ABSTRACT
The phenolic and chromatic characteristics of dry red wines made from native Chinese species (Vitis amurensis and its hybrids, and V. davidii) and V. vinifera were evaluated by high-performance liquid chromatography/triple-quadrupole tandem mass spectrometry and spectrophotometer, respectively. V. amurensis and its hybrids wines had higher phenolic percentage of non-coumaroylated 3, 5-O-diglucosidic anthocyanins, V. davidii wines had higher phenolic percentage of coumaroylated 3, 5-O-diglucosidic anthocyanins, kaempferol-3-O-glucoside, and quercetin-3-O-rhamnoside, V. vinifera wines had higher phenolic percentage offlavan-3-ols and 3-O-monoglucosidic anthocyanins. Wines of native Chinese species had relatively higher blue % value and lower red % value. By the use of principal component analysis and Pearson correlation analysis, specific phenolic compounds could be recognized as phenolic fingerprints of different wines, which not only play an important role in wine differentiation, but also explain their chromatic differences.
Introduction
Phenolic compounds in red wines contribute greatly to both the organoleptic properties as well as health benefits.[Citation1] According to their color expression, phenolics in wines can be classified into two groups: anthocyanins and non-anthocyanin phenolics. Anthocyanins, as a group of important pigments in red grapes and wines, contribute a red to purple color.[Citation2] Non-anthocyanin phenolics comprise mainly flavan-3-ols, flavonols, and phenolic acids. Flavan-3-ols contribute to bitterness and astringency in wines. During aging, polymerization or condensation occurs between flavan-3-ols themselves and other compounds, such as anthocyanins and flavonols, thus altering the wine’s tannin structure and mouthfeel.[Citation3] Flavonols exist in wines mainly in glycosidic forms, the colors of which may change from colorless to yellow. They are responsible for the progressive formation of condensed pigments during the red wine conservation and aging process, which results from the interaction with anthocyanins directly or through acetaldehyde.[Citation4] In addition to contributing astringency and a minor amount of acidity,[Citation5] phenolic acids, such as hydroxybenzoic acids and hydroxycinnamic acids, can also be involved in oxidizing and browning reactions in wines, and have an impact on the color changes.[Citation6,Citation7] Though such non-anthocyanin phenolics have no red color, they can participate in a complex spontaneous association termed co-pigmentation with anthocyanins to stabilize and enhance the color expression, essentially in young red wines and, thus, contribute to red wine color, especially in their early maturation.[Citation8–Citation11]
China has several species of Vitis germplasms which are distributed extensively. These native species, such as V. amurensis and V. davidii, have relatively strong disease resistance and good adaptability to local climatic conditions.[Citation12] V. amurensis is native to northeastern China (Jilin Province, Liaoning Province, and Heilongjiang Province). This species has a high resistance to white rot and anthracnose and can survive even at –40°C. As a wine grape, V. amurensis and their hybrids are unique for making high-quality red and sweet wines, which have a brilliant ruby red color, fine fragrance, and a distinctive taste.[Citation13] V. davidii grapes are mainly found in subtropical rainforest in the Yangtze River Basin (mainly Jiangxi Province and Hunan Province) and they possess good adaptability to hot temperature and humid conditions. Although their vines are susceptible to downy mildew disease, they have strong resistance to powdery mildew, botrytis, spot anthracnose, white rot disease, and anthracnose.[Citation14] The wines produced from V. davidii grapes are dark purple or ruby red and have a distinct aroma reflecting the taste of the variety.[Citation15]
Currently, wines made from V. vinifera grapes take up most of the wine market all over the world. Considering the good adaptability to local climatic conditions, as well as the distinctive sensory characteristics, V. amurensis and V. davidii grapes have huge potential to be developed as major wine grapes for local wine industries in many parts of China. However, up until now, studies to compare phenolic and chromatic characteristics of wines made from these three species (V. amurensis, V. davidii, and V. vinifera) are quite limited.[Citation14,Citation16,Citation17] In order to have a better phenolic and chromatic understanding of these different wine products, the present study aimed to figure out: (1) the phenolic and chromatic characteristics of dry red wines made from some V. amurensis, V. davidii, and V. vinifera grapes; and (2) the phenolic reason which can in part explain the chromatic differences of wines made from different species.
Material and methods
Chemicals
Methanol, acetonitrile, and formic acid of high-performance liquid chromatography (HPLC)-grade were purchased from Fisher (Fairlawn, NJ, USA). Ultrapure water was obtained from a Milli-Q Element water purification system (Millipore, Bedford, MA). Forty-five non-anthocyanin phenolic standards such as quercetin, rutin, myricetin, isorhamnetin, kaempferol, kaempferol-3-O-glucoside, kaempferol-3-O-galactoside, quercetin-3-O-glucoside, quercetin-3-O-glucuronide, quercetin-3-O-galactoside, quercetin-3-O-rhamnoside, myricetin-3-O-galactoside, dihydrokaempferol, dihydromyricetin, dihydroquercetin, (+)-catechin, (-)-epicatechin, (-)-epigallocatechin, gallocatechin, epicatechin gallate, (-)-epigallocatechin-3-O-gallate, procyanidin B1, procyanidin B2, procyanidin C1, 2-hydroxybenzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, gallic acid, protocatechuic acid, vanillic acid, syringic acid, 2-hydroxycinnamic acid, 3-hydroxycinnamic acid, 4-hydroxycinnamic acid, caffeic acid, gentisic acid, ferulic acid, sinapic acid, chlorogenic acid were purchased from Sigma-Aidrich (St. Louis MO, USA); laricitrin, syringetin, syringetin-3-O-glucoside, and syringetin-3-O-galactoside were purchased from ChromaDex (Santa Ana, USA); isorhamnetin-3-O-glucoside and myricetin-3-O-glucoside were purchased from Extrasynthese (Genay, France). All the standards were dissolved in HPLC-grade methanol and stored at –20°C.
Wine samples
Representative commercial wine samples of different species were numbered, together with their detailed information, such as their species and varieties, and listed in . V. amurensi (and their hybrid varieties) wines were kindly provided by Tongtian winery (Jilin Province), V. davidii wines were kindly provided by Tongmu winery (Hunan Province), and V. vinifera wines were kindly provided by CiticGuoan Winery (Xinjiang Province). The winemaking procedures that were used strictly followed the local winemaking standards.
Table 1. Wine sample information.
High-performance liquid chromatography/triple-quadrupole tandem mass spectrometry (HPLC-QqQ-MS/MS) analysis for anthocyanin compounds
Anthocyanin compounds were separated and analyzed by the method of HPLC-QqQ-MS/MS which was reported in our previous study.[Citation18] To introduce the method briefly: HPLC was performed on an Agilent series 1200 instrument (Agilent Technologies, CA, USA) and a Poroshell 120 EC-C18 column (150 × 2.1 mm, 2.7 μm, Agilent Technologies, CA, USA) was used. The gradient elution was water as mobile phase A and acetonitrile/methanol (50:50, v/v) as mobile phase B, both with 0.1% formic acid added, and the elution gradient was from 10 to 46% B for 28 min, from 46 to 10% B for 1 min, column clean-up and re-equilibration at 90% A and 10% B for 5 min, with a flow rate at 0.4 mL/min. The column temperature was set at 55°C. All wine samples of 1 mL were filtered using 0.45 μm polyether sulphone membrane and then injected directly for analysis, with an injected volume of 1 µL. An Agilent 6410 QqQ instrument equipped with an Electrospray Ionization (ESI) source was used. The spray voltage was set at 4 kV in positive mode; the source temperature was kept at 150°C; gas temperature was 350°C; gas flow was 12 L/h; nebulizer pressure was 35 psi. The [M+H]+ ions were selected as precursor ions of anthocyanin compounds. Multiple reactions monitoring (MRM) mode was selected for both identification and quantification. Each wine sample was detected in triplicate.
HPLC-QqQ-MS/MS analysis for non-anthocyanin compounds
Non-anthocyanin phenolic compounds were separated by the use of HPLC-QqQ-MS/MS condition described in HPLC-QqQ-MS/MS analysis for anthocyanin compounds, except that Spray voltage was set at 4 kV in negative mode, because non-anthocyanin phenolic compounds showed a relatively higher response. The [M+H]− ions were selected as precursor ions of non-anthocyanin phenolic compounds. Selective ion monitoring (SIM) is a type of monitoring mode which can scan fixed [M+H]− ions with different fragmentor voltages, and was used to find out the optimum fragmentor voltage according to the corresponding abundance of each ion fragmented under different voltages, while the product ion scan mode was used to find out the optimum collision energy, as well as product ions of each compound. The MRM mode was used to detect the aimed compounds, both for identification and quantification. Each wine sample was detected in triplicate. The selected parameters are shown in supplementary material, Table A1.
Method validation of non-anthocyanin phenolic detecting
The chromatograms of 45 non-anthocyanin phenolic standards were observed (supplementary material, ), each compound exhibited a sharp peak within 29 min. The linearity, detection limit (LOD), quantification limit (LOQ), accuracy and precision of the method were analyzed. As shown in supplementary material Table A2, the regression equations and correlation coefficients (>0.99) showed the method to have good sensitivity and linearity, with LODs in the range of 0.003~3 mg/L, and LOQs in the range of 0.01~10 mg/L. Accuracy and precision studies were assessed by analyzing the recoveries and quantification stabilities of intraday and interday experiments (n = 6) in the form of the relative standard deviation (% RSD) for the 45 non-anthocyanin phenolic compounds at three different concentration levels. As shown in supplementary material Table A3, the recoveries for non-anthocyanin phenolic compounds ranged from 80~116.72%, and the RSD values obtained for quantification experiments ranged from 0.04~5.45.
Determination of chromatic characteristics of wines
A spectrophotometer (Shimadzu, Tokyo, Japan) was used to record the wines’ absorbance spectra (380–700 nm) in a 1 mm path length quartz cuvette. Using absorbance values at 420, 520, and 620 nm, five colorimetric parameters were calculated as described by Glories.[Citation19] These parameters were color intensity (CI), hue, red %, yellow %, and blue %, and were calculated according to:
Each analysis was performed in triplicate.
Statistical analysis
Phenolic identification and quantification analysis was performed by Agilent Mass Hunter workstation software version B.04.00. Averages and standard deviations were calculated using Microsoft Excel 2007 software. IBM SPSS Statistics 20 (IBM, New York, NY, USA) for Windows was used for statistical calculations and principal component analysis (PCA). The differences were considered to be statistically significant when p < 0.05. Pearson correlation analysis was performed by using MetaboAnalyst 3.0.[Citation20]
Results and discussion
Anthocyanin composition analysis
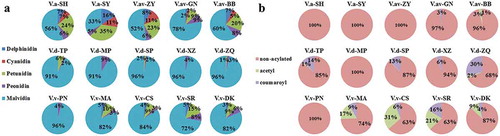
Anthocyanins, as the most important pigments that are responsible for red wine color, are mainly extracted from red grape skins during winemaking, but can also be extracted from flesh when teinturier grapes are used. In total, 27 anthocyanin compounds which belonged to different species were successfully detected (). V. amurensis and its hybrids wines showed a wider concentration range (38.62~863.16 mg/L), by contrast, anthocyanins in wines of V. davidii and V. vinifera ranged from 66.81~397.66 mg/L and 18.53~173.6 mg/L, respectively. Composition of anthocyanins in wines of different species also showed great differences. In V. amurensis and its hybrids wines, 3, 5-O-diglucosidic anthocyanins took a relatively larger percentage (81.41~99.35%) in total anthocyanins, while in V. davidii wines, only limited 3-O-monoglucosidic anthocyanins were detected (0.25~1.09%), as for V. vinifera wines, anthocyanins were all composed of 3-O-monoglucosidic types. In most V. vinifera grape berries, the glycosylation of anthocyanidins takes place exclusively at the 3-position by the activity of 3-O-glucosyltransferase (3GT), whereas both the 3- and 5-positions are glycosylated in non-V. vinifera and their hybrids.[Citation21] Comparing the 3-O-monoglucosidic and 3, 5-O-diglucosidic anthocyanins percentages of different wines, it might be deduced that in non-V. vinifera grapes, the synthesis of 3, 5-O-diglucosidic anthocyanins is more active than the synthesis of 3-O-monoglucosidic anthocyanins, but grape berris of V. amurensis and its hybrids also have a higher bioactive ability than V. davidii grapes in synthesizing 3-O-monoglucosidic anthocyanins. Normally, 3, 5-O-diglucosidic anthocyanins are more stable than their 3-O-monoglucosidic counterparts, but are more susceptible to browning and are less colored,[Citation2] anthocyanins’ glycosylation differences will to a large extent influence the wine color hue and stability.
Table 2. Concentrations (mg/L) of 57 quantified phenolic compounds in different wine samples.
Except for the glycosylation differences in anthocyanins, anthocyanidin composition in wines showed different species features (). According to the different substituents on their B-ring, anthocyanins can be divided into two groups: cyanidin and peonidin based anthocyanins are 3’-substituted anthocyanins, delphinidin-, petunidin-, and malvidin-based anthocyanins are 3’, 5’-substituted anthocyanins.[Citation22] In V. davidii and V. vinifera wines, the anthocyanidin profile from different varieties within one species seemed generally alike: malvidin-based anthocyanins dominated the anthocyanidin profile, while other anthocyanidin-based anthocyanins were quite limited. This was in accordance with previous reports.[Citation2,Citation14] However, in wines of V. amurensis and its hybrids, anthocyanidin composition changed greatly in different varieties, and the percentages of those non-malvidin based anthocyanins generally increased compared with V. davidii and V. vinifera wines. This might be caused by a different transcriptional levels and bioactivities of flavonoid 3’-hydroxylase (F3’H) and the flavonoid 3’, 5’-hydroxylase (F3’5’H) in V. amurensis grape berries, which play a vital role in synthesizing 3’-substituted and 3’, 5’-substituted anthocyanins.[Citation23] It has been reported that delphinidin-, cyanidin-, petunidin-, peonidin-, and malvidin-based anthocyanins just contribute pink, red, purple, bluish purple, and reddish purple in red wines, respectively,[Citation24] the great anthocyanidin composition change in wines of V. amurensis and its hybrids will lead to a relatively more obvious color differences than V. davidii and V. vinifera wines.
shows that except for Pinot Noir, as reported that V. vinifera red grape variety which do not contain acylated anthocyanins,[Citation2] higher acylation ratiosin anthocyanins were found in other V. vinifera wines. However, in wines of V. amurensis and its hybrids, only limited acylated anthocyanin compounds were detected in Gongniangyihao (V.av-GN) and Beibinghong (V.av-BB). Except for Miputao (V.d-MP), V. davidii wines had relatively higher acylation ratios than V.av-GN and V.av-BB, but were still much lower than those of V. vinifera wines. These results were in agreement with previous studies on wines made from V. amurensis and its hybrids and V. davidii for the finite acylation degree of anthocyanins.[Citation14,Citation16]
Flavan-3-ols composition analysis
The results in shows that wines of V. amurensis and its hybrids contained very limited flavan-3-ols compounds, with a concentration ranged from 0.5~12.82 mg/L, while in V. davidii wines, except for the variety Ziqiu (V.d-ZQ), with a flavan-3-ols concentration being 4.42 mg/L, other V. davidii wines contained only trace amount of flavan-3-ols. By comparison, more flavan-3-ols in V. vinifera wines were detected (42.4~225.62 mg/L). The flavan-3-ols synthesis is closely related with leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) in grape berries, the results of flavan-3-ols concentration differences indicate that the behavior of LAR and ANR are largely determined by grape species.[Citation25] V. vinifera grape berries may have more active abilities in synthesizing flavan-3-ols than V. amurensis and its hybrids and V. davidii grape berries. Since different forms and amounts of flavan-3-ols present in grape skins and seeds affect the bitter and astringency mouthfeel of red wines, the extremely low levels of flavan-3-ols in wines of V. amurensis and its hybrids and V. davidii means that the taste parameters of these wines were quite different from the regular dry red wines made from V. vinifera grapes.
Phenolic acids composition analysis
On the basis of their structures, phenolic acids can be divided into two groups: hydroxybenzoic acids and hydroxycinnamic acids. In this study, we detected five hydroxybenzoic acids (2-hydroxybenzoic acid, 4-hydroxybenzoic acid, gallic acid, protocatechuic acid, and vanillic acid) and four hydroxycinnamic acids (4-hydroxycinnamic acid, caffeic acid, gentisic acid, ferulic acid). As shown in , in most wine samples, gallic acid and caffeic acid were the dominate hydroxybenzoic acids and hydroxycinnamic acids, respectively. In all V. davidii wines, hydroxycinnamic acids showed a higher concentration level than hydroxybenzoic acids, which was in agreement with previous study.[Citation14] However, from we could see that in V. vinifera, V. amurensis and its hybrids wines, dominate phenolic acids changed due to variety differences, in most of the non-V. davidii wines, hydroxybenzoic acids’ concentration level was higher than that of hydroxycinnamic acids. As important color co-factors, phenolic acids take part in intermolecular co-pigmentation reaction with anthocyanins, the composition and concentration levels of phenolic acids determine their abilities in enhancing and stabilizing wine color, and thus affect red wines’ color quality.[Citation10,Citation11]
Flavonols composition analysis
Six groups of flavonols were detected in this study, namely kaempferol, myricetin, quercetin, syringetin, isorhamnetin, laricitrin, and their derivatives. From , it can be deduced that concentration levels of each flavonols varied might not due to species differences, but on the basis of variety differences. Because there were almost no flavonols similarities observed within same species, except for kaempferol-3-O-glucoside and quercetin-3-O-rhamnoside, which were detected and quantified in V. davidii wines, in other non-V. davidii wines, these two flavonols were either in trace amount or not detected. The abundance of quercetin-3-O-rhamnoside in V. davidii wines was also reported in Meng’s study;[Citation14] however, in other scientists’ studies for non-V. davidii wines, only a few researches reported the detection of kaempferol-3-O-glucoside and quercetin-3-O-rhamnoside,[Citation26–Citation28] therefore, the existence of kaempferol-3-O-glucoside and quercetin-3-O-rhamnoside may be recognized as flavonols markers for the verification of V. davidii dry red wines.
Chromatic parameters comparison of different wines
shows the chromatic parameters of all wine samples, from which we can see that generally wines of V. amurensis and its hybrids had a relatively higher CI level, while V. davidii wines had a medium CI level, and V. vinifera wines had a relatively lower CI level. Most V. vinifera wines had higher red % value than other wines, but most non-V. vinifera wines had higher blue % value than V. vinifera wines. The yellow % value and hue features of different wines were ambiguous. Although phenolic compounds, especially anthocyanins, are recognized as major color-expressing compounds in red wine, their color quality is largely determined by the concentration and percentage of anthocyanin compounds from different types (such as anthocyanidins composition, glycosylation, and acylation degree differences). Besides, considering the co-pigmentation effect from other non-anthocyanin phenolics, red wines’ color quality should be a cooperative result of both anthocyanin and non-anthocyanin phenolic compounds.[Citation8–Citation11]
Table 3. Chromatic parameters of different wine samples.
Phenolic fingerprints analysis
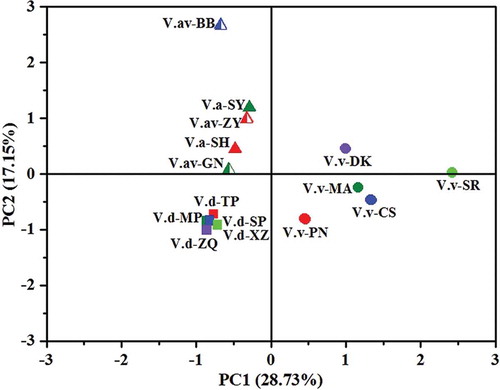
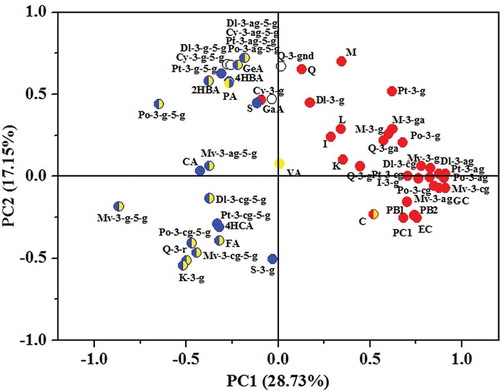
By calculating the percentage of each phenolic compound in total quantified phenols of different wine samples and using PCA, an ideal grape species differentiation of wines according to their phenolic percentage was achieved. As shown in , wines of V. amurensis and its hybrids (sample no. V.a- and V.av-) were located in the top left quadrant of the PCA score plot, V. davidii wines (sample no. V.d-) were located in the bottom left quadrant of the PCA score plot, V. vinifera wines (sample no. V.v-) were located in the right quadrants of the PCA score plot. Because the differentiation of these wines was achieved according to their phenolic percentage, judging from the phenolic loading plot shown in , we can conclude that the percentages of phenolic compounds scattered in different quadrants were positively related with the wines which shared the same quadrants in , and these specific phenolic percentages just represented the phenolic fingerprints of wines from different species.
Phenolic fingerprints’ chemometric relationship with chromatic parameters
On the basis of phenolic percentages and chromatic parameters of different wines, we used Pearson correlation analysis to establish the relationship between phenolic compounds and color parameters. According to the correlation values (data not shown) for the phenolic compounds with the chromatic parameters, phenolics which positively correlated with color properties (correlation value ˃0.1) were all painted with corresponding colors in . From , we could see that the 3-O-monoglucosidic anthocyanins, flavan-3-ols and some flavonols which belonged to the phenolic fingerprints of V. vinifera wines were positively correlated with red %, most 3, 5-O-diglucosidic anthocyanins, phenolic acids and some flavonols which belonged to the phenolic fingerprints of non-V. vinifera wines were positively correlated with and blue %. This was in accordance with our color analysis in chromatic parameters comparison of different wines, that most non-V. vinifera wines had relatively higher blue % but lower red % values than V. vinifera wines. Besides, many of these phenolic compounds were also positively correlated with yellow % value; however, since no obvious yellow % value difference in wines of different species was observed, we think there should be other factors than phenolics that influence the yellow % value more effectively, such as xanthylium salts.[Citation29]
In red wines, both 3-O-monoglucosidic anthocyanins and 3, 5-O-diglucosidic anthocyanins exhibit color directly. Other non-anthocyanin phenolics, although many are colorless compounds, play an important role in co-pigmentation by the physicochemical association with anthocyanin compounds without the formation of a covalent bond.[Citation30] Given that the co-pigmentation effect is usually inconsistent in different wines due to non-anthocyanin phenolics’ special molecular structures and their affinities with anthocyanin compounds, their abilities to influence wine color hue, intensity, and stability are also different.[Citation8–Citation11] By the use of PCA and Pearson correlation analysis, it can provide us some chemometric clues to explain the phenolic impact on chromatic differences of wines from different species, namely, the relatively higher phenolic percentages of 3-O-monoglucosidic anthocyanins (except for cyanidin-3-O-glucoside), flavan-3-ols, and some flavonols (kaempferol, myricetin, myricetin-3-O-galactoside, myricetin-3-O-glucoside, quercetin, quercetin-3-O-galactoside, quercetin-3-O-glucoside, isorhamnetin, isorhamnetin-3-O-glucoside, and laricitrin) affected the V. vinifera wines’ higher red % significantly, whereas the higher blue % of non-V. vinifera wines were mainly affected by the higher phenolic percentage of petunidin-3, 5-O-diglucosides, peonidin-3, 5-O-diglucosides, acetylated 3, 5-O-diglucosidic anthocyanins, 2-hydroxybenzoic acid, 4-hydroxybenzoic acid, protocatechuic acid, caffeic acid, gentisic acid, syringetin (V. amurensis and its hybrids wines) and malvidin-3, 5-O-diglucosides, coumaroylated 3, 5-O-diglucosidic anthocyanins, 4-hydroxycinnamic acid, ferulic acid, quercetin-3-O-rhamnetin, kaempferol-3-O-glucoside, syringetin-3-O-glucoside (V. davidii wines).
Practical significance of the grape species comparison
Currently, wine drinking in China has become increasingly popular and many vineyards and wineries have been built for wine production. Not only the traditional V. vinifera grapes, such as Cabernet Sauvignon, Cabernet Franc, Cabernet Gernischt, Merlot, Chardonnay, and Riesling, are cultivated for winemaking, wines made from V. amurensis (and its hybrids) and V. davidii grapes are also welcomed. By comparing the phenolic and chromatic characteristics of wines made from different species, we can have a basic understanding of their phenolic and chromatic differences, potentially assisting us improve the sensory quality of domestic wines. For instance, from the result of PCA and Pearson correlation analysis ( and ), we can see that the chromatic quality of wines are largely affected by phenolic characteristics, phenolic compounds in non-V. vinifera wines contribute greatly to wines’ relatively higher blue % value, whereas phenolic compounds in V. vinifera wines contribute greatly to wines’ relatively higher red % value. Therefore, a proper blend of wines from different species may lead to a better color quality for wine products. Furthermore, shows that V. vinifera wines had relatively higher flavan-3-ol phenolics, and non-V. vinifera wines had relatively higher 3, 5-O-diglucosidic anthocyanins, flavan-3-ol phenolics are responsible for wines’ astringency and 3, 5-O-diglucosidic anthocyanins own a better color stability than 3-O-monoglucosidic anthocyanins, the blend of wines from different species can also make up the deficiencies of wines made from mono-species.
Conclusions
Phenolic compounds and chromatic parameters of wines from V. amurensis and its hybrids, V. davidii, and V. vinifera were compared in this study. The results indicated that the general phenolic profiles of wines were different due to their species differences. Non-V. vinifera wines’ anthocyanins were mainly comprised of 3, 5-O-diglucosidic anthocyanins, with limited proportions for 3-O-monoglucosidic anthocyanins, nevertheless, V. vinifera wines’ anthocyanins were all 3-O-monoglucosidic type. V. vinifera wines contained more flavan-3-ols than non-V. vinifera wines. In most wine samples, gallic acid and caffeic acid were the dominate hydroxybenzoic acids and hydroxycinnamic acids, respectively. Hydroxycinnamic acids showed a higher concentration level than hydroxybenzoic acids in all V. davidii wines, but a relatively lower concentration level than hydroxybenzoic acids in most wines of non-V. davidii wines. Kaempferol-3-O-glucoside and quercetin-3-O-rhamnoside were more abundant in V. davidii wines compared with wines of other species. The results of PCA and Pearson correlation analysis suggest that phenolic percentages can not only differentiate wines of different species, but also chemometrically explain the relatively higher red % and blue % values of V. vinifera wines and non-V. vinifera wines, respectively.
LJFP_A_1233117_Supplemental_material.docx
Download MS Word (182.4 KB)Funding
This study was supported by the China Agriculture Research System, Grant number CARS-30 to Chang-Qing Duan.
Additional information
Funding
References
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin Pattern of Several Red Grape Cultivars and Wines Made from Them. European Food Research and Technology 2002, 215, 32–37.
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17(2), 1571–1601.
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The Mouth-Feel Properties of Grape and Apple Proanthocyanidins in a Wine-Like Medium. Journal of the Science of Food and Agriculture 2003, 83, 564–573.
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in Grapes, Grape Products and Wines: Burden, Profile and Influential Parameters. Journal of Food Composition and Analysis 2006, 19(5), 396–404.
- Sáenz-Navajas, M.P.; Fernández-Zurbano, P.; Ferreira, V. Contribution of Nonvolatile Composition to Wine Flavor. Food Reviews International 2012, 28(4), 389–411.
- Francia-Aricha, E.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New Anthocyanin Pigments Formed After Condensation with Flavanols. Journal of Agricultural and Food Chemistry 1997, 45, 2262–2266.
- Pissarra, E.J.; Mateus, N.; Rivas-Gonzalo, J.; Santos-Buelga, C.; De Freitas, V. Reaction Between Malvidin-3-Glucoside and (+)-Catechin in Model Solutions Containing Different Aldehydes. Journal of Food Science 2003, 68, 476–481.
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the Copigmentation Between Anthocyanins and Flavan-3-Ols and Their Influence in the Colour Expression of Red Wine. Food Chemistry 2009, 114(2), 649–656.
- Rustioni, L.; Bedgood, D.R.; Failla, O.; Prenzler, P.D.; Robards, K. Copigmentation and Anti-Copigmentation in Grape Extracts Studied by Spectrophotometry and Post-Column-Reaction HPLC. Food Chemistry 2012, 132(4), 2194–2201.
- Zhang, B.; He, F.; Zhou, P.P.; Liu, Y.; Duan, C.Q. Copigmentation Between Malvidin-3-O-Glucoside and Hydroxycinnamic Acids in Red Wine Model Solutions: Investigations with Experimental and Theoretical Methods. Food Research International 2015, 78, 313–320.
- Zhang, B.; Liu, R.; He, F.; Zhou, P.P.; Duan, C.Q. Copigmentation of Malvidin-3-O-Glucoside with Five Hydroxybenzoic Acids in Red Wine Model Solutions: Experimental and Theoretical Investigations. Food Chemistry 2015, 170, 226–233.
- Wan, Y.Z.; Schwaninger, H.; Li, D.; Simon, C.J.; Wang, Y.J.; Zhang, C.H. The Eco-Geographic Distribution of Wild Grape Germplasm in China. VITIS–Journal of Grapevine Research 2008, 47(2), 77.
- Liu, L.; Li, H. Review: Research Progress in Amur Grape, Vitis Amurensis Rupr. Canadian Journal of Plant Science 2013, 93(4), 565–575.
- Meng, J.F.; Xu, T.F.; Qin, M.Y.; Zhuang, X.F.; Fang, Y.L.; Zhang, Z.W. Phenolic Characterization of Young Wines Made from Spine Grape (Vitis Davidii Foex) Grown in Chongyi County (China). Food Research International 2012, 49(2), 664–671.
- Liang, N.N.; Pan, Q.H.; He, F.; Wang, J.; Reeves, M.J.; Duan, C.Q. Phenolic Profiles of Vitis Davidii and Vitis Quinquangularis Species Native to China. Journal of Agricultural and Food Chemistry 2013, 61(25), 6016–6027.
- Zhao, Q.; Duan, C.Q.; Wang, J. Anthocyanins Profile of Grape Berries of Vitis Amurensis, Its Hybrids and Their Wines. International Journal of Molecular Sciences 2010, 11(5), 2212–2228.
- Zhao, Q.; Duan, C.Q.; Wang, J. Components of Non-Anthocyanin Phenolic Compounds in Wines of Vitis Amurensis and Its Hybrids. African Journal of Biotechnology 2013, 10(66), 14767–14777.
- Li, S.Y.; He, F.; Zhu, B.Q.; Xing, R.R.; Reeves, M.J.; Duan, C.Q. A Systematic Analysis Strategy for Accurate Detection of Anthocyanin Pigments in Red Wines. Rapid Communications in Mass Spectrometry 2016, 30(13), 1619–1626.
- Glories, Y. La Couleur des Vins Rouges. Connaissance de la Vigne et du Vin 1984, 18, 253–271.
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making Metabolomics More Meaningful. Nucleic Acids Research 2015, 43(1), 251–257.
- De la Cruz, A.A.; Hilbert, G.; Rivière, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.C.; Delrot, S.; Mérillon, J.M.; Monti, J.P.; Gomès, E.; Richard, T. Anthocyanin Identification and Composition of Wild Vitis spp. Accessions by Using LC-MS and LC-NMR. Analytica Chimica Acta 2012, 732, 145–152.
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin Composition and Anthocyanin Pathway Gene Expression in Grapevine Sports Differing in Berry Skin Colour. Australian Journal of Grape and Wine Research 1996, 2, 163–170.
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. The Plant Journal 2008, 54, 733–749.
- Burns, J.; Mullen, W.; Landrault, N.; Teissedre, P.L.; Lean, M.E.; Crozier, A. Variations in the Profile and Content of Anthocyanins in Wines Made from Cabernet Sauvignon and Hybrid Grapes. Journal of Agricultural and Food Chemistry 2002, 50(14), 4096–4102.
- Bogs, J.; Downey, M.; Harvey, J.; Ashton, A.; Tanner, G.; Robinson, S. Proanthocyanidin Synthesis and Expression of Genes Encoding Leucoanthocyanidin Reductase and Anthocyanidin Reductase in Developing Grape Berries and Grape Vine Leaves. Plant Physiology 2005, 139, 652–663.
- La Torre, G.L.; Saitta, M.; Vilasi, F.; Pellicanò, T.; Dugo, G. Direct Determination of Phenolic Compounds in Sicilian Wines by Liquid Chromatography with PDA and MS Detection. Food Chemistry 2006, 94(4), 640–650.
- Rodrı́guez-Delgado, M.Á.; González-Hernández, G.; Conde-González, J.E.; Pérez-Trujillo, J.P. Principal Component Analysis of the Polyphenol Content in Young Red Wines. Food Chemistry 2002, 78(4), 523–532.
- Rossouw, M.; Marais, J. The Phenolic Composition of South African Pinotage, Shiraz and Cabernet Sauvignon Wines. South African Journal for Enology and Viticulture 2004, 25(2), 94–104.
- Es-Safi, N.E.; Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium Salts Formation Involved in Wine Colour Changes. International Journal of Food Science and Technology 2000, 35(1), 63–74.
- Rustioni, L.; Di Meo, F.; Guillaume, M.; Failla, O.; Trouillas, P. Tuning Color Variation in Grape Anthocyanins at the Molecular Scale. Food Chemistry 2013, 141(4), 4349–4357.