ABSTRACT
The volatile constituents of cocoa liquor and the differences between cocoa liquors from different origins were studied. Direct solvent extraction-solvent assisted flavor evaporation and gas chromatography-olfactrometry-mass spectrometry in conjunction with aroma extract dilution analysis were used to identify the key flavor compounds in five cocoa liquors. There were significant differences of specific compounds between cocoa liquor from different areas of origins. Then, the purge and trap method was used to gathering the aroma-active components of five cocoa liquors from different origins, and one internal standard was used during this process for the further quantitative analysis by gas chromatography-olfactrometry-mass spectrometry. The results indicated that 3-methylbutanal, acetic acid, tetramethylpyrazine, and 3-methylbutanoic acid were the components with high concentrations. The contents of most compounds in the five kinds of cocoa liquor were dramatically different. The content of odorants of cocoa liquors from Papua New Guinea was higher than that of the others and that from Indonesia was the lowest. For sensory evaluation, the overall odor outlines of the five cocoa liquors were very similar, the Papua New Guinea cocoa liquor had higher preference than those of the other four samples. Principal component analysis showed that the characteristics of cocoa liquor from Papua New Guinea, Indonesia, and Ivory Coast were very significant.
Introduction
Chocolate is a kind of food prepared by specified technologies, with cocoa products (cocoa butter, cocoa liquor, or cocoa powder), sugar as main raw materials, adding or not adding dairy products, and food additives. Chocolate is popular and attracts most consumers by its delicate taste and special aroma. Cocoa liquor is a kind of important cocoa product. It is prepared by cocoa beans being ground, fermented, and roasted with cocoa butter. The aroma of cocoa liquor is one of the most important characteristics to impact its quality. Many factors affect the aroma of cocoa liquor, such as cocoa bean origin, postharvest processing (fermentation and drying), roasting, and storage.[Citation1–Citation3] Among them, the cocoa bean origin is the most important factor determining the aroma of all cocoa products, and it is related to both the genetic and environmental factors.
The main cocoa-producing area in the world is West Africa and South America, including Ghana, Nigeria and Cameroon (CM), Brazil, and Ecuador. Asian cocoa production has increased in recent years, the largest output is from Malaysia and Indonesia (ID). Because of the different environmental factors, cocoa liquors from various geographic regions exhibit different aromatic properties. The different producing areas and processing methods impact the composition of cocoa beans, which lead to different aroma and taste properties.[Citation4] Oceanian country such as Papua New Guinea (PNG) produces excellent cacao beans with unique aromas of red wine and brandy. As each cacao bean has a different quality, in order to manufacture chocolate of higher standards and better selling potential, cacao material of better flavor quality should be chosen. Five distinguished cacao liquors are imported to be used as raw material for producing chocolate of high quality-Le conte by China Oil and Foodstuffs Corporation (COFCO), it is essential to investigate the aroma-active compounds of these five cacao liquors.
The aroma constituents of cocoa liquor and chocolate have been investigated for several decades (since the middle of the 20th century) through different isolation techniques. A fast and accurate method of headspace gas chromatography-mass spectrometry (GC-MS) was used to study the alkylpyrazine of 120 cocoa liquor samples, tetramethylpyrazine had the highest concentration.[Citation5]
Purge and trap (P&T) techniques are commonly used for aroma components extraction from samples such as drinking water, honey, chocolate, beverages, etc. With P&T, the analytes are purged out of the sample and trapped onto Tenax tube by a flow of gas (nitrogen or helium) at room temperature. The trapped sample components were desorbed at 250ºC for a certain time, and transferred directly to the GC-MS or gas chromatography-olfactometry-mass spectrometry (GC-O-MS) for analysis of aroma-active compounds., usually cryofocusing (liquid nitrogen, –196ºC) is applied to make the peak appear sharp. P&T is a solvent-free technique which became the technique of choice in the EPA and standard methods for the determination of volatile organic compounds (VOCs).[Citation5,Citation6]
In the aroma extract dilution analysis (AEDA) procedure, stepwise dilutions (such as 1:2n, i.e., 1:2, 1:4, 1:8, … or 1:3n, i.e., 1:3, 1:9, 1:27, …) of an original extract of food are performed and the diluted extracts are then evaluated by GC-O to get flavor dilution (FD) factors (FD = the highest dilution of an extract at which the compound can be detected by panelist at sniffing port), so in this way, the contribution of an odorant to the overall aroma profile of sample is ranked, and the key aroma-active compounds can be screened out.[Citation7,Citation8]
Not all the volatiles may contribute to the aroma of food, therefore, only small amounts of odorants are responsible for the characteristic aroma of cocoa liquor. It revealed that the contribution of one compound to the overall odorants was not only due to its concentration, but also relied on its odor threshold.[Citation9,Citation10] However, to the best of our knowledge, no comparative study was performed focusing on the key aroma-active compounds of cocoa liquors from different regions.
In this study, the aroma-active compounds in cocoa liquors from five areas were qualitatively and quantitatively analyzed by GC-O-MS, and the key aroma-active compounds were identified by AEDA. The aim of present work was to identify the aroma-active compounds in cocoa liquors from different regions (Asia, Africa, and Oceania), so as to provide scientific evidence for the manufacture of chocolate products. Finally, principal component analysis (PCA) was used to achieve origin recognition.
Materials and methods
Materials
Cocoa liquors
Three batches of cocoa liquors from five geographical regions: Ghana (forastero), CM (forastero), ID (forastero), Ivory Coast (CI; forastero), and PNG (forastero) were supplied by Barry Callebaut (Klang, Malaysia). All the products were prepared, including roasting, decladding, and grinding, based on the cocoa liquor preparation stages of the International Office of Cocoa, Chocolate, and Sugar Confectionery (IOCCC) Analytical Method Number 13, 1971.
Chemicals
n-Alkanes (C7-C30) and 2-methyl-3-heptanone (chromatographic reagent, purity >99%) were purchased from Sigma-Aldrich (Santa Clara, CA, USA). The solvents and authentic standard flavor compounds used in volatiles identification were of pure grade (purity >97.7%) from Merck and Sigma-Aldrich companies, respectively.
Methods
P&T extraction
The P&T concentrator (Atomx Teklink, Teledyne Tekmar, Ohio, USA) was interfaced with a system containing a gas chromatograph coupled with a mass spectrometer. A trap tube (No. 9, Agilent Technologies) was used for trapping volatiles. Cocoa liquor (3.0 g) was put in a 40 mL vial, then 1 μL internal standard solution (2-methyl-3-heptanone in n-hexane, 163.2 ng/μL) was added to the sample. The vial was equilibrated at 50ºC in a thermostatic bath for 15 min and high purity nitrogen gas (>99.9992%) was utilized as a purge gas flowing at 50 mL/min. The other analytical conditions were as following: Trap temperature: purge 30ºC; desorption 250ºC; baking 280ºC. Time: purge 25 min; desorption 2 min; trap baking 10 min.
Direct solvent extraction-solvent assisted flavor evaporation (DSE-SAFE)
Cocoa liquor (50.0 g) was placed in a Teflon bottle and then extracted with 150 mL × 2 redistilled dichloromethane. The combined solvent was about 270 mL, and was concentrated using a Vigreux column to approximately 100 mL. Then the concentrate was applied to a modified SAFE apparatus (Glasbläserei Bahr, Manching, Germany) for solvent extraction. The SAFE apparatus used was similar to that described by Engel et al.,[Citation11] consisting of high vacuum pump, diffusion pump, receiving tube, and waste tube, operating at high vacuum (10−4 to 10−5 Torr) and very low temperature (–196ºC) to trap volatile substance and to avoid the production of artifact. Distillation was carried out for 0.5 h under vacuum. After distillation, the distillate was concentrated to about 30 mL by Vigreux column, then dried over Na2SO4 (anhydrous) and concentrated again by gentle nitrogen flow to about 200 μL, ready for further analysis.[Citation12]
GC-O-MS
The GC-O-MS system was consisted of Agilent 7890A gas chromatograph coupled to a Triple Quad 7000B Mass Spectrometer Detector (both Agilent, Palo Alto, CA, USA), and equipped with a Sniffer 9000 Olfactometer (Brechbühler, Switzerland). Separations in GC-O-MS were performed on two columns with the same dimensions (30 m × 0.25 mm i.d., 0.25 µm film thickness) but having different polarities (DB-WAX or DB-5MS, J&W Scientific, Folsom, CA, USA). The carrier gas was ultra-high purity helium (≥99.999%) and the flow rate was 1.2 mL min−1. The oven temperature was programmed from 40ºC held for 3 min, increased at 5ºC min−1 to 200ºC, and then increased at 10ºC min−1 to 230ºC held for 3 min, the total running time was 41 min. The temperatures of the injector and the GC-MS interface were both 250ºC. Electron-impact mass spectra were generated at 70 eV, with m/z scan range from 28 to 250 amu. The ion source temperature was 230ºC and the quadrupole temperature was 150ºC. Compounds were identified according to NIST 08 mass spectra libraries installed in the GC-MS equipment. At the sniff port humidified air was generally supplied at 12 mL min−1. The GC effluent was split 1:1 between mass spectrometry detector (MSD) and sniffing port (working at room temperature). Sniffing was performed by three experienced panelists.
AEDA
Serial dilutions were prepared from the initial SAFE extracts at a ratio of 1:3n in dichloromethane. One microliter of original distillate and serial dilutions (1:3, 1:9, 1:27, etc.) were subjected to GC-O-MS on the DB-WAX column to detect and evaluate the aroma-active compounds. The highest dilution in which the compound was detectable was the flavor dilution factor (FD) of that compound, FD = Log33n so the Log3FD values were 1, 2, 3, and so on. According to Ferreira et al.,[Citation13] compounds with higher Log3 FD are considered to be more important. The FD factor of each compound was determined by three experienced panelists by performing sniffing test on GC-O. Each panelist had received at least 2 years of weekly GC-O training.
Identification of aroma-active compounds
The identification of aroma-active compounds was based on the mass spectrum, linear retention index (LRI), and odor descriptions of aroma-active compounds.[Citation14] n-alkanes (C7–C30) were analyzed on DB-WAX and DB-5MS column under the same conditions with equation below to calculate LRIs.
where n represent the number of carbon atoms whose peaks were in front of the identified compounds. The variables tRa, tRn, tR(n+1), respectively, represent for the retention time of the identified compounds, upper alkane, and lower alkane, which was described by Dool and Krazt.[Citation15] An aroma-active compound was positively identified only when its mass spectra, retention indices and odor properties could match with those of authentic standards at the same time. A compound was considered to be tentatively identified when only one or two of the above criteria were met, e.g., if a compound was identified only by retention index (RI)/odor property, this compound was regarded as tentatively identified; if MS data was present, even if in coordination with standard compound verification, then the compound was positively identified.
Semi-quantification of volatile compounds
The concentration of each positively identified odorant was semi-quantified by using 2-methyl-3-heptanone as an internal standard.[Citation16] The volatile compounds in cocoa liquor were quantified as the following: 3.0 g cocoa liquor was weighted accurately and transferred into a pre-cleaned 40-mL amber vial. Then, 1 μL internal standard solution (2-methyl-3-heptanone in hexane) was added to the sample. And the final concentration of the internal standard was 54 ng/g. The following equation was used to calculated the mean value of triplicates:
where Sa was stand for the peak area of compound a, Ss was stand for the peak area of the internal standard, and Cs represent for the concentration of internal standard, RF = 1.
Sensory evaluation of cocoa liquor
Sensory evaluation was carried out by 10 panelists who ranged in age from 24- to 35-years-old (4 men and 6 women) having extensive experience in the sensory evaluation of food products. To train the sensory panel to recognize the aroma character of cocoa liquor, daily training sessions were conducted for 2 months. Sensory evaluation was performed by the method recently described with a little modification.[Citation17] The method was described as follows: 5 g of cocoa liquor was introduced into the mouth for 15 s, followed by chewing 10 times, and deep expiration was performed several times to obtain the aroma.[Citation18] A 5-point universal intensity scale was used in this evaluation (0–1: very weak; 1–2: weak; 2–3: middle; 3–4: strong; 4–5: very strong). Sensory evaluations were performed in a testing room without other odors. Samples were taken out of refrigeration approximately 1 h prior to evaluation. Random Arabic numerals were given to the five types of samples. All the samples were evaluated in a one-time evaluation, three independent sessions were also included. Differences of the same result between sessions and panels were no more than two category scales.
Statistical analysis
One-way analysis of variance (ANOVA) was used for the quantitative results to determine significant differences in the amounts of predominant aroma-active compounds between samples from different origin. Tukey’s multi-range test was performed to grade samples when the samples exhibited a significance difference, with the level of significance set at p < 0.05. Clustering analysis was done by PCA. All the ANOVAs, PCA, and Tukey’s multiple range tests were performed with IBM SPSS Version 19 software.
Results and discussion
Sensory evaluation
Odor attributes of the cocoa liquors from five regions were evaluated in a descriptive sensory evaluation by 10 trained panelists. As shown in , the odor properties of cocoa liquors were divided into roast, caramel, milky, floral, sour, and overall according to sensory reference standards (). The overall odor outlines of the five cocoa liquors were very similar, but the intensity of each note between the cocoa liquor was different. It revealed, from , that the cocoa liquor of PNG had higher preference than those of the other four samples, the milky odor note of PNG was lower than that of Ghana and CM. The cocoa liquor of ID had the lowest intensity of the whole aroma profile.
Table 1. Sensory reference standards.
Figure 1. Sensory evaluation of five cocoa liquors (The scores of aroma were 0, 1, 2, 3, 4, and 5, which represent intensities of none, very weak, weak, middle, strong, and very strong, respectively).
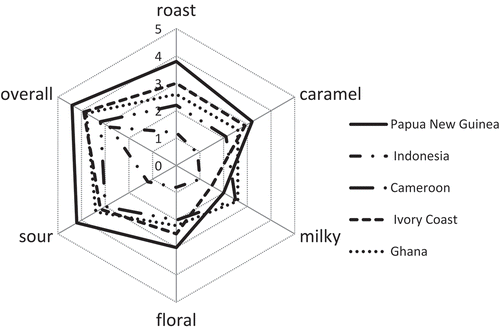
Malty, roast, and nutty are important notes of chocolate, which come from Maillard reaction of cocoa beans during roasting. Flavor research of cocoa beans from different regions showed that cocoa liquors from West Africa (Ghana and CI) were regarded as the most standard benchmark cocoa aroma.[Citation1] In our study, the PNG cocoa liquor had the strongest preference with modest roast and caramel flavor. Pulp sugar fermentation could yield high levels of acids, particularly acetic acid.[Citation19] Acid is an important part of chocolate flavor, but stronger acidity was viewed as an off-flavor of the chocolate.[Citation20] The result of this study showed that, all the cocoa liquor had higher score of sour flavor, among them, cocoa liquor of PNG was the highest.
Identification of predominant aroma-active compounds
As shown in , 43 aroma active compounds were detected by GC-O-MS technique. Among them, 25 compounds with average log3 FD-factor ≥3 were identified as predominant odorants in the extracted fractions. The odorants of 3-methylbutanal (malty), linalool (floral), β-phenylethyl alcohol (rosy), benzaldehyde (almond-like), benzeneacetaldehyde (rosy), β-phenylethyl acetate (fruity), 3-methylbutyl benzoate (fruity), 2,5/6-dimethylpyrazine (potato-like), ethylpyrazine (popcorn), trimethylpyrazine (roasted), 3-ethyl-2,5-dimethylpyrazine (roasted), tetramethylpyrazine (nutty), 3,5-diethyl-2-methylpyrazine (cocoa), furfural (potato), acetic acid (sour), 3-methylbutanoic acid (stink), and dimethyl trisulfide (onion) reached higher FD factors in all the samples, considered as the key aroma-active compounds contributing to the overall odor of the cocoa liquor. It was shown that the most important compounds were pyrazines and aldehydes. Other compounds such as hexanal, ethyl phenylacetate, 2-pentyfuran, and 3-acetyl-1H-pyrroline also present the floral or nutty aroma.
Table 2. Sensory evaluation of five cocoa liquors.
Table 3. Aroma-active compounds in the volatile extracts of five cocoa liquors obtained by DSE-SAFE.
The diagram of total ion chromatography (TIC) showed that the major peaks were 3-methylbutanal (LRI: 938), acetic acid (LRI: 1428), tetramethylpyrazine (LRI: 1483), and 3-methylbutanoic acid (LRI: 1669). They were protruded both in peak areas and FD factors. There were also some compounds of very small peak areas but with high FD factors, such as benzeneacetaldehyde (rosy), β-phenylethyl alcohol (rosy), β-phenylethyl acetate (honey-like), ethylpyrazine (popcorn-like), dimethyl trisulfide (onion-like), and so on. It revealed that the contribution of one compound to the overall odorants was not only due to its concentration but also relayed on its odor threshold.[Citation9,Citation10]
Most of those compounds listed in had been reported as the aroma-active compounds in cocoa products except for 1H-pyrrole-2-carboxaldehyde (caramel, cocoa), massoia lactone (floral). Bonvehí studied the volatile components in roasted cocoa powder and proved that the major components of cocoa aroma were tetramethylpyrazine, benzaldehyde, benzeneacetaladehyde, acetophenone, 3-methylbutanoic acid, 5-methyl-2-phenyl-2-hexenal, ethyl phenylacetate, and maltol.[Citation21] By using solid-phase micro extraction (SPME)/GC-MS method, Misnawi found that the specific aroma of cocoa liquor such as sweet, nutty, caramel, and chocolate-like notes were associated with trimethylpyrazine, tetramethylpyrazine, 2,3-butanediol, dodecanoic acid, β-phenylethyl alcohol, 2-acetylpyrrole, and benzeneacetaldehyde.[Citation22] These differences might result from the various kinds of raw materials.
Besides the common higher FD value odorants, some other components were only showed higher FD value in some samples. There were six compounds existing only in PNG cocoa liquors, including ethyl 3-hydroxybutyrate, 2-nonanone, 3-hydroxy-2-butanone, 2,5-dimethyl-4-hydroxy-3(2H)-furanone, 2-methoxy-4-vinylphenol, and 4-methyl-5-thiazoleethanol. It was worth mentioning that 2,5-dimethyl-4-hydroxy-3(2H)-furanone (HDMF; caramel-like) was only important for the overall profile aroma of PNG cocoa liquor. It was formed when fructose-1, 6-bisphosphate was heated.[Citation23] Counet et al. found that the concentration of HDMF was higher in the chocolate after conching.[Citation4] Our result also indicated that the producing area of cocoa liquors has important influenced on the kind of aroma compounds. Besides, there were certain odorants found only in CI cocoa liquor, such as γ-undecalactone (peach-like) and (E)-2-decenal (fatty). (E)-2-decenal was frequently identified in food stuff rich in fat, such as peanut,[Citation24] hazelnuts,[Citation25] and so on, which might generate from the degradation of fatty acid. Forss found thatγ-undecalactone was an important aroma-active compound for milk products and could generate from hydroxy acids or triglycerides by heating,[Citation26] octanal was only exist in sample of ID. Saturated aldehydes generally show green-like, hay-like, paper-like odor, while unsaturated aldehydes present frying, fatty, or oily odor. The saturated aldehydes can be formed together with the unsaturated aldehydes, by autoxidation of unsaturated fatty acids.[Citation27] Linalool, 3-methylbutyl acetate, ethyl phenylacetate, which contributed to pleasant aroma, have been reported existing in the unroasted cocoa beans.[Citation28] The specific aroma-active compounds profile mentioned previously in different cocoa liquor samples showed the aroma difference between different regions.
Quantitative analysis of five cocoa liquors
The concentrations of predominant odorants in cocoa liquors from five different regions were quantified by internal standard method via the P&T extraction. The predominant odorants quantitated is only the compounds can be detected by GC-MS and their contents in five cocoa liquors were listed in . Each value was the average value of triplicate trials results and the difference significance of each result was calculated by ANOVA. As shown in , most components in the cocoa liquor of different regions were significantly different (p < 0.05). The amounts of 3-methylbutanal and acetic acid were the highest, being 1055.68 ± 20.85 and 1415.67 ± 20.75 ng/g respectively; while the amount of 3-methylbutyl benzoate in ID cocoa liquor was only 4.9 ± 0.18 ng/g. Moreover, the amounts of benzeneacetaldehyde and 3,5-diethyl-2-methylpyrazine were trace, meaning that they were too low to be detected, but their smells were a little intense, due to their low detection thresholds. Almost all the compounds had the highest concentration in the cocoa liquor of PNG, while the concentrations were lowest in the sample of ID, it was consistent with sensory evaluation. The difference between five cocoa liquors might be resulted from the difference of bean constituents which could be caused by growth location, climatic condition, soil condition, and so on.
Table 4. Relative concentrations of predominant aroma-active compounds in five cocoa liquors measured by the P&T extraction followed by GC-O-MS.
Aldehydes
The aldehydes quantified in were mostly Strecker degradation aldehydes. Among them, 2-methylpropanal, 3-methylbutanal, and benzeneacetaldehyde were generally considered originating from Strecker degradation of valine, leucine, and phenylalanine in the cocoa liquor, respectively. They were considered as important contributors of the typical cocoa-like aroma of cocoa powder.[Citation29] The content of 3-methylbutanal was highest and had the biggest difference between five cocoa liquors, it ranged from 165.7 to 1055.68 ng/g. This might be due to the different content of leucine and pH value of cocoa liquor.[Citation27]
Pyrazines
Pyrazines are important flavor compounds in baked goods such as roasted peanuts, coffee beans, hazelnuts, and cocoa beans. It have been reported that pyrazine generated during the roasting of cocoa and contributed desirable nutty and roast odor to the cocoa products.[Citation17,Citation23] Ten kinds of pyrazine were quantitated in , and tetramethylpyrazine had the highest concentration. The results of this study showed that under the same standard process, cocoa liquor from different regions had different features. For example, trimethylpyrazine and tetramethylpyrazine had the highest level in PNG cocoa liquor, while CI cocoa liquor had the highest level of 2,5-dimethylpyrazine, ethylpyrazine and 2-ethyl-6-methylpyrazine. These differences between these five cocoa liquors might be due to the different geographical environment in different soil and humidity conditions. Torres-Moreno also found that cocoa bean of different regions roasted under the same conditions would produce great differences in flavor when applied to the chocolate products.[Citation30] The research of Brunetto indicated that different regions of cocoa beans being processed in the same baking conditions would generate significant differences on pyrazine content.[Citation31]
Acids
Acids is usually considered generating in the fermentation process, and the content of acids, especially acetic acid, had a significant relationship with fermentation conditions.[Citation32] Meanwhile, acids will also directly affect the formation of other flavor substances in the subsequent processing. The quantitative results showed that the Oceania country of PNG had the highest level of total acid, and the Asian country of ID had the least amount of total acid, meanwhile those of African country such as CM, Ghana, and CI were in the middle. The result also showed that the content of total acid was the lowest in ID cocoa liquor, this might be due to the missing of some special operations to the materials, such as rapid artificial drying.
PCA
To evaluate the possibility of differentiating the samples based on their origin, PCA was applied for data analysis. The first two principal components (PCs) explained the 66.8 and 15.6% of date variance, respectively. The aroma-active compounds analyzed by PCA for cocoa liquors from different origins were shown in . The figure showed that the three batch samples from the same origin had fallen to the same areas. The characteristics of cocoa liquor from PNG, ID, and CI were very significant. Linalool oxide, linalool, acetic acid, 3-methylbutanoic acid, dimethyl trisulfide, β-phenylethyl acetate, 3-methylbutyl acetate, and tetramethylpyrazine were character-impact compounds of PNG cocoa liquor. Phenethanol, limonene, 2-pentyfuran, 2-methyl-2-butenal, ethylpyrazine, and 2,5-dimethylpyrazine were key odorants of CI cocoa liquor. Except for hexanal, concentration of each odorant of ID cocoa liquor was the lowest. The content of flavor compounds of CM and Ghana cocoa liquor were mediate, and had no significant features.
Figure 2. Sensory evaluation of five cocoa liquors (The scores of aroma were 0, 1, 2, 3, 4, and 5, which represent intensities of none, very weak, weak, middle, strong and very strong, respectively).
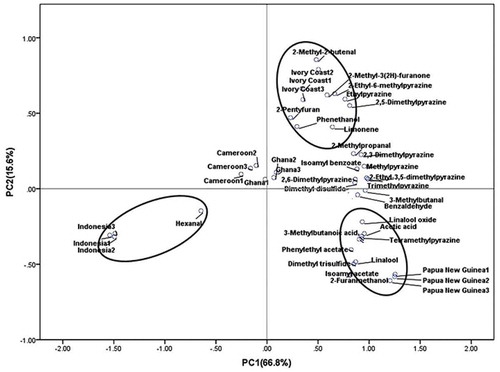
Combined (sensory evaluation) with and (qualitative and quantitative analysis), it could be seen that the sensory score of PNG (especially roast, floral notes) were the highest, accordingly the contents of aroma-active compounds such as pyrazines (except for 2,5-dimethylpyrazine and ethylpyrazine), esters and aldehydes were the highest; while the sensory score of ID was the lowest, the corresponding aroma-active compounds had the lowest concentration.
Each cacao bean variety has a unique potential flavor character. But environmental factors such as climate, the amount and time of sunshine and rainfall, soil conditions, ripening, time of harvesting, and the time between harvesting and bean fermentation all have great impact on the final flavor formation.[Citation33,Citation34] The review of Afoakwa et al.[Citation1] stated that the differences in genetic origin, cocoa variety, and the duration of the fermentation influenced the composition and contents of flavor precursors such as polyphenols and alkaloids (including caffeine, theobromine, and theophylline), protein (low molecular weight)/free amino acids, carbohydrates, etc., therefore, made considerable variations on the flavor profile of cocoa and chocolate.
Conclusion
In this research, the key aroma-active compounds of cocoa liquors from five different regions were studied and compared qualitatively and quantitatively. Differences in the species and concentration of flavor substances between each other was observed. The flavor properties of cocoa liquors from Ghana and CI have the closest correlation. The result of sensory evaluation also showed the difference of odor characteristics among them. The origin of raw material had a great influence on the quality of cocoa liquor aroma profile. Counet et al.[Citation35] have shown that for cocoa liquor, the total content of volatile compounds varied for certain geographic origins. The compounds that impact cocoa and chocolate flavors are molecules such as pyrazines (nutty), aldehydes (cocoa aroma), and esters (fruity). In this study, our funding showed that pyrazines, aldehydes, and esters were the predominant aroma-active compounds of the five cocoa liquor tested. Afoakwa et al.[Citation1] reviewed that Asian and Oceanian beans exhibited a range of flavor profiles ranging from subtle cocoa and nutty/sweet notes in java beans to the intense acid, and PNG beans were specifically higher in total aroma. In our investigation, the total aroma content of the PNG cocoa liquor was the highest, the sensory score was the highest. These results were in agreement with the literature. The final quality of cocoa liquors had great relationship with the cocoa nuts’ growing condition and processing after they were collected. Growing environment, soil, temperature, humidity, microorganisms, enzymes, and so on all may affect the flavor precursors content of cocoa mass; and fermentation, roasting conditions of cocoa beans may also affect the species and content of volatile compounds. So, further studies will be focused on the odorant precursors in cocoa liquors and their formation pathways, they are critical for the control of key technique process and the final quality of production of cocoa liquor. Moreover, comparison of the quality difference of chocolate manufactured by different cocoa liquors from different origins should be further studied.
Besides, further research should be focused on different variety of cacao bean, different growing areas, different roasting conditions, and digestion conditions, etc., and by using the same techniques involved in this article to study these differences; and by using techniques such as ultrafiltration/size exclusion chromatography/reverse-phase high-performance liquid chromatography (RP-HPLC)/LC-MS, the flavor precursors such as polyphenols, free amino acids, and carbohydrates should be investigated.
Nomenclature
PNG Papua New Guinea
CM Cameroon
ID Indonesia
CI Ivory Coast
References
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Critical Reviews in Food Science and Nutrition 2008, 48, 840–857.
- Ziegleder, G.; Biehl, B. Analysis of Cocoa Flavor Components and Flavor Precursors. Modern Methods of Plant Analysis 1988, 8, 321–393.
- Cros, E.; Chanliau, S.; Jeanjean, N. Post Harvest Processing: A Key Step in Cocoa. In Confectionary Science II. Proceeding of An International Symposium; Ziegler, G.R.; Ed.; Pennsylvania State University: State College, PA, 1999; 80–95 p.,.
- Counet, C.; Callemien, D.; Ouwerx, C.; Collin, S. Use of Gas Chromatography-Olfactometry to Identify Key Odourant Compounds in Dark Chocolate. Comparison of Samples Before and After Conching. Journal of Agricultural and Food Chemistry 2002, 50, 2385–2391.
- Campillo N.; Viñas P.; López-García, I.; Aguinaga, N.; Hernández-Córdoba, M. Purge-and-Trap Capillary Gas Chromatography with Atomic Emission Detection for Volatile Halogenated Organic Compounds Determination in Waters and Beverages. Journal of Chromatography A 2004, 1035, 1–8.
- Lara-Gonzalo, A.; Sánchez-Uría, J.E.; Segovia-García, E.; Sanz-Medel, A. Critical Comparison of Automated Purge and Trap and Solid-Phase Microextraction for Routine Determination of Volatile Organic Compounds in Drinking Waters by GC-MS. Talanta 2008, 74, 1455–1462.
- Schieberle, P.; Grosch, W. Evaluation of the Flavour of Wheat and Rye Bread Crusts by Aroma Extract Dilution Analysis. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1987, 185, 111–113.
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chemical Senses 2001, 26, 533–545.
- Buttery, R.G.; Takeoka, G.R.; Ling, L.C. Furaneol: Odor Threshold and Importance to Tomato Aroma. Journal of Agricultural and Food Chemistry 1995, 43, 1638–1640.
- Leonardos, G.; Kendall, D.; Barnard, N. Odor Threshold Determinations of 53 Odorant Chemicals. Journal of the Air Pollution Control Association 1969, 19, 91–95.
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation—A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. European Food Research and Technology 1999, 209, 237–241.
- Song, H.L.; Cadwallader, K.R. Aroma Components of American Country Ham. Journal of Food Science 2008, 73, C29–C35.
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rose´ Wines. Aroma Extract Dilution Analysis, Quantitative Determination and Sensory Reconstitution Studies. Journal of Agricultural and Food Chemistry 2002, 50, 4048–4054.
- Xu, Q.Q.; Liu, J.B.; Song, H.L.; Zou, T.T.; Liu, Y.; Zhang, S.P. Formation Mechanism of Volatile and Non-Volatile Compounds in Peptide–Xylose Maillard Reaction. Food Research International 2013, 54, 683–690.
- van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. Journal of Chromatography A 1963, 11, 463–471.
- Chen, G.; Song, H.L.; Ma, C.W. Aroma-Active Compounds of Beijing Roast Duck. Flavour and Fragrance Journal 2008, 24, 186–191.
- Liu, J.B.; Liu, M.Y.; He, C.C.; Song, H.L.; Guo, J.; Wang, Y.; Yang, H.Y.; Su, X.X. A Comparative Study of Aroma-Active Compounds Between Dark and Milk Chocolate: Relationship to Sensory Perception. Journal of the Science of Food and Agriculture 2015, 95, 1362–1372.
- Piccone, P.; Rastelli, S.L.; Pittia, P. Aroma Release and Sensory Perception of Fruit Candies Model Systems. Procedia Food Science 2011, 1, 1509–1515.
- Voigt, J.; Biehl, B.; Heinrichs, H. In-Vitro Formation of Cocoa-Specific Aroma Precursors—Aroma-Related Peptides Generated from Cocoa-Seed Protein by Cooperation of An Aspartic Endoprotease and a Carboxypeptidase. Food Chemistry 1994, 49, 173–180.
- Jinap, S.; Dimick, P.S.; Hollender, R. Flavour Evaluation of Chocolate Formulated from Cocoa Beans from Different Countries. Food Control 1995, 2, 105–110.
- Bonvehí, J.S. Investigation of Aromatic Compounds in Roasted Cocoa Powder. European Food Research and Technology 2005, 221, 19–29.
- Misnawi, B.T.S.; Ariza, B.T.S. Use of Gas Chromatography-Olfactometry in Combination with Solid Phase Micro Extraction for Cocoa Liquor Aroma Analysis. International Food Research Journal 2011, 18, 829–835.
- Schieberle, P. Formation of Furaneol in Heat-Processed Foods. ACS Symposium Series 1992, 490, 164–174.
- Chetschik, I.; Granvogl, M.; Schieberle, P. Quantitation of Key Peanut Aroma Compounds in Raw Peanuts and Pan-Roasted Peanut Meal. Aroma Reconstitution and Comparison with Commercial Peanut Products. Journal of Agricultural and Food Chemistry 2010, 58, 11018–11026.
- Burdack-Freitag, A.; Schieberle, P. Characterization of the Key Odorants in Raw Italian Hazelnuts (Corylus Avellana L. var. Tonda Romana) and Roasted Hazelnut Paste by Means of Molecular Sensory Science. Journal of Agricultural and Food Chemistry 2012, 60, 5057–5064.
- Forss, D.A. Mechanisms of Formation of Aroma Compounds in Milk and Milk Products. Journal of Dairy Research 1979, 46, 691–706.
- Cremer, D.R.; Eichner, K. The Influence of the pH Value on the Formation of Strecker Aldehydes in Low Moisture Model Systems and in Plant Powders. European Food Research and Technology 2000, 211, 247–251.
- Frauendorfer, F.; Schieberle, P. Changes in Key Aroma Compounds of Criollo Cocoa Beans During Roasting. Journal of Agricultural and Food Chemistry 2008, 56, 10244–10251.
- Frauendorfer. F.; Schieberle, P. Identification of the Key Aroma Compounds in Cocoa Powder Based on Molecular Sensory Correlations. Journal of Agricultural and Food Chemistry 2006, 54, 5521–5529.
- Torres-Moreno, M.; Tarrega, A.; Costell, E.; Blanch, C. Dark Chocolate Acceptability Influence of Cocoa Origin and Processing Conditions. Journal of the Science of Food and Agriculture 2012, 92, 404–411.
- Brunetto, M.D.R.; Cayama, Y.D.; Gutierrez, L.; Roa, S.C.; Mendez, Y.C.; Gallignani, M. Headspace Gas Chromatography-Mass Spectrometry Determination of Alkylpyrazines in Cocoaliquor Samples. Food Chemistry 2009, 112, 253–257.
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Critical Reviews in Food Science and Nutrition 2004, 44, 205–221.
- Clapperton, J.F. A Review of Research to Identify the Origins of Cocoa Flavour Characteristics. Cocoa Growers’ Bulletin 1994, 48, 7–16.
- Cambrai, A.; Marcic, C.; Morville, S.; Houer, P.S.; Bindler, F.; Marchioni, E. Differentiation of Chocolates According to the Cocoa’s Geographical Origin Using Chemometrics. Journal of Agricultural and Food Chemistry 2010, 58, 1478–1483.
- Counet, C.; Ouwerx, C.; Rosoux, D.; Collin, S. Relationship Between Procyanidin and Flavor Contents of Cocoa Liquors from Different Origins. Journal of Agricultural and Food Chemistry 2004, 52, 6243–6249.
