of other sugars, such as sucrose and fructose, which are not products of starch modification but are already present in the grain. α-amylases contained in malt hydrolyze starch into these saccharides, which can be used by yeast as a source of carbon in the substrate.[Citation6] That is why in the preparation of traditional Kyrgyz Bozo sugar solution is not used. Generally, malt for Bozo is prepared from wheat by steeping it in water and allowing it to germinate up to 4 days at 20–22°C. During the germination process, wheat grains produce hydrolytic enzymes, such as β-glucanases and α-amylases.[Citation7] Thus, germinated grains are dried at room temperature, and then milled and sieved. Dried wheat malt (14% w/w) is added into the cooked cereal porridge. During this process, a liquefaction of the cooked cereal porridge occurs, as a result of degradation of starch by α-amylases in malt. The remaining stages of the production of Bozo are described by Hancioglu and Karapinar.[Citation5] Consequently, the malting process is an important step that contributes to the taste and flavor of the final product.
α-amylase is the major form of amylase found in humans and other mammals as well as an enzyme present in seeds, and yeasts. All α-amylases are glycoside hydrolases and act on α-1.4-glycosidic bonds. Both α-amylase and β-amylase are present in seeds; β-amylase is present in an inactive form prior to germination, whereas α-amylase and proteases appear once germination has begun.[Citation8]
α-amylase activity can vary dramatically depending on cereal origin and may also be different in individual grains.[Citation6] For the characterization of α-amylase activity a number of methods are available. Amylase action is characterized by simultaneous changes of the following properties of the substrates: change in iodine color reaction, change in optical rotatory power, decrease of viscosity, increase of reducing power, and decrease in the turbidity of starch solution.[Citation8] α-amylase activity can be measured by a colorimetric method.[Citation9,Citation10] In colored solutions, however, α-amylase activity can be determined by the spectrophotometric method.[Citation11] An electrochemical technique to detect enzymes involves the use of electrodes. A photometric method, chemiluminescence, chromatography, fluorescence, ultraviolet (UV) absorbance, and radiochemical techniques are also effective in detecting enzyme activity.[Citation12,Citation13] On the other hand, α-amylase activity can be determined indirectly as a result of its solubilizing effect on starch, leading to a reduction in paste viscosity. The most widely adopted method uses the “falling number” apparatus and other relative rheometers like the rapid viscosity analyzer, Brabender viscometer, or Bohlin CS-50 rotational rheometer to detect starch liquefaction in a heated aqueous suspension of flour or ground grain.[Citation6,Citation14–Citation17] Another instrument, the Rheoswing RSD 1-1, has been used to characterize the quality of a malt, to show correlations between laboratory congress mashing results and rheological curves and to determine viscosity trends during the progress of an industrial mashing process.[Citation18,Citation19] Currently, there are more sensitive rheology methods for material characterization, since flow behavior is responsive to chemical reactions, such as hydrolysis of biopolymers.[Citation20–Citation22]
Rheological oscillatory measurements are more sensitive methods than rotational methods since the sample’s structure will not be damaged during the experiment. In oscillatory measurements substances are studied in the linear viscoelastic (LVE) range (small deformation, i.e., small deformation amplitude such as γ = 0.1%, frequency f = 10 Hz), which do not interfere with biochemical or thermal structural change during the transient evolution of complex systems. These measurements are a nearly non-destructive method.[Citation20] This technology has become a widely used tool for the product development engineer during the last 35 years.[Citation23] For determining α-amylase activity the rheological oscillatory method has not been used before. The aim of this study was to determine capability for measuring α-amylase activity of malt produced from various cereal grains (barley, wheat, millet, and maize) using rheological parameters such as yield stress, deformation, storage, and loss moduli. The colorimetric method was used as a reference method.
Materials and methods
Materials
For preparing malt millet and other cereals, wheat (Triticum aestivum), barley (Hordeum vulgare), millet (Panicum miliaceum), and maize (Zea mays) were obtained from local markets in Bishkek, Kyrgyzstan. For preparation of the porridge, millet groats were milled and sieved using a 1 mm diameter test sieve (Armfield, Hampshire, UK). The milled grains (100 g) were boiled in 0.5 L of water for 50 min under continuous stirring. The porridge was then cooled to room temperature. The average moisture content of the porridge was determined as 23% (w/w). The moisture content in the porridge was determined in triplicate by drying 2.5–5 g of sample material in an electronic moisture analyzer IR 30 (Denver Instrument, Arvada, USA).
Malt was prepared according to a traditional method. Cereals were selected, weighed, washed, and steeped in water (grain/water ratio 1:2 w/v for 24 h and allowed to germinate up to 4 days at 20–22°C. The germinated grains were subsequently dried without forced ventilation at 20–22°C to moisture content of 14%. The height of the grain bed was 2 cm during germination and 0.5 cm during drying. Dried malt was milled using a laboratory blender (Waring Laboratory, Torington, USA) and sieved through a test sieve with a pore diameter of 710 μm (Armfield, Hampshire, UK).
Soluble starch supplied by Merck (Darmstadt, Germany) was used in 0.1% concentration in acetate buffer solution at pH 5.5, which contained CaCl2 in a concentration of 0.1 M. KI (potassium iodide), I2 (iodine crystals), CH3COOH (glacial acetic acid; Merck, Darmstadt, Germany), CН3СООNa-3H2O (sodium acetate 3-hydrate), CaCl2 (calcium chloride), and C7H8 (toluene) were of analytical grade (Reahim, Ural, Russia).
Standard α-amylase activity method
The α-amylase activity method was based on the International Association for Cereal Chemistry (ICC) method (Wohlgemuth method with some modifications by Perten).[Citation10] In this method, the α-amylase activity is expressed in terms of the hydrolysis time required for the enzyme to convert limit dextrins in the presence of an excess of β-amylase to products which give a red-brown coloration when mixed with iodine. The end-point can be determined by use of dextrin-iodine standard or permanent glass color standard.
The α-amylase activity (A) is expressed as a function of α-amylase concentration and the velocity constant for the hydrolytic degradation of limit dextrin at 30°C. In this study, the light absorbance at 575 nm of investigated samples was measured using the UV-visible Spectrophotometer Specord 50 (Analytik Jena, Jena, Germany). The reaction time was 5 min according to Perten’s method.[Citation10] The α-amylase activity was calculated using Eq. (1):
where A is the α-amylase activity and f is the dilution factor if the enzyme extract has been diluted. c is the concentration of enzyme extract (normally c = 5, i.e., 5 g sample extracted with 100 mL of calcium chloride solution), and the extinction values are recorded at measuring times. The dilution factor f without dilution would be f = 1 (i.e., 5 g sample extracted with 100 mL of calcium chloride solution), while f = 2 with dilution of 1 part of normal extract with 1 part of calcium chloride solution.
Rheological fingerprint of α-amylase activity
For the determination of α-amylase activity, rheological measurements were used as an alternative method. Two types of rheological measurements were performed: (1) rotational and (2) oscillatory measurements. These measurements were carried out by using the rheometer MCR 302 (Anton Paar, Graz, Austria) with concentric cylinder geometry CC27. The data from the rheological measurements were analyzed with the supporting rheometer software Rheoplus 32 Multi 6 version 3.40.
Rotational rheological measurements
The rotational measurement condition was used to obtain flow behaviors of millet porridge without malt, by measuring steady shear viscosity () and shear stress (
) at 25, 35, 45, 55, and 65°C. The measurements were performed in three intervals. In interval 1, the shear rate was progressively increased linearly from 0.1 to 30 s−1 over a span of 90 s. In interval 2, the shear rate was constant at shear rate 30 s−1. In interval 3, the shear rate was progressively decreased from 30 to 0.1 s−1. To obtain the rheological parameters, the Casson model was used, which describes the flow behavior of shear thinning materials with yield stress (Eq. [2]).
where τ0 is yield stress, ηCa is the Casson’s coefficient of viscosity, and is shear rate (s−1). Changes in viscosity after addition of malt were measured at a constant shear rate of 5 s−1 over 17 minutes at temperatures of 50, 55, and 60°C. The effect of temperature on dynamic viscosity and activation energy was calculated using an Arrhenius-type equation (Eq. [3]).
where η is the viscosity, Ea is the activation energy of flow (J mol−1), R is the gas constant, and T the absolute temperature in degrees Kelvin. The activation energy Ea is the energy barrier that must be overcome before the flow process is initiated.[Citation24]
Oscillatory rheological measurements
Mechanical oscillatory measurements allow the determination of structural properties of millet porridges without addition and after addition of malt. The sample to be tested is subjected to a sinusoidal deformation and the complex shear modulus G* can be calculated from the response to the oscillatory load. Mathematical separation into a real and imaginary part provides a measure of the stored elastic energy (storage modulus G′) and the energy lost through viscous flow (loss modulus G″). If substances are studied in the viscoelastic range, oscillatory measurements offer a nearly non-destructive measuring method, which allows structural studies on complex systems.[Citation25]
The rapid breakdown of the structure of millet porridge after addition of malt can be determined with an amplitude sweep test. In this test, first the crossover point G′/G″ and upper boundary of the LVE range was determined. After reaching the crossover point G′/G″ the flow of samples commences. Rapid achievement of this point means decomposition of starch with enzymes. This oscillatory test was carried out with constant frequency of 1 Hz and variable strain amplitude from 0.01 to 100%. The optimal mashing temperature was determined using the temperature sweep method at temperatures of 25 to 75°C (deformation amplitude γ = 0.1%, frequency f = 10 Hz).
Statistical data analysis
Partial least squares regression (PLSR) was applied in order to predict α-amylase activity of the malt samples from rheological data. PLSR searches the relationship and interdependence of two (X, Y) or more (X, Y, Z, etc.) random variables and describes their common structure. The accuracy of the regression is expressed with a correlation coefficient (R2). In PLSR the “leave-one-out” cross-validation process was used for validation, that is, leaving out one sample of the calibration set at a time for prediction.[Citation26] The custom-designed versions of PLSR (“SAISIR” package) programmed in MatLab (The MathWorks Inc., MA, USA) were utilized in the statistical data treatment.
Results and discussion
Determination of α-amylase activity of malt by colorimetric method
The results of colorimetric methods are presented in . The α-amylase activity is expressed as a function of α-amylase concentration and the velocity constant for the hydrolytic degradation of a 0.1% (w-v) starch solution. The hydrolysis reaction was carried out at 30ºC, in accordance with Perten.[Citation10] α-amylase activity of barley, wheat, maize, and millet malt was calculated by measuring the change in absorbance using Eq. (1). The results obtained at 30°C show that the α-amylase activity is decreasing in the following order: barley, wheat, millet, and maize malt. The highest α-amylase activity was found in barley malt (32.83 A) and wheat malt (32.09 A), the lowest in maize malt (10.76 A). The α-amylase activities of wheat and barley malt are almost the same. Therefore, for the production of the beverage Bozo, traditionally used wheat malt could be replaced by cheaper barley malt if α-amylase activity is the main criteria for malt selection.
Table 1. Comparison of amylase activity different malt types from colorimetric method with crossover point G′/G and LVE-range parameters of porridge + malts from amplitude sweep.
Rheological fingerprint of α-amylase activity
Flow behavior of millet porridge
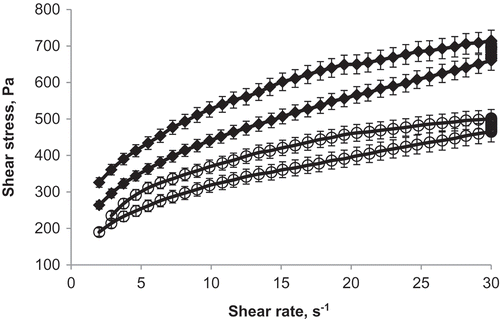
Prior to our investigations of the α-amylase activity of malt with rheological methods, the flow behavior of millet porridge without malt was measured to determine its viscosity. The viscosity curves of millet porridges without malt at 25 and 65°C are shown in . Yield stress was measured at all temperatures (from 19.94 to 17.8 Pa). Generally, porridge is assumed to be a non-Newtonian fluid as reported earlier.[Citation15,Citation27,Citation28] The regression analysis was done according to the models of Herschel-Bulkley, Casson, Bingham, and Ostwald-De-Waele for all porridge samples. Of these, the Ostwald-De-Waele model is least frequently used to describe flow curves of porridge. Among the rheological models applied, the best fit (R = 0.994 /0.997) was achieved by applying the Casson model, which was superior to the Bingham and Herschel-Bulkley models (R = 0.8). Therefore, the flow curves can be described as a semi-solid structure (Casson model). The rheological parameters are presented in .
Table 2. Adjustment of experimental data of millet porridge to the Casson model n = 0.5.
The temperature effect on viscosity was estimated using Eq. (3). The activation energy, Ea and coefficient A was calculated as 7.3 kJ mol−1 and 1.2 Pa·s, respectively, with a correlation coefficient R2 of 0.967. From the rotational flow test of millet porridge the largest decrease in the effective viscosity (η) was observed between 45 and 65°C, from 20.6 to 16.8 Pa·s. Lower viscosity allows for easier mixing. At these temperatures, the porridge has optimal consistency for the addition of malt. Therefore, further rotational measurements of millet porridge with addition of malt were obtained at 50, 55, and 60°C.
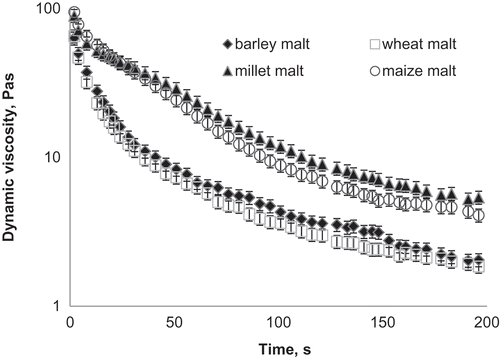
shows the decrease of the viscosity after addition of 15% (w/w) of different malt at 55°C, displaying liquefaction of the porridge due to hydrolysis of starch. The rapid decrease in viscosity in comparison to pure porridge demonstrates the high α-amylase activity of barley malt. The viscosity reduction by maize and millet malt is about the same with the rate 24.5 Pa·s/min. After addition of wheat and barley malt, a rapid reduction in viscosity at a rate of 39.5 Pa·s/min was observed during the first 2 min, followed by a rate of 5 Pa·s/min within 15 min. Thus, it can be concluded that barley malt has the highest α-amylase activity, which confirms the results of the colorimetrical method. In the current study, only starch degradation by malting was taken into consideration as a viscosity dependent factor. Besides starch degradation, cell wall polymers including beta glucans and arabinoxylans are able to influence the viscosity of porridge during malting. This subject will be investigated in future studies.
Oscillatory rheological behavior of porridge
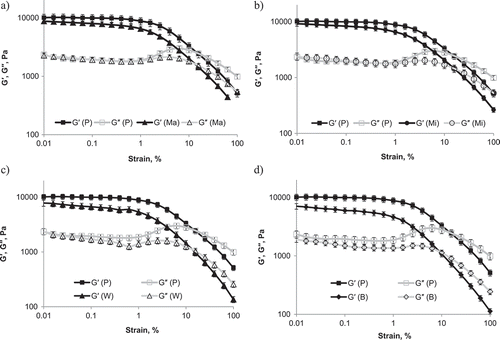
During amplitude-sweeps, the goal was to determine the lowest values of yield stress τ and deformation γ, which can detect weak structures of the system and consequently the highest α-amylase activity of malt. The amplitude-sweeps were performed at 55°C. As shown in , G′ and G″ are nearly parallel in the lower strain range, called the LVE region. The storage modulus of the porridge with barley malt leaves the LVE range at 152 Pa and the storage modulus of the porridge without malt leaves this range at 542 Pa. After the crossover point, the storage modulus G′ decreases and is less than loss modulus G″. The stronger structure of the system, the higher the deformation at this point, and vice versa. A decrease of the storage modulus at a lower deformation is also associated with high amylolytic activities, because during hydrolysis of starch with malt, liquefaction of the system occurs. Crossover point and LVE-range parameters of porridge with the addition of the malt from amplitude sweeps are presented in . Comparing rheological parameters (γ, τ, G′) obtained from yield stress τ, it can be concluded that barley malt possesses higher α-amylase activity than other malts (). It has the lowest storage modulus G′ and yield stress τ.
Figure 4. Amplitude sweep of millet porridge (P) before and after addition of different malts: a: maize (Ma); b: millet (Mi); c: wheat (W); and d: barley (B).
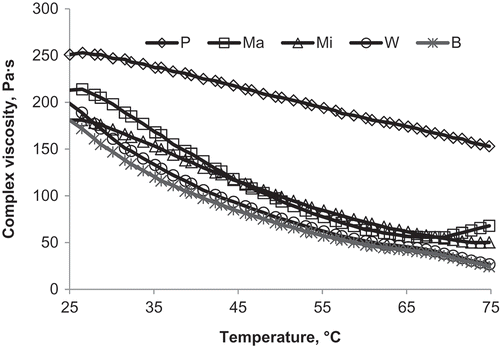
For determination of the optimal temperatures for mashing, temperature-sweep measurements were performed. The optimal temperature for hydrolysis of millet porridge’s starch with malt is defined as a temperature at which during measurement the highest reduction of viscosity was observed. The results from the temperature-sweeps are presented in . distinctly shows the decrease of complex viscosity of porridge with malt due to immediate action of the α-amylases of malt. Increase in temperature also leads to structural changes as a result of the activation of α-amylase. At 66°С, the sample with barley malt has the lowest viscosity, while the sample with maize malt shows the highest complex viscosity. At temperatures above 66°С, the decrease in complex viscosity of the sample with maize malt ends with the inactivation of the enzymes. Other types of malt indicate α-amylase activity even at 75°С.
Comparison of colorimetric and rheological method
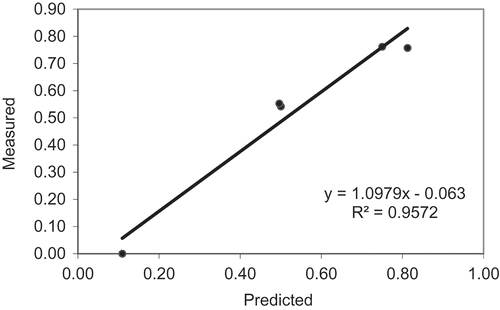
As rotational measurement has demonstrated, the viscosity of porridge depends not only on temperature and α-amylase activity, it also depends on the shear rate. Using oscillatory measurements structural change of millet porridge with malt was studied in the viscoelastic range with small deformations (deformation amplitude γ = 0.1%, frequency f = 10 Hz), an almost non-destructive method. Results of the oscillation measurements were used for comparison with measurements from the colorimetric and rheological methods. PLSR was applied in order to predict α-amylase activity of the malt samples from rheological data such as yield stress, deformation and storage modulus. Correlation coefficient (R2) of the predicted data from rheological oscillation methods and measured α- amylase activity from colorimetric method was R2 = 0.9572. The linear relationship between the two methods was y = 1.0979x – 0.063 (). Consequently, results from PLSR revealed that oscillatory rheological parameters could be an effective indicator to predict the α-amylase activity of investigated malt.
Conclusion
The α-amylase activities of maize, millet, wheat, and barley malt were investigated by rheological and colorimetric methods (ICC-108). Colorimetric methods show that the α-amylase activity is decreasing in the following order: barley, wheat, millet, and maize malt. The highest α-amylase activity was found in barley malt (32.83 A) and wheat malt (32.09 A), the lowest in maize malt (10.76 A). The α-amylase activities of wheat and barley malt are nearly the same. Therefore, for the production of the beverage Bozo, traditionally used wheat malt could be replaced by cheaper barley malt if α-amylase activity is the main criteria for malt selection. It is noteworthy that the colorimetric method is a very time consuming method for the determination of α-amylase activity. Rheological measurements were used as an alternative method for the determination of α-amylase activity. Using rotational measurements, the millet porridge presented semi-solid structure behavior and the rheological parameters were well-represented by the Casson model. The rheological properties of millet porridge before and after addition of malt from millet, maize, wheat, and barley were analyzed. The rapid reduction of the porridge viscosity with barley and wheat malt as a result of starch degradation demonstrates their higher α-amylase activity. The oscillatory measurements also show that barley malt possesses the highest amylolytic activity. This study demonstrates the possibility of detecting the optimal temperature of amylase activities for malt by oscillatory measurement. The optimal temperature of α-amylase activities for both wheat and barley malt was found at 66°C, which corresponds to literature data. At temperatures above 66°C inactivation of enzymes occurs and the viscosity reduction of porridge is slowed down. In determination of α-amylase activity of malt, the oscillation test is advantageous in terms of short measurement time (about 20 min) and the possibility of “online” observation of the consistency changing during the malting process. Thus, it is possible to use rheological measurements, especially the oscillatory measurements, to determine the α-amylase activity of grain malt.
Funding
The financial support of Kyrgyz-Turkish Manas University is gratefully acknowledged.
Additional information
Funding
References
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-Based Fermented Foods and Beverages. Food Research International 2003, 36, 527–543.
- Gotcheva, V.; Pandiella, S.S.; Angelov, A.; Roshkova, Z.G.; Webb, C. Microflora Identification of the Bulgarian Cereal-Based Fermented Beverage Boza. Process Biochemisrty 2000, 36, 127–130.
- Botes, A.; Todorov, S.D.; Mollendorff, J.W.; Botha, A.; Dicks, L.M.T. Identification of Lactic Acid Bacteria and Yeast from Boza. Process Biochemistry 2007, 42, 267–270.
- Arici, M.; Daglioglu, O. Boza: A Lactic Acid Fermented Cereal Beverage As a Traditional Turkish Food. Reviews International 2002, 18, 39–48.
- Hancioglu, O.; Karapinar, M. Microflora of Boza, a Traditional Fermented Turkish Beverage. International Journal of Food Microbiology 1997, 35, 271–274.
- Kent, N.L.; Evers, A.D. Technology of Cereals: An Introduction for Students of Food Science and Agriculture, 4th Ed; Pergamon Press: Oxford, Great Britain, 1994; 336 pp.
- Mayolle, J.E.; Lullien-Pellerin, V.; Corbineau, F.; Boivin, P.; Guillard, V. Water Diffusion and Enzyme Activities During Malting of Barley Grains: A Relationship Assessment. Journal of Food Engineering 2012, 109, 358–365.
- Yoo, Y.J.; Hong, J.; Hatch, R.T. Comparison of α-Amylase Activities from Different Assay Methods. Biotechnology and Bioengineering 1987, 30(1), 147–151.
- ICC–108. Colorimetric Method for the Determination of Alpha-Amylase Activity, Cereals or Cereal Products. Verlag Moritz Schaefer, Detmold, Germany, 1968.
- Perten, H. A Colorimetric Method for the Determination of Alpha-Amylase Activity. Cereal Chemistry 1966, 43, 336–342.
- Safarik, I. Spectrophotometric Determination of Amylase Activity in Coloured Solutions. Journal of Biochemical and Biophysical Methods 1991, 22, 61–67.
- Kroger, S.; Setford, S.J.; Turner, A.P. Electrochemical Assay Method for the Rapid Determination of Oxidase Enzyme Activities. Biotechnology Techniques 1998, 12, 123–127.
- Huang, X.; Choi, Y.; Im, H.; Yarimaga, O.; Yoon, E.; Kim, H. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782.
- ICC–107. Determination of the “Falling Number” According to Hagberg—As a Measure of the Degree of Alpha-Amylase Activity in Grain and Flour. Verlag Moritz Schaefer, Detmold, Germany, 1968, 1995.
- AACC International Method, 56-81.03. Determination of Falling Number. Approved Methods of the American Association of Cereal Chemist Inc.: St. Paul, MN, 2000.
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Effect of Germination Time on Alpha-Amylase Production and Viscosity of Maize Porridge Food Research International 2002, 35, 315–321.
- Goode, D.L.; Ulmer, H.M.; Arendt, K.E. Model Studies to Understand the Effects of Amylase Additions and pH Adjustment on the Rheological Behaviour of Simulated Brewery Mashes. Journal of the Institute of Brewing 2005, 111(2), 153–164.
- Senge, B.; Schwarzlos, B.; Blochwitz, R.; Annemuller, G. Rheological Examination of Mashing in the Brewery Process. Applied Rheology 1996, 6, 11–18.
- Hoog, D.; Senge, B.; Annemuller, G. Rheologische Kontrolle von Labormaischen. Brauwelt 1997, 37, 1606–1610.
- Fischer, P.; Windhab, J.E. Rheology of Food Materials. Current Opinion in Colloid & Interface Science 2011, 16, 36–40.
- Doucet, D.; Gauthier, F.; Foegeding, E.A. Rheological Characterization of a Gel Formed During Extensive Enzymatic Hydrolysis. Journal of Food Science 2001, 66(5), 711–715.
- Fischer, P.; Rehage, H. Rheological Master Curves of Viscoelastic Surfactant Solution by Varying the Solvent Viscosity and Temperature. Langmuir 1997, 13(26), 7012–7020.
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd Ed; Wiley: New York, NY, 1980.
- Mezger, G.T. The Rheology Handbook, 3rd Ed; Vincentz Network: Hannover, Germany, 2011.
- Sai Manohar, R.; Manohar, B.; Haridas Rao, P. Rheological Characterization of Wheat Porridge (Cooked Dalia), a Semi-Liquid Breakfast Food. Journal of Cereal Science 1998, 27, 103–108.
- Dijkstra, T.K. Latent Variables and Indices: Herman Wold’s Basic Design and Partial Least Squares, in Handbook of Partial Least Squares: Concepts, Methods and Applications (Springer Handbooks of Computational Statistics Series, Vol. II), Esposito, V.V.; Chin, W.W.; Henseler, J.; Wang, H.; Eds.; Springer, Heidelberg: Dordrecht/London/New York, NY, 2010.
- Sai Manohar, R.; Devi, U.G.R.; Bhattacharya, S.; Rao, G.V. Wheat Porridge with Soy Protein Isolate and Skimmed Milk Powder: Rheological, Pasting and Sensory Characteristics. Journal of Food Engineering 2011, 103, 1–8.
- Ojijo, N.K.; Shimoni, E. Rheological Properties of Fermented Finger Millet (Eleucinecoracana) Thin Porridge. Carbohydrate Polymers 2011, 57, 135–143.