ABSTRACT
Recombined dairy cream, which primarily comprises anhydrous milk fat and milk protein, has significant advantages compared to natural cream; however, its most notable disadvantage is poor stability. The objective of this study was to investigate the effects of lecithin on the stability and physical properties of recombined dairy cream (20% fat, and 1.5% protein) in terms of the creaming rate, mean oil droplet size and distribution, surface protein concentration, ζ-potential, and apparent viscosity. The results clearly showed that lecithin can significantly improve the stability of recombined dairy cream by decreasing the creaming rate, especially at a concentration of 0.6% (w/w). Increasing the lecithin concentration decreased the mean oil droplet size and the surface protein concentration but slightly increased the ζ-potential. The apparent viscosity decreased and surprisingly increased at 0.6% (w/w). We can infer that lecithin initially displaces proteins from the oil surface and may interact with both proteins and polysaccharides, forming a much more stable structure.
Introduction
Native dairy cream is traditionally considered a luxury product, particularly in areas where milk sources are scarce; therefore, the application and popularization of cream is restricted to certain regions. Recombined dairy cream primarily comprises anhydrous milk fat (AMF) and milk protein powder. Both of materials can be conveniently stored, transported, and developed into food products. Its convenience lies in the fact that the composition and desired properties of recombined dairy cream can be standardized and adapted independent of the milking season.[Citation1,Citation2] As a result, recombined dairy cream is becoming a common alternative to natural dairy cream.[Citation3]
Both native dairy cream and recombined dairy cream are types of food emulsions and are, thus, thermodynamically unstable systems in which various destabilization phenomena (creaming, aggregation, coalescence, partial coalescence, and Ostwald ripening) occur during production or storage.[Citation4,Citation5] The fat globule membrane is important for the stability of cream. Native fat globule membranes are composed of phospholipids and proteins[Citation6–Citation8] that act as natural emulsifying agents, prevent the flocculation and coalescence of fat globules in milk, and protect the fat against enzyme action.[Citation9] In contrast, AMF, which is one of the most important components in recombined cream, lacks membranes because they were broken down and removed during the production process.[Citation10] To acquire stable recombined dairy cream, a suitable thermodynamic system must be rebuilt during the production process. Researchers have developed various methods of ensuring that recombined dairy cream is thermodynamically stable and has a prolonged shelf life, typically by utilizing proteins, emulsifiers, and thickening materials.
Lecithin is a small molecule surfactant that is one of the most commonly used emulsifiers in the food industry.[Citation11,Citation12] It is widely accepted as a natural ingredient by consumers, and legislators classify it as generally recognized as safe.[Citation13] Lecithin is composed of many different phospholipids, and the two most abundant phospholipid species are phosphatidylcholine and phosphatidylethanolamine.[Citation14] Lecithin has been proven to be useful for enhancing the heat stability of emulsions,[Citation15–Citation17] which may be explained by the interactions between lecithin and proteins. Dickinson studied the effect of lecithin on the viscoelastic properties of β-lactoglobulin-stabilized emulsions and determined that the addition of lecithin after emulsification increases the mechanical strength of the emulsion with no significant protein displacement from the oil–water interface.[Citation18] Seamus compared the effect of lecithin and monoglycerides on the heat stability of emulsions and postulated that lecithin increases heat stability by forming protein–phospholipid complexes.[Citation19]
The primary goal of this study was to investigate the effects of soybean lecithin on the stability and physical properties of recombined cream, including the creaming stability, mean oil droplet size and distribution, surface protein concentration, ζ-potential, and apparent viscosity. The relationship between the stability and characteristics of recombined dairy cream was also examined.
Materials and methods
Materials
AMF was purchased from Fonterra Cooperative Group (Auckland, New Zealand). Milk protein powder (MPC which contains 72.1% [w/w] protein, 1.4% [w/w] fat, 16.9% [w/w] lactose, 7.46% [w/w] ash) and microcrystalline cellulose (MCC) were kindly donated by Cezanne Co., Ltd. (Ningxia, China). Soybean lecithin was purchased from Cargill Co. (Beijing, China).
Preparation of recombined cream
The recombined cream consisted of 20% (w/w) AMF, 1.5% (w/w) MPC, 0.25% (w/w) MCC, and various concentrations of soybean lecithin (0, 0.20, 0.40, 0.60, 0.80, and 1.0% w/w). The MPC (1.5% w/w) was dissolved in deionized water and stirred with an overhead stirrer (RW 20.n, IKA Co., Ltd., Guangzhou, China) at 600 rpm and 50°C for 1 h to complete the hydration process. The MCC (0.25%) was dissolved in 70°C deionized water and then thoroughly dispersed with a XHF-D homogenizer (Ningbo Scientz Biotechnology Co. Ltd., Ning Bo, China) at 8000 rpm for 4 min. The AMF was heated to 80°C to ensure complete melting and to dissolve the soybean lecithin in the liquid AMF. Next, the oil phase, water phase, and MCC solution were mixed with a stirrer at 800 rpm and 50°C for 15 min. The emulsion was immediately homogenized via a two-step, laboratory-scale, high-pressure homogenizer (Shanghai Shen Lu homogenizer Co. Ltd., Shanghai, China) at 15 MPa during the first stage and 4 MPa during the second stage. After homogenization, the mixtures were sealed in bottles, sterilized at 115°C for 15 min, and cooled to room temperature in a water bath. The samples were subjected to further measurements and analyses after being stored at room temperature for 1 day.
Determination of creaming stability
The creaming stability of the emulsions was represented by creaming rate which was measured using a LUMiSizer (LUM LUMGmbH, Germany) (Executed standards: ISO TR13097). The multisample analytical centrifuge used in this study employs STEP technology, which allows the measurement of the intensity of transmitted near-infrared (NIR) light as function of time and position over the entire sample length without scanning. The measurement scheme was described by Sobisch and Lerche.[Citation20] The following instrument parameters were used for the measurements: (1) 1500 rpm, (2) 3600 s timeExp, (3) 10 s time interval, (4) 25°C temperature, and (5) a light factor of 1.00.
Determination of oil droplet size and distribution
A Malvern MasterSizer 3000 (Malvern Instruments Co., Ltd., Worcestershire, UK) (Executed standards: ISO 13320) was used to determine the droplet size distribution and average diameter of the emulsion droplets. We used the method described by Moreno[Citation21] with slight modifications. Each sample was measured in triplicate. The refractive index, dispersed phase adsorption, and continuous phase refractive index were set to 1.52, 0.1, and 1.33, respectively. The emulsion was diluted in recirculating water until 10–20% obscuration was reached. The mean oil droplet size D3,2 (μm) was calculated using the following equation:
where ni is the number of particles with the same diameter, and di is the particle size.
Determination of surface protein concentration
The surface protein concentration of the emulsion samples was measured using previously described methods[Citation3,Citation22] with some modifications. Each emulsion was centrifuged at 6560g for 30 min at 4°C in a temperature-controlled centrifuge; then, the subnatant was carefully removed with a syringe and separated by filtration. The protein concentration of the subnatant was then measured using the Kjeldahl nitrogen procedure. The specific surface area (SSA; m2 g−1) was obtained from the particle size distribution as measured with a MasterSizer 3000, and the surface protein concentration (Γ, mg m−2) was calculated with the following equation:
where P0 is the total protein in the emulsion (g), Ps is the amount of protein measured in the subnatant (g), and MC is the weight of the creaming layer (g).
Determination of ζ-potential
The ζ-potential of the recombined dairy cream solutions was measured by laser Doppler velocimetry and dynamic light-scattering techniques with a Malvern Zetasizer Nano-ZS90 instrument (Malvern Instruments, Worcestershire, UK) (Executed standard: ISO 13321) at 25°C. The solution samples were diluted with deionized water at a ratio of 1:1000, and then, 1 mL of each diluted sample was placed in a visibly clear disposable zeta cell (Model DTS 1060C, Malvern Instruments Co., Ltd., Worcestershire, UK) without air bubbles. The equilibrium time was 180 s before the particle charge data were collected over 20 continuous readings. Measurements were performed in triplicate.
Determination of apparent viscosity
The apparent viscosity of the emulsion samples was determined using a Physica MCR 301 rheometer (Anton Paar Co., Ltd., Graz, Austria) with a CC27 rotor. The temperature was controlled at 25°C using a Peltier System Viscotherm VT2 (Paar Physica), and the rheological measurement data were analyzed with RheoPlus software (Anton Paar Co., Ltd., Graz, Austria). The dependence of the apparent viscosity on the shear rate was observed in controlled rate mode. The shear rate increased linearly from 0 to 200 s−1 over 420 s, and 60 points were obtained.
Statistical analysis
All of the measurements were conducted at least in duplicate, and the entire study was performed in two replicates. Statistical analysis (one-way analysis of variance [ANOVA]) was conducted using IBM SPSS 21 (IBM SPSS Inc., Chicago, IL, USA). The difference between the means was determined by Bonferroni’s test and significance was defined at P < 0.05.
Results and discussion
Creaming stability
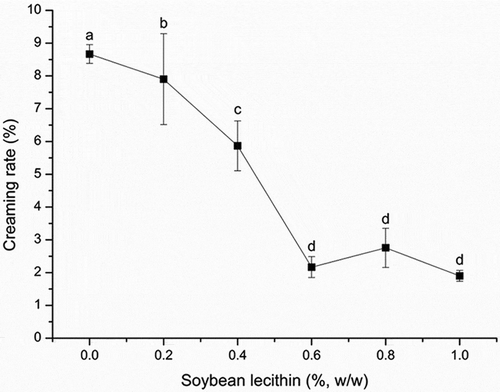
Creaming is a function of oil-in-water instabilities, which originate from the respective densities of the two liquids in the emulsion and gravitational force.[Citation23] Measuring the amount of cream separated during a certain period of time is a useful way to assess the emulsion’s thermodynamic stability. Creaming rate was evaluated by NIR transmittance of samples. A high creaming rate means a high transmittance, which means that more creamed layer is formed after the centrifugation and indicates that the recombined cream has poor stability. The results showed that when the concentration of soybean lecithin increased from 0% to 0.6% (w/w), the creaming rate decreased from 8.6 ± 0.3% to 2.2 ± 0.3% (). Therefore, lecithin improved the stability of the recombined cream, especially at a concentration of 0.6% (w/w). As the concentration was increased further, the creaming rate remained stable. A slight increase in the creaming rate was noted at a concentration of 0.8%, but this difference was not statistically significant. Therefore, once the lecithin concentration exceeded 0.6%, it did not further improve the stability. To explain why lecithin increases the stability of recombined cream and determine the mechanism in recombined dairy cream, we analyzed its physical properties using additional indicators.
Oil droplet size and distribution
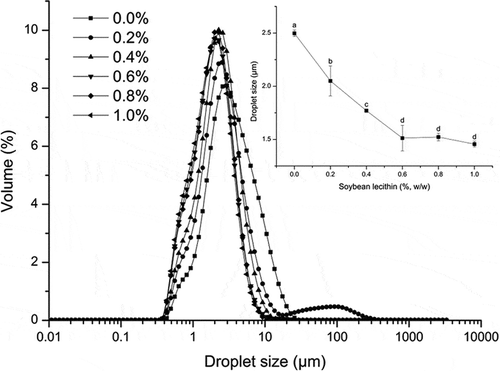
Small droplets are beneficial for emulsion stability because they exhibit greater Brownian movement against gravity.[Citation24] Moreover, as reported by Truong,[Citation25] the droplet size influences the crystallization of milk fat. The effect of crystallization on emulsion stability was confirmed by Rousseau;[Citation26] briefly, smaller crystals provided better protection to droplets than larger crystals and thus contribute to emulsion stability. The influences of soybean lecithin on the mean oil droplet size and distribution of the recombined cream are depicted in . The droplet size distribution at each concentration had a single peak in between 1 and 10 μm. The holistic peak shifted left and exhibited a narrower distribution as the concentration increased from 0% to 0.6% (w/w), whereas the average diameter decreased significantly (P < 0.05) from 2.50 ± 0.03 to 1.51 ± 0.12 μm. Lecithin, as discussed above, comprises phospholipids; the phospholipid head is attracted to water, whereas the tail, which consists of a long hydrocarbon chain, is repelled by water. These species can decrease the surface tension because of their amphiphilic nature.[Citation27] In tandem with homogenization, transferring kinetic energy into cream generates abundant smaller droplets and a larger surface area in the emulsion.[Citation28,Citation29] However, when the lecithin concentration was 0.6–1.0%, the distribution curves almost overlapped, and the mean oil droplet size did not differ (P > 0.05). Thus, it is likely that the lecithin reached the critical micelle concentration (CMC) at that point and could no longer decrease the interfacial tension.[Citation30]
Figure 2. Effect of soybean lecithin on the mean droplet size distribution of recombined dairy cream. The line-map in the top-right corner is the mean oil droplet size. Data are expressed as the mean ± standard deviations. Different superscript letters over the data points represent significant differences (P < 0.05) among the various lecithin levels.
Surface protein concentration
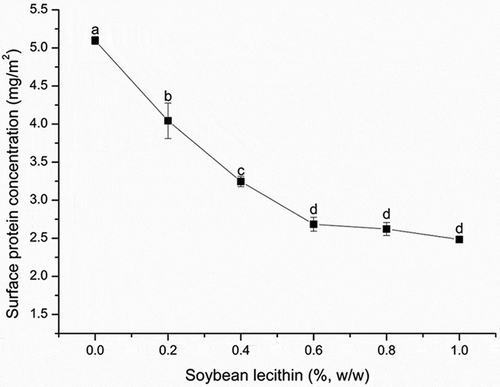
The surface protein concentration substantially affects the stability of cream because proteins absorb onto the surfaces of oil droplets, thereby forming an interfacial layer that prevents droplet coalescence.[Citation31,Citation32] We measured the surface protein concentration to investigate the interactions between lecithin and milk proteins (). The amount of surface protein gradually and significantly (P < 0.05) decreased from 5.10 ± 0.05 to 2.68 ± 0.09 mg m−2 until the concentration of lecithin reached 0.6%, suggesting that some portion of the protein at the interface was substituted by phospholipids. This result differs from that reported by Dickinson[Citation18] but is in agreement with the findings of Yuan and Moreno.[Citation21,Citation33] Paradoxically, the surface protein concentration and creaming rate decreased at the same time in this study. This means although more proteins disassociated from the oil droplets, the recombined cream remained stable. This is different from ancient researches. Actually, proteins are usually either bound at the interfacial layer of the globules or located unbound in the serum phase. The interactions between lecithin and serum protein are often ignored, but they have great significance, especially in recombined dairy cream, and may contribute to its stabilization. Combined with the result of ζ-potential and apparent viscosity, we would mention the interactions in section “ζ-potential” and “Apparent viscosity”. Here, lecithin concentrations of 0.6%, 0.8%, and 1.0% did not result in significant changes, which indicated that the competitive adsorption was complete at 0.6% lecithin. The protein remained at the interface was likely to be β-lactoglobulin, since β-lactoglobulin is able to form intermolecular disulphide bonds at the interface[Citation34,Citation35] and has a combination with phosphatidylcholine[Citation36] which makes it difficult to be displaced in the surface of oil. Comparing and clearly shows that the mean oil droplet size and surface protein concentration had similar tendencies, with both exhibiting a threshold at a lecithin concentration of 0.6%. We can infer that 0.6% lecithin is the critical point at which the system approached equilibrium with respect to the mean oil droplet size and surface protein concentration.
ζ-Potential
The influence of soybean lecithin concentration on the ζ-potential of recombined cream is shown in . The oil droplets became increasingly positive as the lecithin concentration increased. Specifically, as the lecithin concentration increased from 0% to 0.4%, the ζ-potential increased from −34.38 ± 1.13 to −31.57 ± 0.90 mV, and this change became significant (P < 0.05) at a concentration of 0.4% (compared to the value at 0%). Above 0.4%, the ζ-potential fluctuated slightly but did not change significantly throughout the rest of the experiment (P > 0.05).
Figure 4. Effect of the soybean lecithin concentration on the ζ-potential of recombined dairy cream. The data are expressed as the mean ± standard deviations. Different superscript letters over the data points indicate significant differences (P < 0.05) among the various lecithin levels.
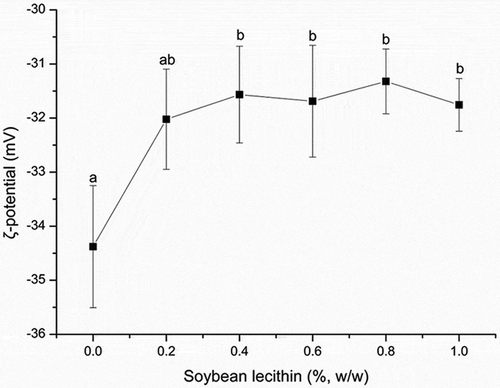
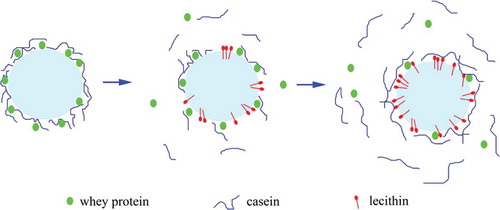
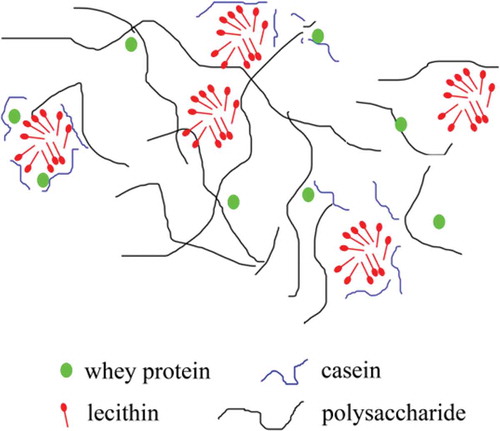
MPC is composed of casein and whey protein, has an isoelectric point of approximately pH 4.6, and is negatively charged below the natural pH of recombined cream (pH 7.0, data not shown).[Citation37] Similarly, in mixtures of phospholipid and phosphatidylinositol, lecithin is also negatively charged at pH 7.0.[Citation38] Electrostatic repulsion forces between droplets have been shown to enhance the stabilization effect;[Citation39] however, this was not the primary stability mechanism of lecithin observed here. The potential did not change when the lecithin concentration increased from 0.2% to 1.0% (P > 0.05). Same phenomenon observed in Mcsweeney’s literature.[Citation40] It may be explained by that the increase in the net negative charge of the emulsion droplets due to protein displacement is likely to be offset by lecithin.[Citation40] However, casein particles are preferentially absorbed into the interface of oil droplets and have a high competitive displacement by surfactant molecules.[Citation41,Citation42] Especially, β-casein is more readily to be displaced by emulsifier.[Citation43] So, when the lecithin concentration was 0.2% (w/w), β-casein was possibly preferentially displaced in a large amount resulting in a slight increase of ζ-potential. This is also evidenced by a sharp decrease of surface protein concentration at 0.2% lecithin (). Here, in order to explain the phenomenon, we also propose another new hypothesis. In Mackie’s literature,[Citation44] proteins interact and combine with surfactant whatever in interface or serum phase. As the concentration of lecithin increased, the proteins were displaced and transferred into the serum phase, thereby increasing the serum protein concentration. As a result, more proteins will have a tendency to return to the oil droplets to combine with lecithin by hydrophilic groups (), thus forming an electrostatic shield which made ζ-potential remain constant. But this kind of combination may not be strong and is breakable, which cannot be observed in this study. Further study using undamaged method to determine surface protein concentration without centrifugation is needed.
Apparent viscosity
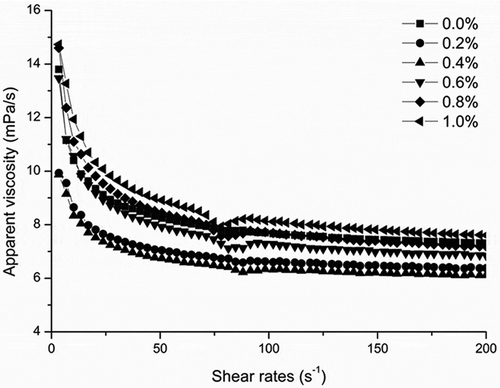
The steady shear rheological behavior of the creams was also determined. All of the samples displayed shear-thinning behaviors (), which can be explained by the utilization of MCC, a polysaccharide that can absorb water to form a gel solution with a space grid structure.[Citation45] This grid structure was generally broken at elevated shear rates, resulting in shear-thinning behaviors. Adding lecithin reduced the interfacial tension and enhanced the breakage of oil droplets such that the droplets became smaller and more uniform, thereby decreasing the overall viscosity of the cream at lower lecithin concentrations. In accordance with these expectations, the viscosity of the cream decreased as the lecithin concentration increased (from 0% to 0.4%) in the initial phase. However, as the concentration increased from 0.4% to 1.0%, the viscosity increased abnormally. This behavior was unexpected and has not been reported before. We suggest that lecithin micelles, which have hydrophilic groups on the outside and hydrophobic groups on the inside, formed when the lecithin concentration increased to 0.6% (see the discussion in section ‘Oil droplet size and distribution’) and that these micelles might connect the proteins and polysaccharides in a continuous phase[Citation19,Citation46–49] (). This complicated polymer could reinforce the stable structure of the emulsion and, thus, increase the viscosity of the cream.
Conclusion
The results of this study indicated that soybean lecithin positively affects the stability of recombined dairy cream, especially at a concentration of 0.6%. We found that soybean lecithin has favorable emulsification abilities and can significantly reduce the droplet sizes of recombined cream at optimal concentrations. Lecithin displaced interfacial proteins and slightly increased the ζ-potential through interfacial activities. However, ζ-potential remained constant when lecithin concentration increased from 0.2% to 1.0% which may be the result of charge offset by lecithin. We also hypothesize that the displacement is followed by protein recovery as the serum protein concentration increases. The viscosity of the cream decreased as the lecithin concentration increased (from 0% to 0.4%) because of the lecithin-induced decrease in interfacial tension. However, as the concentration increased from 0.4% to 1.0%, the viscosity increased abnormally. This behavior could be explained by the interactions between the proteins, lecithin micelles, and polysaccharides that resulted in a much more stable network structure. However, these interactions must be confirmed by additional studies. Here, we focused exclusively on the effects of lecithin on recombined cream over a short-time period. Meaningful and time-consuming storage experiments should be performed in the future.
Fundings
This work was supported by the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-37) and the Planning Subject of the Twelfth Five-Year-Plan from the National Science and Technology for Rural Development in China (Grant numbers 2013BAD18B05-02 and 2013BAD18B12-05, respectively).
Additional information
Funding
References
- Fredrick, E.; Moens, K.; Heyman, B.; Fischer, S.; Van der Meeren, P.; Dewettinck, K. Monoacylglycerols in Dairy Recombined Cream: I. The Effect on Milk Fat Crystallization. Food Research International 2013, 51(2), 892–898.
- van Lent, K.; Le, C.T.; Vanlerberghe, B.; Van der Meeren, P. Effect of Formulation on the Emulsion and Whipping Properties of Recombined Dairy Cream. International Dairy Journal 2008, 18(10), 1003–1010.
- Wu, S.; Wang, G.; Lu, Z.; Li, Y.; Zhou, X.; Chen, L.; Zhang, L. Effects of Glycerol Monostearate and Tween 80 on the Physical Properties and Stability of Recombined Low-Fat Dairy Cream. Dairy Science & Technology 2016, 96(3), 377–390.
- Fredrick, E.; Walstra, P.; Dewettinck, K. Factors Governing Partial Coalescence in Oil-in-Water Emulsions. Advances in Colloid & Interface Science 2010, 153(1), 30–42.
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence Stability of Emulsions Containing Globular Milk Proteins. Advances in Colloid & Interface Science 2006, 123–126(21), 259–293.
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Characterization of Protein Components of Natural and Heat-Treated Milk Fat Globule Membranes. International Dairy Journal 2002, 12(4), 393–402.
- Keenan, T.W.; Mather, I.H. Intracellular Origin of Milk Fat Globules and the Nature of the Milk Fat Globule Membrane; Springer: New York, US, 2006.
- Rombaut, R.; Camo, J.V.; Dewettinck, K. Analysis of Phospho- and Sphingolipids in Dairy Products by a New HPLC Method. Journal of Dairy Science 2005, 88(2), 482–488.
- Singh, H. The Milk fat Globule Membrane—A Biophysical System for Food Applications. Current Opinion in Colloid & Interface Science 2006, 11(s 2–3), 154–163.
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, 2003.
- Szuhaj, B.F. Lecithins: Sources, Manufacture & Uses; American Oil Chemists’ Society: Urbana, US, 1989.
- Whitehurst, R.J. Emulsifiers in Food Technology; John Wiley & Sons: Oxford, UK, 2008.
- Bylaite, E.; Nylander, T.; Venskutonis, R.; Jönsson, B. Emulsification of Caraway Essential Oil in Water by Lecithin and β-Lactoglobulin: Emulsion Stability and Properties of the Formed Oil–Aqueous Interface. Colloids & Surfaces B Biointerfaces 2001, 20(4), 327–340.
- Yamamoto, Y.; Araki, M. Effects of Lecithin Addition in Oil or Water Phase on the Stability of Emulsions Made with Whey Proteins. Bioscience Biotechnology & Biochemistry 1997, 61(11), 1791–1795.
- Mccrae, C.H.; Muir, D.D. Heat Stability of Recombined Milk: Influence of Lecithins on the heat Coagulation Time-pH Profile. Journal of Dairy Research 1992, 59(2), 177–185.
- Hardy, E.E.; Sweetsur, A.W.; West, I.G.; Muir, D.D. Heat Stability of Concentrated Milk: Enhancement of Initial Heat Stability by Incorporation of Food Grade Lecithin. International Journal of Food Science & Technology 1985, 20(1), 97–105.
- Singh, H.; Sharma, R.; Tokley, R.P. Influence of Incorporation of Soya Lecithin into Skim Milk Powder on the Heat Stability of Recombined Evaporated Milk. Australian Journal of Dairy Technology 1992, 47(1), 33.
- Dickinson E, Yamamoto Y. Effect of Lecithin on the Viscoelastic Properties of β-Lactoglobulin-Stabilized Emulsion Gels. Food Hydrocolloids 1996, 10(3), 301–307.
- Mcsweeney, S.L.; Healy, R.; Mulvihill, D.M. Effect of Lecithin and Monoglycerides on the Heat Stability of a Model Infant Formula Emulsion. Food Hydrocolloids 2008, 22(5), 888–898.
- Sobisch, T.; Lerche, D. Thickener Performance Traced by Multisample Analytical Centrifugation. Colloids & Surfaces A Physicochemical & Engineering Aspects 2008, 331(1–2), 114–118.
- Garca-Moreno, P.J.; Anna, F.H.; Jacobsen, C. Influence of Casein-Phospholipid Combinations as Emulsifier on the Physical and Oxidative Stability of Fish Oil-in-Water Emulsions. Journal of Agricultural & Food Chemistry 2014, 62(5), 1142–1152.
- Ye, A.; Srinivasan, M.; Singh, H. Influence of NaCl Addition on the Properties of Emulsions Formed with Commercial Calcium Caseinate. Food Chemistry 2000, 69(3), 237–244.
- Tadros, T. Application of Rheology for Assessment and Prediction of the Long-Term Physical Stability of Emulsions. Advances in Colloid and Interface Science 2004, 108, 227–258.
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Critical Reviews in Food Science & Nutrition 2011, 51(4), 285–330.
- Truong, T.; Bansal, N.; Sharma, R.; Palmer, M.; Bhandari, B. Effects of Emulsion Droplet Sizes on the Crystallisation of Milk Fat. Urology 2014, 66(66), 397–401.
- Rousseau, D. Fat Crystals and Emulsion Stability—A Review. Food Research International 2000, 33(33), 3–14.
- Wiacek, A.E.; Adryanczyk, E. Interfacial Properties of Phosphatidylcholine-based Dispersed Systems. Industrial & Engineering Chemistry Research 2015, 54(25), 6489–6496.
- Elwell, M.W.; Roberts, R.F.; Coupland, J.N. Effect of Homogenization and Surfactant Type on the Exchange of Oil between Emulsion Droplets. Food Hydrocolloids 2004, 18(3), 413–418.
- Mortimer, R.G. Physical Chemistry; Elsevier LTD: Oxford, 2008.
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; Wiley: Hoboken, US, 2012.
- Sherman, P. Emulsion Science; Academic Press: New York, US, 1968.
- Van Aken, G.A.; Zoet, F.D. Coalescence in Highly Concentrated Coarse Emulsions. Langmuir 2000, 16(18), 7131–7138.
- Yuan, F.; Dalgleish, D.G. Casein Adsorption on the Surfaces of Oil-in-Water Emulsions Modified by Lecithin. Colloids & Surfaces B Biointerfaces 1993, 1(6), 357–364.
- Dickinson,E.; Rolfe, S.E.; Dalgleish, D.G. Competitive Adsorption in Oil-in-Water Emulsions Containing α-Lactalbumin and β-Lactoglobulin. Food Hydrocolloids 1989, 3(3), 193–203.
- Murray, B.S.; Cros, L. Adsorption of β-Lactoglobulin and β-Casein to Metal Surfaces and their Removal by a Non-Ionic Surfactant, as Monitored via a Quartz Crystal Microbalance. Colloids & Surfaces B Biointerfaces 1998, 10(4), 227–241.
- Brown, E.M.; Carroll, R.J.; Pfeffer, P.E. Complex Formation in Sonicated Mixtures of β-Lactoglobulin and Phosphatidylcholine. Lipids 1983, 18(2), 111–118.
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. Journal of Agricultural & Food Chemistry 2003, 51(6), 1696–1700.
- Wang, G.; Wang, T. Oxidative Stability of Egg and Soy Lecithin as Affected by Transition Metal Ions and pH in Emulsion. Journal of Agricultural & Food Chemistry 2008, 56(23), 11424–11431.
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of Emulsions Containing Sodium Caseinate and Dextran Sulfate: Relationship to Complexation in Solution. Food Hydrocolloids 2008, 22(4), 647–659.
- Mcsweeney, S.L.; Healy, R.; Mulvihill, D.M. Effect of Lecithin And Monoglycerides on the Heat Stability of a Model Infant Formula Emulsion. Food Hydrocolloids 2008, 22(5), 888–898.
- Goff, H.D.; Kinsella, J.E.; Jordan, W.K. Influence of Various Milk Protein Isolates on Ice Cream Emulsion Stability. Journal of Dairy Science 1989, 72(2), 385–397.
- Britten, M.; Giroux, H.J. Emulsifying Properties of Whey Protein and Casein Composite Blends. Journal of Dairy Science 1991, 74(10), 3318–3325.
- Cornec, M.; Wilde, P.J.; Gunning, P.A.; Mackie, A.R.; Husband, F.A.; Parker, M.L.; Clark, D.C. Emulsion Stability as Affected by Competitive Adsorption between an Oil-Soluble Emulsifier and Milk Proteins at the Interface. Journal of Food Science 1997, 63(1), 39–43.
- Mackie, A.; Wilde, P. The Role of Interactions in Defining the Structure of Mixed Protein–Surfactant Interfaces. Advances in Colloid & Interface Science 2006, 117(1), 3–13.
- Chen, J.; Yan, S.; Hu, J.; Yuan, J. A Study on the Properties of Microcrystalline Cellulose Gel. Journal of Cellulose Science & Technology 1994, 2, 1–6.
- Kristensen, A.; Nylander, T.; Paulsson, M.; Carlsson, A. Calorimetric Studies of Interactions between β-Lactoglobulin and Phospholipids in Solutions. International Dairy Journal 1997, 7(1), 87–92.
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of Milk Protein-Polysaccharide Interactions on the Stability Of Ice Cream Mix Model Systems. Food Hydrocolloids 2015, 45(45), 327–336.
- Perez, A.A.; Carrara, C.R.; Sanchez, C.C.; Patino, J.M.R.; Santiago, L.G. Interactions between Milk Whey Protein and Polysaccharide in Solution. Food Chemistry 2009, 116(1), 104–113.
- Otzen, D. Protein-Surfactant Interactions: A Tale of Many States. Biochimica et Biophysica Acta 2011, 1814(5), 562–591.