ABSTRACT
Increased reactive oxygen species causes cells or tissue damages and is associated with some degenerative diseases, such as coronary heart diseases and cancer. This research is intended to isolate and to identify the antiradical compounds from the bark of Jujube tree (Zizyphus mauritiana Auct. non Lamk.). The methanol extract of the bark was successively fractionated with n-hexane and ethyl acetate to give n-hexane fraction, ethyl acetate fraction, and methanol extract residue, respectively. The extract and fractions were examined for its radical scavenging activity using 2,2’-diphenyl-1-picrylhydrazil radical. The ethyl acetate fraction, having the most active 2,2’-diphenyl-1-picrylhydrazil radical scavenging, was further repeatedly separated using vacuum liquid chromatography, and the purification was carried out using preparative thin layer chromatography (TLC) in order to obtain an active isolate. The purity of the isolate was evaluated using thin layer chromatography method and melting point measurement. The structure of the isolate was identified chemically using various spray reagent and ultraviolet-visible, infrared, infrared, Liquid Chromatography-Mass Spectrometry (LC-MS) and Proton Magnetic Resonance (1H-NMR) spectroscopic methods. The extract, fraction, and sub-fractions were determined for its total contents of phenolic and flavonoid. The results showed that the methanol extract residue contained the highest total phenolic (36.29% wt/wt gallic acid equivalent), while fraction E4 of the ethyl acetate extract contained the highest total flavonoids (21.31% wt/wt quercetin equivalent). The active isolate showed to have 2,2’-diphenyl-1-picrylhydrazil radical scavenging activity with IC50 of 7.58 μ/mL and is identified as trans-p-coumaroyl triterpene. Detail structure of the triterpene needs to be identified further.
Introduction
Reactive oxygen species (ROS) is a result of the normal aerobic metabolism in the human body which potentially cause molecular damages.[Citation1] Compunds that are categorized as ROS, such as superoxide anion radicals, singlet oxygen, hydrogen peroxide, and hydroxyl radicals, are very reactive.[Citation2] ROS in excessive amount may attack cells and tissues and damage them, which, in turn, can cause some degenerative diseases, such as coronary heart diseases and cancer.[Citation3] In order to supress ROS production, some antioxidants are needed in the human body to prevent degenerative diseases. Antioxidants are compounds, either synthetic or natural, capable of delaying, inhibiting, or preventing the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidative chain reactions.[Citation4] Plants may become the source of potential natural antioxidant, due to a number of phenolic compounds mainly flavonoid and phenolic acids contained.[Citation5] A Jujube plant (Ziziphus mauritiana Auct. non Lamk) is traditionally used as herbal medicine, especially in Bali Province, Republic of Indonesia.
Dahiru et al.[Citation6] reported that the ethanol extract of Z. mauritiana leaves have hepatoprotector activity, which prevents liver damage in mice treated with carbon tetrachloride. Compound with hepatoprotector activities can be taken into account as antioxidants by slowing down the process of free radical oxidation[Citation7] An oxidative stress is a consequence of unbalance pro-oxidant amounts and the oxidant in the organism body.[Citation8] The potential use of Z. mauritiana as antioxidant has been reported by some investigators. A previous study reported that the 2,2’-diphenyl-1-picrylhydrazil (DPPH) radical scavenging activity from the ethanol and hexane extracts of Z. mauritiana leaf has IC50 value of 101.02 and 124.21 µg/mL, respectively; compared with ascorbic acid (IC50 value of 78.12 μg/mL).[Citation9] Perumal et al.[Citation10] reported the DPPH radical scavenging activity from methanol extract of leave and bark of Z. mauritiana with IC50 values of 21.40 ± 0.15 μg/mL and 20.09 ± 0.19 μg/mL, respectively; compared to that of positive control of butyl hydroxytoluene with IC50 value of 18.50 ± 0.19 μg/mL. The ethanol extract of the Z. mauritiana bark is also reported to have antioxidant activity toward the free radical scavenging of DPPH with the IC50 of 27.47 μg/mL, compared with the radical scavenging activity of ascorbic acid with IC50 value of 18.63 μg/mL.[Citation11]
Jain et al.[Citation12] and Bhatt and Dhyani[Citation13] reported that the bark of Z. Mauritiana contained compounds of alkaloid, saponin, flavonoid, phenolics acids, lignin, steroid, and tannin. Antioxidant activity is often correlated with the presence of phenolic compounds, such as a flavonoid, phenolic acid, and triterpenoat acid;[Citation14–Citation16] therefore, the contents of phenolics and flavonoids are also determined during antioxidant studies from plants. Although the antioxidant activity of Z. Mauritiana has been reported, the active compounds and their mechanism of action is largely unknown. This research is intended to isolate the antioxidant compounds from the bark of Z. Mauritiana by bioassay-guided fractionation and to determine its DPPH radical scavenging activity.
Materials and methods
Materials
The bark of the Jujube tree was collected from Bukit Jimbaran, Bali and its authenticity is determined in the Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada. The plant samples used were aged from 5–6 years from five different locations in Jimbaran, Badung Regency of Bali Province, Indonesia. The plant sample is taken in the morning during February 2013, it is during this time that the plant is active growing. The sample of bark is taken at the main stem of the plant (in the center). The chemicals used in this research were ethyl acetate and n-hexane (technical grade), n-hexane, methanol, chloroform, ethyl acetate, TLC plates silica gel GF254, Silica Gel 60 (0.040–0.063 mm), Silica Gel 40 (0.063–0.200 mm) and Silica gel PF254 (Merck Darmstadt, Germany). The solvents used were of analytical grade unless otherwise specified.
Extraction and isolation
The bark samples are dried in the oven at controlled temperature (60ºC) for 3 days. The dried Z. mauritiana barks were powdered and extracted using methanol. The methanol extract was fractionated successively with n-hexane and ethyl acetate to give corresponding fractions and methanol extract residue. These extract and fractions were further determined for its DPPH radical scavenging activity along with phenolics and flavonoid contents. The ethyl acetate fraction was then fractioned by vacuum liquid chromatography (VLC) over silica gel 60, eluted with solvents with increasing polarity (different mixtures of n-hexane-chloroform and chloroform-methanol). Fractions with the same TLC profiles were combined (designated with E1-E5) and were evaluated for its DPPH radical scavenging activity along with phenolics and flavonoid contents. The most active fraction (E4) was further separated to give active isolate.
DPPH radical scavenging activity assay
Determination of the DPPH free radical scavenging activity was carried out as in Kikuzaki et al.[Citation17] A certain volume of sample is added to 1.0 mL of 0.4 mM DPPH and 3.950 mL methanol. The mixture is subsequently subjected to vortex and left for 30 min. The absorbance (Abs) is measured at wavelength of 515 nm using methanol as blank (Abs sample). The control solution consisting of 1.0 mL DPPH and 4.0 mL methanol was prepared and its absorbance was measured Abs control.
The percentage of DPPH radical scavenging activity was plotted against the sample concentrations (μg/mL) to obtain IC50, defined as the concentration of the samples necessary to cause 50% scavenging of DPPH radical, calculated by an equation generated from linear regression.[Citation18]
Determination of the total phenolic content
Total phenolic content of studied extract and fractions was determined using visible spectrophotometric methods as in Chun et al.[Citation19] In volumetric flask 10 mL, a portion of diluted samples (methanol extract, n-hexane and ethyl acetate fractions, methanol extract residue, and fractions of the separation of ethyl acetate fraction) was added. The solution is then added with 0.4 mL of Folin-Ciocalteu reagent and left for 5–8 min. The solution was then added with 4.0 mL Na2CO3 7% and aquabidest until volume was reached. After 90 min, the absorbance of each solution was read at wavelength of 765 nm. The mixture of distilled water and Folin-Ciocalteu reagent was used as blank. The contents of total phenolic of samples were expressed as grams of gallic acid equivalent (GAE)/100 g dry samples. The samples were analyzed in triplicate.
Determination of the total flavonoid content
The total flavonoid content was determined by visible spectrophotometry as reported by Zou et al.[Citation20] The studied samples are methanol extract, n-hexane fraction, ethyl acetate fraction, methanol extract residue, and fractions obtained during separation of ethyl acetate fraction. Into volumetric flask 10.0 mL, a portion volume of samples was added into volumetric flask, followed by 4.0 mL of aquabidest and 0.3 mL of AlCl3 and was left for 6 min. The solution was then added with 4.0 mL of 4% NaOH and distilled water until volume. The solution was left for 15 min and then its absorbance was measured at 510 nm. All reagents without samples are used as blank. Total flavonoid contents of extracts and fractions were expressed as grams of quercetin equivalent (QE)/100 g dry material.
Results and discussion
The methanol extract of Jujube bark (814.0 g) were successively fractionated with n-hexane and ethyl acetate to give hexane fraction (27.5 g), ethyl acetate fraction (167.5 g), and methanol extract residue (604.8 g). Both fractions and residue were evaluated for their antioxidant activity using DPPH free radical scavenging method. Ethyl acetate fraction showed the strongest antioxidant activity (IC50 23.70 µg/mL), followed by methanol extract (IC50 26.83 µg/mL) and residue (IC50 40.71 µg/mL), while the hexane fraction was not active. The ethyl acetate fraction was further fractionated using VLC to give 5 fractions, E1 (0.3 g), E2 (5.3 g), E3 (3.9 g), E4 (8.7 g), and E5 (1.8 g). Fractions E4 and E5 were active with the IC50 values of of 17.60 and 32.23 µg/mL, respectively ().
Table 1. The activity of DPPH free radical scavenging activity of methanol extract and fractions of the Jujube tree bark.
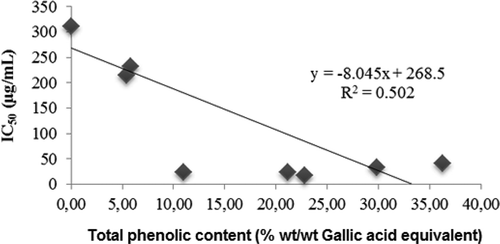
Antiradical activity of plant extracts is due to the presence of phenolic compounds which have the capability to donor radical hydrogen, therefore, in this study, the levels of phenolics and flavonoids contents are determined using visible spectrophotometry. compiled the phenolics and flavonoid contents of the methanol extract, fractions, and residue. The result showed that the residual methanol extract contains the highest phenolic content (36.29% wt/wt GAE), followed by fraction E5 (29.87% wt/wt GAE), E4 (22.83% wt/wt GAE), ethyl acetate (21.20% wt/wt GAE), and E3 (12.64% wt/wt GAE), methanol extract (11.01% wt/wt GAE), E2 (5.85% wt/wt GAE), and E1 (5.41% wt/wt GAE). The correlation between the total phenolic content (x-axis) and IC50 value (y-axis) of the samples gave linear regression equation of y = –8.045x + 268.5 with the coefficient of determination (R2) of 0.502 (). It means that 50.20% of DPPH free radical scavenging activity was contributed by phenolic compounds. DPPH radical scavenging activity of the samples is influenced not only by the phenolic compounds but also by other compounds, such as vitamins and carotenoids, which possibly gave 49.80% contribution toward DPPH radical scavenging activity of Jujube bark.[Citation14,Citation21]
Table 2. The total of phenolic and flavonoid content of methanol extract and fractions.
Figure 1. The relation between the total of phenolic content expressed as gallic acid equivalent with IC50.
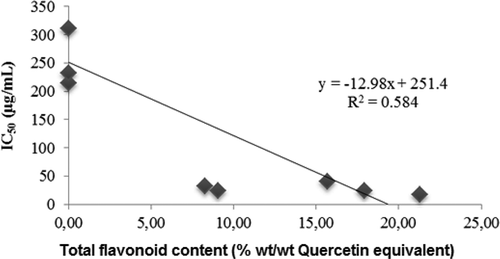
The total flavonoid content of methanol extract, fractions, and residue were also shown in . Fraction E4 contains the highest flavonoid (21.31% wt/wt QE), followed by ethyl acetate fraction (17.92% wt/wt QE), residue (15.69% wt/wt QE), methanol extract (9.07% wt/wt QE), and then fraction E5 (8.29% wt/wt QE) and E3 (5.73% wt/wt QE). No flavonoid is detected in E1 and E2 fractions. The correlation between total flavonoid content (x-axis) and IC50 value (y-axis) gave linear regression equation of y = –12.98x + 251.4 with the coefficient of determination (R2) of 58.40% (). It means that 58.40% contribution of DPPH free radical scavenging activity comes from flavonoid compounds, while 41.60% of DPPH antiradical activity is due to contribution of other secondary metabolites.
Figure 2. Relation between the total flavonoid content expressed as % quercetin equivalent (% wt/wt) with IC50.
The fraction E4 exhibited the highest antioxidant activity and contained high phenolic and flavonoids contents, therefore, this fraction was further separated using VLC. The fractions obtained were examined for its DPPH free radical scavenging activity. The most active fractions (E44) was then separated using coloumn chromatography to give E441, E442, and E443 fractions, respectively. The most active fraction, E443 was separated using preparative TLC to give active isolate. The active isolate was obtained as brownish white solid with the melting point of 287–292°C. The isolate gave positive test with FeCl3 (purple-black) and Liebermann Bourchard reagent which indicated the presence of phenolic and triterpenoid compounds, respectively. Lee et al.[Citation22] have isolated 11 terpenoids from the fruit of the Z. jujube, four of which are condensed with cis or trans p-coumaric acid, namely (1) 3-O-cis-p-coumaroyl alphitolic acid; (2) 3-O-trans-p-coumaroyl alphitolic acid; (3) 3-O-cis-p-coumaroyl maslinic acid; and (4) 3-O-trans-p-coumaroyl maslinic acid.
The chemical structure of isolate was characterized using spectroscopic methods based on its ultraviolet-visible (UV-Vis), infrared (IR), mass and Proton Magnetic Resonance (1H-NMR) spectra. The mass spectrum (ESI+) showed a quasi-molecular ion peak at m/z 641.7 [M+Na]+. Other peaks at m/z 165.1 and 473.6 were particularly informative with coumaric acid and triterpene fragments, respectively. The UV-Vis spectrum of the active isolate gave absorption maxima at 203 (methanol), 261 and 296 nm, which are typical for benzenenoid type chromophore (hydroxyphenyl and p-coumaroyl, respectively). The IR spectrum showed specific absorption bands at 3442 cm−1 (OH); 1544 cm−1 (C = C aromatic); 1697 cm−1 (conjugated C = O); 1292 cm−1 (C-O); 2949 cm−1 (C-H aliphatic); 1382 cm−1 (CH3). The complex peaks at δ 0.7 to 2 in 1H-NMR spectrum was typical for triterpene protons. Nevertheless, the existance of p-coumaroyl moiety was fully supported by the appearance of two doublet signals each with 2H integration and coupling constant of 8.4 Hz at δ 6.82 and δ 7.36 which are characteristic for H-3″; H-5″; and H-2″; H-6″ of p-disubstituted benzen, respectively. The other two doublet signals (1H, 16.2 Hz, each) at δ 7.42 and δ 6.77 belong to H 3′ (H-β) and H-2′ (H-α) of the α,β-unsaturated carbonyl, respectively. The very high coupling constant of these doublets indicated trans configuration of the double bond. Based on the UV-Vis, IR, 1H-NMR, and mass spectroscopic data, the isolate was determined as trans-p-coumaroyl triterpene. Further purification and a detailed NMR analysis of the active isolate need to done to determine the structure of triterpenene. The isolate has the DPPH free radical scavenging activity with IC50 value of 7.58 µg/mL.
Conclusion
The active constituent of Jujube bark (Ziziphus mauritiana Auct, non Lamk.) was trans-p-coumaroyl triterpene. This isolate showed to have DPPH free radical scavenging activity with the IC50 of 7.58 mg /mL. In addition, there is a correlation between radical scavenging activity and phenolics and flavonoid contents, as indicated with R2 value of 0.502 and 0.584, respectively. This finding support that the extract of Jujube bark can be used as food supplement with antioxidant activity.
Funding
Putu Oka Samirana would like to thank the Directorate of Higher Education, the Ministry of Research, Technology and Higher Education, Republic of Indonesia for the scholarship during the study.
Additional information
Funding
References
- Benzie, I.F.; Strain., J.J. The Ferric Reducing Ability of Plasma (FRAP) As a Measure of “Antioxidant Power:” The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Waris, G.; Ahsan, W. Reactive Oxygen Species: Role in the Development of Cancer and Various Chronic Conditions. Journal of Carcinogenesis 2006, 5, 1–14.
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-Radical Scavenging Capacity and Antioxidant Activity of Selected Plant Species from the Canadian Prairies. Food Chemistry 2004, 84, 551–562.
- Kumaran, A.; Karunakaran, R.J. Antioxidant and Free Radical Scavenging Activity of Aquoeus Extract of Coleus Aromaticus. Food Chemistry 2006, 97, 109–114.
- Martin, K.R.; Appel, C.L. Polyphenol As Dietary Supplements: A Double-Edge Sword. Nutrition and Dietary Supplement 2010, 2, 1–12.
- Dahiru, D.; William, E.T.; Nandro, M.S. Protective Effect of Ziziphus Mauritiana Leaf Extract on Carbon Tetrachloride-Induced Liver Injury. African Journal Biotechnology 2005, 4, 1177–1179.
- Murray, R.K.; Granner, D.K.; Rodwel, V.W. Harper’s Biochemistry (Biokimia Harper), 27th Ed; Wulandari, N.; Trans.; EGC: Jakarta, 2009.
- Urquiaga, I.; Leighton, F. Plant Polyphenol Antioxidants and Oxidative Stress. Biological Research 2000, 33, 55–64.
- Abalaka, M.E.; Mann, A.; Adeyomo, S.O. Studies on in-Vitro Antioxidant and Free Radical Scavenging Potential and Phytochemical Screening of Leaves of Ziziphus Mauritiana L. and Ziziphus Spinachristi L. Compared with Ascorbic Acid. Journal of Medical Genetics and Genomics 2011, 3, 28–34.
- Perumal, S.; Mahmud, R.; Piaru, S.P.; Cai, L.W.; Ramanathan, S. Potential Antiradical Activity and Cytotoxycity Assesment of Ziziphus Mauritiana and Syzygium Polyanthum. International Journal of Pharmacology 2012, 8, 535–541.
- Rahman, S. Antioxidant, Analgesic, Cytotoxic and Antidiarrheal Activities of Ethanolic Ziziphus Mauritiana Bark Extract. Oriental Pharmacy and Experimental Medicine 2012, 12, 67–73.
- Jain, A.; Bhatt, S.; Dhyani, S. Phytochemical Screening of Secondary Metabolites of Ziziphus Mauritiana Lam. Bark. International Journal of Current Pharmaceutical Research 2012, 4, 156–159.
- Bhatt, S.; Dhyani, S. Quantification of Secondary Metabolites from Ziziphus Mauritiana Lam. Bark. International Journal of Bio-Technology and Research 2013, 3(2), 1–6.
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant Activity and Total Phenolic Content of Iranium Ocimum Accessions. Food Chemistry 2003, 83, 547–550.
- Gujral, H.S.; Angurala, M.; Sharma, P.; Singh, J. Phenolic Content and Antioxidant Activity of Germinated and Cooked Pulses. International Journal of Food Properties 2011, 14, 1366–1374.
- Bozalan, N.K.; Karadeniz, F. Carotenoid Profile, Total Phenolic Content, and Antioxidant Activity of Carrots. International Journal of Food Properties 2011, 14, 1060–1068.
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidants Properties of Ferulic Acid and Its Related Compounds. Journal of Agricultural and Food Chemistry 2002, 50, 2161–2168.
- Rohman, A.; Riyanto, S.; Utari, D. Antioxidant Activities, Total Phenolic and Flavonoid Contents of Ethyl Acetate Extract of Mengkudu (Morinda Citrifolia, L) Fruit and Its Fractions. Majalah Farmasi Indonesia (Indonesian Journal of Pharmacy) 2006, 17(3), 136–142.
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide Radical Scavenging Activity of the Major Polyphenols in Fresh Plums. Journal of Agricultural and Food Chemistry 2003, 51, 8067–8072.
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of Flavonoid-Rich Extract of Hypericum Perforatum L. in Vitro. Journal of Agricultural and Food Chemistry 2004, 52, 5032–5039.
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. Journal of Agricultural and Food Chemistry 2001, 49, 5165–5170.
- Lee, S.M.; Park, J.G.; Lee, Y.H.; Lee, C.G.; Byung-Sun Min, B.S.; Kim, J.H.; Lee, H.K. Anti-Complementary Activity of Triterpenoides from Fruits of Zizyphus Jujuba. Biological and Pharmaceutical Bulletin 2004, 27, 1883–1886.
