ABSTRACT
Human physiology normally contains pathogenic and non-pathogenic microorganisms in the gastrointestinal flora. Disturbance of these microorganism balance results in the formation of infection. Extensive use of antibiotics for cure of these disturbances like Helicobacter pylori (H. pylori) infection leads to patient discomfort and associated side-effects. There is a need to adopt adjunct or alternative approach in order to minimize such conditions. Probiotics is one of the potential therapies to cure gastrointestinal discomforts especially associated with H. pylori. It competes through non-immune and immune systems. This review article concludes that probiotics are used to eradicate the infection at increased rate, and decreased associated side-effects are caused by triple therapy. A proper evaluation of these probiotics is demanded before their use in future as a commercial product. Furthermore, their effect on immune system requires more research work so that their usage for other chronic disorders can also be considered.
Introduction
The study of intestinal microbial flora and its impact on human health has become an interesting approach for scientist. Normal flora contains more than 500 species which includes pathogens as well as friendly bacteria.[Citation1] Helicobacter pylori is one of the major causative agents for peptic ulcer, adenocarcinoma, and chronic gastritis by disturbing normal flora. Its ability to develop different strains shows resistant to conventional therapies.[Citation2,Citation3] Classical treatments of H. pylori include combination of antibiotics and proton pump inhibitor.[Citation4,Citation5] The standard treatment strategy used as first-line management of H. pylori is triple therapy which includes proton pump inhibitor, metronidazole, amoxicillin or clarithromycin twice daily. Alternative treatments include quinolone-based treatment or quadruple therapy containing bismuth.[Citation6] The use of triple therapy is continuously decreasing throughout the world. The main reason is the declining eradication rate and increase in the associated side-effects related to gastrointestinal (GIT) tract. Thus, need of novel therapy is intensely felt recently.[Citation7] The novel and ideal approach to treat H. pylori is that the infection should be cured without causing the antibiotic resistance as well as side–effects.[Citation8–Citation10]
Eradication rate of most classical approaches of H. pylori treatment fails due to antibiotics resistance and poor patient acceptance toward therapy.[Citation11] This failure with conventional triple therapy proposes to find alternative treatment plans.[Citation12] Lactic acid bacteria (LAB) mainly present in dairy products shows therapeutic activity against H. pylori.[Citation2] Friendly bacteria also called “probiotics” have been found beneficial for the cure of different GIT conditions.[Citation1] They show their activity in different ways either interfering the bacterial site of action or by producing antimicrobial molecule.[Citation13] Their efficiency was proved by minimizing the side-effects and enhancing patient compliance.[Citation14,Citation15] Remarkable effect is seen as probiotics being adjunctive to the triple therapy of antibiotics or may be used as prophylaxis.[Citation16,Citation17] The meta-analysis for evaluating the probiotics’ role for cure of H. pylori infection showed that the eradication rate for standardized treatment and combined therapy with supplemental probiotics were 74.8% and 82.6%, respectively.[Citation16] Probiotics is not only effective for eradication but also responsible for reduced adverse effects due to the antibiotic therapy like GIT disturbances, including diarrhoea, lose bowels, etc.[Citation18] Probiotics had an in vitro inhibitory effect on H. pylori but did not show complete removal of intestinal barrier abnormalities.[Citation16,Citation19] Patient compliance was unaffected by this therapy.[Citation20]
Method
All data were collected from the published research and review papers regarding the field of probiotics and H. pylori infection in 2016. The databases searched were PubMed, ScienceDirect, Google scholar, Medline, ISI web of science, and Scopus. H. pylori, resistance due to antibiotics, probiotics and GIT discomfort, side-effects of triple therapy, effects and uses of probiotics were the keywords used to search the data.
Helicobacter pylori
H. pylori is a globally widespread pathogen. It is a gram-negative and spiral-shaped bacteria.[Citation21] It is the major causative microorganism of chronic gastritis, peptic ulcer, and the risk factor for other gastric malignancies.[Citation19,Citation22] It has tendency to withstand the stomach acidity and adhere to gastric mucosa.[Citation23] Its prolonged stay in stomach leads to increase release of IL-8, which in turn increases neutrophils, thus results in chronic gastritis.[Citation24] Several functional and physiological alterations have been observed due to increased stay of H. pylori in the stomach and epithelial cell lines of intestine.[Citation19,Citation25] Some of these alterations have been seen even after bacterial removal from the host, thus showing post-infection and activated immune system for prolonged time.[Citation25] Development of different bacterial strains shows resistance to classical therapies.[Citation2]
Probiotics
Probiotics can be defined as the live food supplement microbial in nature used for microbial balance in the body thus responsible for better health.[Citation8,Citation15,Citation26,Citation27] These have the tendency to interact the microbial flora of GIT tract to produce beneficial effect.[Citation28–Citation30] The risk of re-infection by H. pylori can also be reduced by increase in intake of probiotics present in dietary products.[Citation31] Significant decrease in average frequencies and intensities with nausea, vomiting, and diarrhoea were observed due to probiotics.[Citation4] They inhibit the growth of various gastric, intestinal, and urinary pathogens. Their antagonistic activity mainly depends on environmental conditions that are aerobic or non-aerobic.[Citation28] Prebiotics can be defined as the particular fermented dietary fibres used to exhibit the required changes in the structure or function of intestinal microbial flora.[Citation32] Most commonly used prebiotics is inulin, which is the main representative of the prebiotic range used to improve the intestinal health.[Citation33] Synbiotics is the particular term used for combination of probiotics and prebiotics.[Citation34,Citation35] The combination also shows increase in mineral adsorption and maintain lipid metabolism.[Citation34] Symptomatic treatment of dyspepsia after H. pylori infection can be treated successfully by use of particular probiotics.[Citation19,Citation36] Probiotics supplementation increases patient compliance and their adhesion to treatment.[Citation5]
Composition of probiotics
Probiotics are mainly found in the dietary preparations like fermented milk that includes LAB and bacteria from other genera and bacterial species.[Citation26] Lactobacillus, Streptococcus, Enterococcus, Bifidobacteria, Eschericia, Bacillus, and some fungal strains of Sacchromyces are the main genera present in probiotics.[Citation35] Different probiotic strains are Lactobacillus acidophilus, Lactobacillus johnsonii, Lactobacillus gasseri, and Lactobacillus rhamnosus.[Citation37] These strains have the capability of conversion of linoleic acid to conjugated linoleic acid (CLA). CLA has distinct physiological properties of being antidiabetic, anticancerous, anti-inflammatory, etc.[Citation24]
Meta-analysis of different probiotics
Probiotic strains increase the eradication rate of H. pylori by 5–10% and found successful in reducing associated side-effects[Citation38,Citation39] such as Lactobacillus,[Citation40] Bifidobacterium,[Citation41] Lactoferrin.[Citation42,Citation43] While fermented milk[Citation44] shows significant decrease in eradication but no effect on side-effect reduction.[Citation45] The supplementation of probiotics with triple therapy helps reducing side-effects,[Citation46] and bovine lactoferrin improves eradication therapy.[Citation47] The use of Saccharomyces boulardii with triple therapy is recommended option for H. pylori extermination and reduction of side–effects.[Citation48]
Mechanism of action
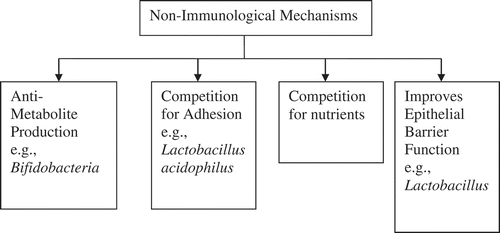
The use of probiotics as modification to conventional treatment strategy has solid biological and physiological background. It harmonizes the intestinal ecosystem mainly by invigorating immune and non-immune systems[Citation16,Citation49]: (i) invigorating non-immune system: It comprises of further different modes of action ().
Antimicrobial molecule and metabolite production
Probiotics are responsible for decrease of number of cells affecting metabolism or toxins produced by intestinal bacteria.[Citation26] LAB present in intestinal microflora produces volatile fatty acids which show antibacterial activity.[Citation50] Bifidobacteria is responsible for production of bacteriocin which has ability to kill infectious microorganisms, may be gram positive, gram negative, or yeast, etc.[Citation51]
Competitors for adhesion receptors
H. pylori adhesion with gut receptors by producing specific type of toxins like vacuolating toxin (VacA) and cytotoxin-associated antigen (CagA) so activates the pro-inflammatory response of cell resulting in cell death.[Citation52] H. pylori produces ammonia from urea in stomach thus withstanding gastric acidity. This leads to production of pro-inflammatory cytokines interlukin-8 (IL-8) attracting neutrophils toward inflammation site. Persistent stay of H. pylori leads to the development of severe conditions like peptic ulcer, chronic gastritis, etc.[Citation2,Citation53] Adhesion of non-pathogenic microorganisms like probiotics to the epithelial gut wall inhibits adhesion of pathogens such as H. pylori thus inhibiting their action in vitro.[Citation26,Citation31] Probiotics decreases the production of IL-8 cytokines thus suppressing the inflammation.[Citation2]
Competitors for nutrients
The intestine is the rich source of nutrients and contains microbial flora. It is also the competition site for the pathogens and probiotics.[Citation26] Biopsy studies of different patients revealed that Lactobacillus and H. pylori are the mutual resident of intestinal mucosa so evidence shows the competition for nutrients there.[Citation54,Citation55] The antibiotics disturbs intestinal flora therefore leads to super infection or sometimes recurrence of H. pylori infection and probiotics helps to balance this microflora.[Citation39]
Reinforcement of epithelial barrier function
Persistent exposure to H. pylori causes disruption of epithelial barrier function which if remained may lead to the progression of cancer. Probiotics improve this barrier function, thus treat or prevent cancer.[Citation52]
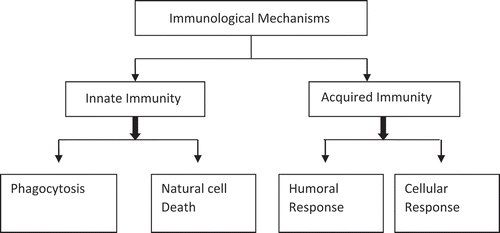
Invigorating immune system
The immune system is activated by two mechanisms: innate immunity, or acquired immunity. GIT has the ability to keep non-pathogenic organisms and eradicating the infectious one through active immune system[Citation1] (). H. pylori infected cells activate inflammatory mediators like cytokines, eicosanoids, and chemokines causing local inflammation and tissue damage.[Citation56] These mediators attract and activate neutrophils and monocytes/macrophages at the site of infection where probiotics inhibit PGE-2 release by H. pylori.[Citation52] So, when probiotics are orally administrated, the bacteria present in them activate macrophage activity thus activating inflammatory mediators.[Citation26] They also inhibit the activity of alpha tumour necrosis factor (TNF-a).[Citation57] Probiotics increases the IgA and immunoglobulin secreting cells.[Citation1,Citation35] This mechanism combats inflammation.[Citation31,Citation58] Activity of immune response depends largely on status of host defense.[Citation59]
Advantages
Clinical studies show improvement in clinical responses when probiotics are co-administered with triple therapy. Apparent decrease in GIT conditions related to other antibiotic therapy is also observed, like diarrhoea or loose bowels.[Citation60] Probiotics including B. infantis remarkably enhance the bowel activity. It also increases the regeneration of colonic mucosa mainly after the cessation of chemotherapeutic agent 5-flouro-uracil used for colonic carcinoma.[Citation16] Probiotics are used for reduction of cholesterol in body.[Citation61] It is used to treat constipation, heart disease, cancer, and ulcerative colitis.[Citation26] Remarkable decrease in the frequency of nausea, vomiting, and epigastric pain is observed.[Citation62] Significant decrease in antibiotic-associated diarrhoea (AAD) is noticed with probiotics.[Citation37,Citation63] It increases the gastrointestinal health condition along with decrease in inflammation associated with H. pylori and other pathogens.[Citation56] Lactobacillus johnsonii La1 (LC1) inhibits H. pylori motility.[Citation64]
Probiotics are also used for prophylaxis and treatment of various pathological conditions like acute infectious diarrhea,[Citation65] atopic disease,[Citation66] H. pylori infection,[Citation67] chronic inflammatory bowel disease,[Citation68] chronic ulcerative colitis,[Citation69,Citation70] Crohn’s disease,[Citation70,Citation71] inflammatory bowel syndrome, pouchitis,[Citation70] atopic dermatitis[Citation72] and constipation,[Citation73] cancer,[Citation33] and allergy.[Citation74] Probiotics are also used to ameliorate dental problems, prevent hepatic encephalopathy and urogenital conditions.[Citation75] They are used to cure infections related to respiratory tract and other infectious diseases.[Citation34] The are also used to relieve anxiety.[Citation76]
Discussion
The use of antibiotics as over-the-counter (OTC) medicine has made the microorganisms more resistant, and associated side-effects of antibiotics like GIT discomfort result in patient reluctance for completing the course of therapy. Gastrointestinal tract is full of microorganisms that may be pathogenic or non-pathogenic, where non-pathogens inhibit pathogenic activities and their growth. Helicobacter is extensively widely spread pathogen and causative agent of many malignancies of human body from gastritis to ulcerative colitis. The classical therapies to treat H. pylori infection has shown to be less effective with passage of time because of developing resistant strains and patient non-compliance toward therapy. Probiotics are the live microorganisms used as food supplements to maintain better health conditions. They are found to be effective in various pathological conditions of human body, mainly of GIT tract. They help to maintain balance in the normal microbial flora in stomach and gut by inhibiting H. pylori infection in various ways either by immunological or by non-immune system. Different strains of probiotics show different degrees of activity, so they can be administered as required such as Lactobacillus, Bifidobacteria. Probiotics are used to treat number of disorders like GIT infections, respiratory tract infections, cancer, allergies, etc.
Future prospects
The future consideration of probiotics require more work on their standardized use like selection of suitable strain, its beneficial dose, and required effects, etc. Effectiveness of different strains should be checked to select better strain for health benefits. Thorough study and data are also required to analyse the possible infections and side-effects caused by probiotics as they are live microorganisms. More extensive work is required on immunological parameters of probiotics so that their use to different immune system–related disorders such as allergy, asthma, etc., can also be considered.
Conclusion
Probiotics are being used to cure many disorders such as GIT infections, respiratory infections, allergies, cancer, etc. Different strains of probiotics are effective against different microorganisms like H. pylori either by interfering immune system or not. But the need of time is to identify standardized ways to use specific probiotic strains as therapeutic agents without causing side-effects.
Acknowledgments
We thank all the researchers who helped us to understand the use of probiotics. The authors have no conflict of interest.
References
- Saulnier, N.; Zocco, M.A.; Di Caro, S.; Gasbarrini, G.; Gasbarrini, A. Probiotics and Small Bowel Mucosa: Molecular Aspects of their Interactions. Genes Nutrition 2006, 1(2), 107–115.
- Rokka, S.; Myllykangas, S.; Joutsjoki, V. Effect of Specific Colostral Antibodies and Selected Lactobacilli on the Adhesion of Helicobacter pylori on AGS Cells and the Helicobacter-Induced IL-8 Production. Scandinavian Journal of Immunology 2008, 68(3), 280–286.
- Patel, A.; Shah, N.; Prajapati, J.B. Clinical Application of Probiotics in the Treatment of Helicobacter Pylori Infection—A Brief Review. Journal of Microbiology, Immunology and Infection 2014, 47(5), 429–437.
- Nista, E.C.; et al. Bacillus Clausii Therapy to Reduce Side-Effects of Anti-Helicobacter pylori Treatment: Randomized, Double-Blind, Placebo Controlled Trial. Alimentary Pharmacology and Therapeutics 2004, 20(10), 1181–1188.
- Ziemniak, W. Efficacy of Helicobacter pylori Eradication Taking into Account its Resistance to Antibiotics. Journal of Physiology and Pharmacology 2006, 57(Suppl 3), 123–141.
- Egan, B.J.; et al. Treatment of Helicobacter pylori. Helicobacter 2007, 12(Suppl 1), 31–37.
- Scaccianoce, G.; et al. Triple Therapies Plus Different Probiotics for Helicobacter pylori Eradication. European Review for Medical and Pharmacological Sciences 2008, 12(4), 251–256.
- Drouin, E. Helicobacter pylori: Novel Therapies. Candian Journal of Gastroenterology 1999, 13(7), 581–583.
- Efrati, C.; et al. Helicobacter pylori Eradication: Sequential Therapy and Lactobacillus reuteri Supplementation. World Journal of Gastroenterology: WJG 2012, 18(43), 6250–6254.
- Lionetti, E.; et al. Probiotics and Helicobacter pylori Infection in Children. Journal of Biological Regulators and Homeostatic Agents 2012, 26(1 Suppl), S69–S76.
- Roma, E.; Miele, E. Helicobacter pylori Infection in Pediatrics. Helicobacter 2015, 20(S1), 47–53.
- Malfertheiner, P.; et al. Current Concepts in the Management of Helicobacter pylori Infection: The Maastricht III Consensus Report. Gut 2007, 56(6), 772–781.
- Dore, M.P.; et al. Lactobacillus reuteri in the Treatment of Helicobacter pylori Infection. Internal and Emergency Medicine 2014, 9(6), 649–654.
- Ahmad, K.; et al. Probiotics for the Treatment of Pediatric helicobacter pylori Infection: A Randomized Double Blind Clinical Trial. Iranian Journal of Pediatrics 2013, 23(1), 79–84.
- O’Connor, A.; et al. Treatment of Helicobacter pylori Infection 2013. Helicobacter 2013, 18(Suppl 1), 58–65.
- Dajani, A.I.; et al. Do Probiotics Improve Eradication Response to Helicobacter pylori on Standard Triple or Sequential Therapy? Saudi Journal of Gastroenterology 2013, 19(3), 113–120.
- Hamilton-Miller, J.M. The Role of Probiotics in the Treatment and Prevention of Helicobacter pylori Infection. International Journal of Antimicrobial Agents 2003, 22(4), 360–366.
- Tursi, A.; et al. Effect of Lactobacillus Casei Supplementation on the Effectiveness and Tolerability of a New Second-Line 10-Day Quadruple Therapy after Failure of a First Attempt to Cure Helicobacter pylori Infection. Medical Science Monitor 2004, 10(12), CR662–CR666.
- Verdu, E.F.; et al. The Role of Luminal Factors in the Recovery of Gastric Function and Behavioral Changes after Chronic Helicobacter pylori Infection. American Journal of Physiology, Gastrointestinal and Liver Physiology 2008, 295(4), G664–G670.
- Zhang, M.-M.; et al. Probiotics in Helicobacter pylori Eradication Therapy: A Systematic Review and Meta-Analysis. World Journal of Gastroenterology: WJG 2015, 21(14), 4345.
- Mehling, H.; Busjahn, A. Non-Viable Lactobacillus Reuteri DSMZ 17648 (Pylopass) as a New Approach to Helicobacter Pylori Control in Humans. Nutrients 2013, 5(8), 3062–3073.
- Lesbros-Pantoflickova, D.; Corthesy-Theulaz, I.; Blum, A.L. Helicobacter pylori and Probiotics. Journal of Nutrition 2007, 137(3Suppl 2), 812S–818S.
- Blaser, M.J. Helicobacters are Indigenous to the Human Stomach: Duodenal Ulceration is Due to Changes in Gastric Microecology in the Modern Era. Gut 1998, 43(5), 721–727.
- Kim, J.M.; et al. Conjugated Linoleic Acids Produced by Lactobacillus Dissociates IKK-[Gamma] and Hsp90 Complex in Helicobacter pylori-Infected Gastric Epithelial Cells. Labortary Investigation 2008, 88(5), 541–552.
- Bercik, P.; et al. Immune-Mediated Neural Dysfunction in a Murine Model of Chronic Helicobacter pylori Infection. Gastroenterology 2002, 123(4), 1205–1215.
- Fuller, R. Probiotics in Human Medicine. Gut 1991, 32(4), 439–442.
- Hungin, A.P.; et al. Systematic Review: Probiotics in the Management of Lower Gastrointestinal Symptoms in Clinical Practice – An Evidence-Based International Guide. Alimentary Pharmacology and Therapeutics 2013, 38(8), 864–886.
- Hutt, P.; et al. Antagonistic Activity of Probiotic Lactobacilli and Bifidobacteria against Entero- and Uropathogens. Journal of Applied Microbiology 2006, 100(6), 1324–1332.
- Johnson-Henry, K.C.; et al. Probiotics Reduce Bacterial Colonization and Gastric Inflammation in H. pylori-Infected Mice. Digestive Diseases and Sciences 2004, 49(7–8), 1095–1102.
- Butel, M.-J. Probiotics, Gut Microbiota and Health. Médecine et maladies infectieuses 2014, 44(1), 1–8.
- Jarosz, M.; et al. Dietary and Socio-Economic Factors in Relation to Helicobacter pylori Re-Infection. World Journal of Gastroenterology: WJG 2009, 15(9), 1119–1125.
- Thomas, D.W.; et al. Probiotics and Prebiotics in Pediatrics. Pediatrics 2010, 126(6), 1217–1231.
- Burns, A.J.; Rowland, I.R. Anti-Carcinogenicity of Probiotics and Prebiotics. Current Issues of Intestinal Microbiology 2000, 1(1), 13–24.
- de Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics, and Synbiotics. Advancs in Biochemical Engoineering/Biotechnology 2008, 111, 1–66.
- Gupta, V.; Garg, R. Probiotics. Indian Journal of Medical Microbiology 2009, 27(3), 202–209.
- Caramia, G. Metchnikoff and the Centenary of Probiotics: An Update of their Use in Gastroenteric Pathology during the Age of Development. Minerva Pediatrica 2008, 60(6), 1417–1435.
- Videlock, E.J.; Cremonini, F. Meta-Analysis: Probiotics in Antibiotic-Associated Diarrhoea. Alimentary Pharmacology and Therapeutics 2012, 35(12), 1355–1369.
- Buzas, G.M. [Probiotics in Gastroenterology – From a Different Angle]. Orvosi Hetilap 2013, 154(8), 294–304.
- Zheng, X.; Lyu, L.; Mei, Z. Lactobacillus-Containing Probiotic Supplementation Increases Helicobacter pylori Eradication Rate: Evidence from a Meta-Analysis. Revista Espanola de Enfermdades Digestivas 2013, 105(8), 445–453.
- Tong, J.L.; et al. Meta-Analysis: The Effect of Supplementation with Probiotics on Eradication Rates and Adverse Events during Helicobacter pylori Eradication Therapy. Alimentary Pharmacology and Therapeutics 2007, 25(2), 155–168.
- Wang, Z.H.; Gao, Q.Y.; Fang, J.Y. Meta-Analysis of the Efficacy and Safety of Lactobacillus-Containing and Bifidobacterium-Containing Probiotic Compound Preparation in Helicobacter pylori Eradication Therapy. Journal of Clinical Gastroenterology 2013, 47(1), 25–32.
- Sachdeva, A.; Nagpal, J. Meta-Analysis: Efficacy of Bovine Lactoferrin in Helicobacter pylori Eradication. Alimentary Pharmacology and Therapeutics 2009, 29(7), 720–730.
- Zou, J.; Dong, J.; Yu, X.F. Meta-Analysis: The Effect of Supplementation with Lactoferrin on Eradication Rates and Adverse Events During Helicobacter pylori Eradication Therapy. Helicobacter 2009, 14(2), 119–127.
- Sachdeva, A.; Nagpal, J. Effect of Fermented Milk-Based Probiotic Preparations on Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. European Journal of Gastroenterology and Hepatology 2009, 21(1), 45–53.
- Molina-Infante, J.; Gisbert, J.P. Probiotics for Helicobacter pylori Eradication Therapy: Not Ready for Prime Time. Revista Espanola de Enfermdades Digestivas 2013, 105(8), 441–444.
- Li, S.; et al. Meta-analysis of Randomized Controlled Trials on the Efficacy of Probiotics in Helicobacter pylori Eradication Therapy in Children. European Journal of Pediatrics 2014, 173(2), 153–161.
- de Bortoli, N.; et al. Helicobacter pylori Eradication: A Randomized Prospective Study of Triple Therapy Versus Triple Therapy Plus Lactoferrin and Probiotics. The American Journal of Gastroenterology 2007, 102(5), 951–956.
- Szajewska, H.; Horvath, A.; Piwowarczyk, A. Meta-Analysis: The Effects of Saccharomyces boulardii Supplementation on Helicobacter pylori Eradication Rates and Side Effects During Treatment. Alimentary Pharmacology and Therapeutics 2010, 32(9), 1069–1079.
- Pacifico, L.; et al. Probiotics for the Treatment of Helicobacter pylori Infection in Children. World Journal of Gastroenterology: WJG 2014, 20(3), 673–683.
- Pongpech, P.; Hentges, D.J. Inhibition of Shigella sonnei and Enterotoxigenic Escherichia coli by Volatile Fatty Acids in Mice. Microbial Ecology in Health and Disease 1989, 2(3), 153–161.
- Collado, M.C.; Hernandez, M.; Sanz, Y. Production of Bacteriocin-Like Inhibitory Compounds by Human Fecal Bifidobacterium Strains. Journal of Food Protection 2005, 68(5), 1034–1040.
- Myllyluoma, E.; et al. Effects of Multispecies Probiotic Combination on helicobacter pylori Infection In Vitro. Clinical and Vaccine Immunology 2008, 15(9), 1472–1482.
- Rieder, G.; et al. Role of Adherence in Interleukin-8 Induction in Helicobacter pylori-Associated Gastritis. Infection and Immununity 1997, 65(9), 3622–3630.
- Garcia, C.A.; et al. [Probiotic Properties of Lactobacillus spp Isolated from Gastric Biopsies of Helicobacter pylori Infected and Non-Infected Individuals]. Revista Medica de Chile 2009, 137(3), 369–376.
- Mack, D.R.; et al. Probiotics Inhibit Enteropathogenic E. coli Adherence In Vitro by Inducing Intestinal Mucin Gene Expression. American Journal of Physiology 1999, 276(4 Pt 1), G941–G950.
- Yang, Y.J.; et al. Lactobacillus acidophilus Ameliorates H. pylori-Induced Gastric Inflammation by Inactivating the Smad7 and NFkappaB Pathways. BMC Microbiology 2012, 12, 38.
- Pena, J.A.; Versalovic, J.; Lactobacillus Rhamnosus GG Decreases TNF-Alpha Production in Lipopolysaccharide-Activated Murine Macrophages by a Contact-Independent Mechanism. Cell Microbiology 2003, 5(4), 277–285.
- Gill, H.S. Probiotics to Enhance Anti-Infective Defences in the Gastrointestinal Tract. Best Practice and Research Clinical Gastroenterolgy 2003, 17(5), 755–773.
- Haller, D.; et al. Non-Pathogenic Bacteria Elicit a Differential Cytokine Response by Intestinal Epithelial Cell/Leucocyte Co-Cultures. Gut 2000, 47(1), 79–87.
- Li, S.; et al. Meta-analysis of Randomized Controlled Trials on the Efficacy of Probiotics in Helicobacter pylori Eradication Therapy in Children. European Journal of Pediatrics 2014, 173(2), 153–161.
- Adams, C.A. The Probiotic Paradox: Live and Dead Cells are Biological Response Modifiers. Nutrition Research Reviews 2010, 23(1), 37–46.
- Tolone, S.; et al. Evaluation of Helicobacter Pylori Eradication in Pediatric Patients by Triple Therapy Plus Lactoferrin and Probiotics Compared to Triple Therapy Alone. Italian Journal of Pediatrics 2012, 38, 63.
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in Prevention of Antibiotic Associated Diarrhoea: Meta-Analysis. BMJ 2002, 324, 1361.
- Isobe, H.; et al. Reduction of Overall Helicobacter pylori Colonization Levels in the Stomach of Mongolian Gerbil by Lactobacillus Johnsonii La1 (LC1) and its In Vitro Activities against H. pylori Motility and Adherence. Bioscience Biotechnology Biochemistry 2012, 76(4), 850–852.
- Allen, S.J.; Okoko, B.; Martínez, E.; Gregorio, G.; Dans, L.F. Probiotics for Treating Infectious Diarrhoea (Cochrane Review). In: The Cochrane Library, issue 4, 2003 [online]. Available at http://www.update-software.com/abstracts/AB000333.htm.
- Kalliomaki, M.; et al. Probiotics and Prevention of Atopic Disease: 4-Year Follow-Up of a Randomised Placebo-Controlled Trial. Lancet 2003, 361(9372), 1869–1871.
- Sykora, J.; et al. Effects of a Specially Designed Fermented Milk Product Containing Probiotic Lactobacillus Casei DN-114 001 and the Eradication of H. pylori in Children: A Prospective Randomized Double-Blind Study. Journal of Clinical Gastroenterology 2005, 39(8), 692–698.
- Weng, M.; Walker, W.A. Bacterial Colonization, Probiotics, and Clinical Disease. The Journal of Pediatrics 2006, 149(5), S107–S114.
- Mallon, P.; McKay, D.; Kirk, S.; Gardiner K. Probiotics for Induction of Remission in Ulcerative Colitis. Cochrane Database Systematic Reviews 2007, 17(4), CD005573.
- Shen, J.; Zuo, Z.X.; Mao, A.P. Effect of Probiotics on Inducing Remission and Maintaining Therapy in Ulcerative Colitis, Crohn’s Disease, and Pouchitis: Meta-Analysis of Randomized Controlled Trials. Inflammatory Bowel Diseases 2014, 20(1), 21–35.
- Schultz, M.; et al. Lactobacillus GG in Inducing and Maintaining Remission of Crohn’s Disease. BMC Gastroenterology 2004, 4, 5.
- Kim, H.J.; et al. Clinical Efficacy and Mechanism of Probiotics in Allergic Diseases. Korean Journal of Pediatrics 2013, 56(9), 369–376.
- O’Mahony, L.; et al. Lactobacillus and Bifidobacterium in Irritable Bowel Syndrome: Symptom Responses and Relationship to Cytokine Profiles. Gastroenterology 2005, 128(3), 541–551.
- van der Aa, L.B.; et al. Probiotics and Prebiotics in Atopic Dermatitis: Review of the Theoretical Background and Clinical Evidence. Pediatric Allergy and Immunology 2010, 21(2 Pt 2), e355–e367.
- Narayan, S.S.; et al. Probiotics: Current Trends in the Treatment of Diarrhoea. Hong Kong Medical Journal 2010, 16(3), 213–218.
- Kim, J.Y.; et al. Lipoteichoic Acid Isolated from Lactobacillus plantarum Suppresses LPS-Mediated Atherosclerotic Plaque Inflammation. Molecular Cells 2013, 35(2), 115–124.