ABSTRACT
The present study aimed to investigate the effects of oxidation on physicochemical properties of Sheldrake meat and secondary structure of myofibrillar proteins. Sheldrake breast meat was treated with hydroxyl radical generating systems containing different hydrogen peroxide (H2O2) concentrations. Upon oxidation, the water-holding capacity of Sheldrake meat decreased while toughness increased. Myofibrillar proteins were extracted from the non-oxidized and oxidized meat and heat-treated to form myofibrillar protein gels. It was found that 2-thiobarbituric acid (TBARS), protein carbonyl content, and disulfide bond values increased with H2O2 concentration. Raman spectroscopy analysis of the myofibrillar protein gels showed that oxidation led to protein unfolding, decreased α-helix structure, and increased β-sheets, β-turns, and random coils structures. Changes in the secondary structures of proteins affected the water-holding capacity and textural properties of myofibrillar protein gels. Water-holding capacity of the myofibrillar protein gels decreased with H2O2 concentration. Oxidized myofibrillar protein gels showed reduced hardness, springiness, and cohesiveness.
Introduction
Sheldrake meat is famous for its delicate taste and texture. It has about 16–25% protein content which is much higher than that of chicken and lamb.[Citation1] Muscle proteins are susceptible to oxidations caused by different initiators including oxidizing lipids, metal ions, and other pro-oxidants generated during meat processing, handling, and storage.[Citation2]
Protein oxidation leads to alteration in protein structure and formation of amino acid derivatives and polymers which subsequently changes the functional properties of protein (hydration, solubility, and digestibility). Protein oxidation also affects the organoleptic and nutritional properties of meat products.[Citation3] For example, sensory properties of dry-cured hams were compromised by high hydrostatic pressure (HHP)–induced oxidation.[Citation4] Furthermore, protein oxidation in meat systems has also been related to color and texture deterioration.[Citation5] Mild oxidation caused by hydroxyl radical generating system (HRGS) was found to promote protein network formation and enhance gelation of myofibrillar protein (MP) involving disulfide linkages.[Citation6]
Protein oxidation can be assessed through manifestation of chemical changes such as loss of sulfhydryl groups and tryptophan fluorescence, gain of carbonyl derivatives, and formation of intra- and intermolecular cross-linkings.[Citation7] It is not possible to obtain the structural information of proteins through chemical methods. Raman spectroscopy has become a popular technique which provides structural information on the secondary and tertiary structures of proteins. It is a convenient method with high degree of sensitivity and accuracy.[Citation8,Citation9]
Present study aimed to investigate the effects of protein oxidation on Sheldrake meat quality and structural and functional properties of extracted MP gels. Sheldrake breast muscle was treated with different HRGS. The effects of protein oxidation on color, textural properties, water-holding capacity (WHC), and 2-thiobarbituric acid (TBARS) value of Sheldrake meat were studied. To better understand the changes of protein structure during oxidation, MP was extracted from Sheldrake meat and heat-treated to form MP gels. MP gels were evaluated in terms of the secondary structures of proteins using Raman spectroscopy technique. The extracted MP was also investigated in terms of chemical properties (protein carbonyls and total sulfhydryl contents), surface hydrophobicity, textural properties, and WHC of MP gels.
Materials and methods
In order to investigate the effects of oxidation on Sheldrake meat properties and structure of MPs, two separate studies were performed:
Study 1: The effect of different oxidation on Sheldrake meat properties.
Study 2: The effects of oxidation on the indicator of MPs oxidation, the properties of gels, and MPs structure. For this purpose, Raman spectroscopy was performed.
Study 1
Samples
Fresh Sheldrake breast muscle meat (aged 360 days) was purchased from Jiangnan Poultry Breeding Co. Ltd. (Zhejiang, China). The meat was trimmed of fat and connective tissue prior to the experiment. Sheldrake breast muscles were cut in parallelepipeds (5 cm × 5 cm × 1 cm) and immersed in HRGS (0.1 mM ascorbic acid, 0.01 mM FeCl3, 0.1 M NaCl, 15 mM PIPES buffer (pH 6.2)) with different H2O2 concentrations (5, 10, 15, 20, 25, and 30 mM H2O2). Oxidation process was conducted at 4°C for 20 min and terminated with addition of 1 mM ethylene diamine tetraacetic acid (EDTA). In the control, muscle blocks were placed in buffer solution (0.1 M NaCl, 15 mM PIPES buffer (pH 6.2)).
Chemical composition
The official methods numbered 950.46, 960.39, and 992.15 of the Association of Official Analytical Chemists[Citation10] were used for the determination of moisture, fat, and protein contents of fresh Sheldrake breast muscle meat, respectively.
Color
A portable colorimeter (CR-400, Konica Minolta Investment Ltd., Japan) was used to estimate meat color including lightness (L*), redness (a*), and yellowness (b*). The measured illuminants and the diameter of the instrument were D65 and 8 mm, respectively.
Water-holding capacity (WHC)
WHC was measured according to a previously described method.[Citation11] WHC was calculated as a percentage of weight loss before and after compression of meat, and expressed as:
Shear force
Oxidized meat samples (1 cm × 1 cm × 1 cm) were trimmed along the muscle fiber direction. Muscle tenderness meter (C-LMB digital display type, Northeast Agricultural University, China) was used to record the shear force value along the vertical shear of muscle fiber. Each measurement was calculated as an average of five measurements.
Lipid oxidation
TBARS assay was carried out according to a previously described method.[Citation12] Ground meat sample (2.0 g) was homogenized with 200 µL butyl hydroxyanisole (BHA, 7.2%, w/v 98% ethanol) and 7 mL trichloroacetic acid (TCA, 5%) for 30 s in an ice bath. The solution was then filtered using gauze of 100 mesh. The obtained filtrate was washed with 1 mL of distilled water and diluted to 10 mL. Five millilitres of the filtrate was mixed with 0.2 M TBA (5.00 mL), heated at 80°C for 60 min, and cooled using running water. Absorbance was measured at 532 nm using a spectrophotometer (M200, Multiscan Spectrum, Tecan, America) against a distilled water blank.
Study 2
Extraction of myofibrillar protein (MP)
MP was extracted according to a previously described method.[Citation13] The purified MP samples were stored at 4°C and used within 48 h of preparation.
Protein carbonylation, sulfhydryl group, surface hydrophobicity assay
Protein carbonyls were determined according to a previously described method.[Citation14] Myofibril protein was dispersed in deionized water and stirred using magnetic stirrer in ice bath for 2 h. The mixture was centrifuged, and protein concentration of the resulting supernatant was determined by BCA Protein Assay Kit. Absorbance of the protein solution was measured at 367 nm using spectrophotometer. The concentration of 2,4-dinitrophenylhydrazine (DNPH)–derivatized proteins was determined by the molar extinction coefficient of 22,000 M–1 cm–1.
Sulfhydryl groups (SH) of MP were determined using a previously described method with slight modifications.[Citation15] MP solution (1 mL) was mixed with 50 µL thioglycol and 4 mL phosphate buffer (0.1 M Na2HPO4/NaH2PO4, 8 M urea, 5 M guanidine hydrochloride, pH 8.0). The mixture was kept in a water bath at 25°C for 25 min. Following that, 10 mL of TCA (12 %) was added to the mixture. The reaction solution was incubated in a water bath at 25°C for 1 h and then centrifuged at 3000g for 10 min. The obtained pellets were dissolved in 10 mL of phosphate buffer (0.1 M Na2HPO4/NaH2PO4, l M EDTA, 1% SDS, pH 8.0) and 0.08 mL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). After incubation at 25°C for 30 min, absorbance was measured at 412 nm to calculate the total SH groups using a molar extinction coefficient of 13,600 M–1cm–1.
Surface hydrophobicity of MP was determined according to a previously described method.[Citation16] BPB (40 µL; 1 mg/mL, in PBS, pH 7.0) was added to 1 mL of MP suspension (10 mg/mL, in 15 mM Tris-HCl). Samples were then centrifuged for 20 min at 4°C. Absorbance of the MP supernatant (diluted 1/10) was measured at 595 nm against a blank Tris-HCl without MP.
Preparation of myofibrillar gel (MP gels)
Five milliliters of MP solutions (40 mg/mL) were suspended in phosphate buffer (0.6 mol/L NaCl) and placed in a glass bottle (25 mm × 25 mm). The solutions were heated at a rate of 0.5°C/min from 25 to 70°C and kept at 70°C for 30 min. Following that, the solutions were cooled for 30 min in an ice bath and kept overnight at 4°C before further analysis.
WHC and textural properties of the MP gels
MP gels (2 g) were centrifuged at 10,000g for 20 min at 4°C.[Citation17] WHC (%) was expressed as the ratio of gel weight after centrifugation to the initial weight.
Instrumental texture profile analysis (TPA) of MP gels was carried out according to Dondero et al.[Citation18] using a texture analyzer (Model TA- XT2, Stable Micro Systems Ltd., UK). MP gels were subjected to a compression test using a cylindrical probe (P/5) at a trigger type button with a 1.0 mm/s pretest speed, a l.0 mm/s test speed, a l.0 mm/s post-test speed, a 3 mm distance, and a 5 g trigger force. TPA parameters including springiness, hardness, and cohesiveness were determined.
Raman spectroscopy analysis
Protein secondary structures were determined using a Via-Reflex spectrometer with an excitation at 532 nm at room temperature (Horiba Jobin-Yvon, France). Samples were placed on microscope slides and laser was then focused on the samples. Raman spectra of at least three different positions were collected from 500 to 2100 cm–1.[Citation19] Figure S1 shows the Raman spectra of the non-oxidized and oxidized MP gels in the 600–1800 cm−1 region. Spectral data from the scanning were baseline corrected and normalized according to the protein phenylalanine peak at 1003 cm–1.[Citation20] Protein secondary structures were calculated by integration of the corresponding fitted band. Baseline correction, normalization, derivation, curve fitting, and area calculation were carried out by means of PeakFit, version 4.12, software.
Statistical analysis
Significance of the data was determined through analysis of variance (ANOVA) using SPSS 16.0. Differences between means were compared by using SPSS at a significance level of 0.05.
Results and discussion
Study 1
Chemical composition
Sample compositions are consistent with meat batter formulations. The composition (%) of the control meat was: protein 20.88 ± 1.12, moisture 77.32 ± 1.64, and crude fat 2.13 ± 0.62.
Color
Color of meat products depends on the concentration and type of myoglobin and hemoglobin.[Citation21] shows L*, a*, and b* values of Sheldrake breast meat following oxidation with different levels of HRGS. Significant decrease (p < 0.05) in L* value can be observed with the increase in hydrogen peroxide content of the HRGS. Myoglobin is characterized by a purplish-red or purplish-pink color. In the presence of oxygen, myoglobin (Mb) forms oxymyoglobin (MbO2) which is bright cherry-red in color. Decrease in L* value of Sheldrake meat following oxidation might be due to endogenous removal of oxygen to achieve low-oxygen partial pressures (oxygen consumption) which resulted in formation of the darker color metmyoglobin (MetMb).[Citation22] However, L* value increased not significantly with further oxidation, while a* value decreased significantly (p < 0.05) with the increased concentration of hydrogen peroxide. Meanwhile, b* remained unchanged regardless of the concentration of hydrogen peroxide. The increased MetMb% could lead to the discoloration of fresh meat. The retardation of the formation of MetMb could lead to a higher L* and a* values.[Citation23] It is known that lightness (L*) is an achromatic color component. Fluctuations in the proportions of the forms of Mb in the surface layer of the meat had little effect on lightness (L*). The effect of the forms of myoglobin on redness (a*) depended mainly on the ratio between the amount of MetMb and MbO2, while their impact on yellowness (b*) depended mainly on the proportion of the amounts of reduced form (Mb), oxygenated (MbO2), and oxidized (MetMb) forms. A greater impact on yellowness (b*) was exerted by fluctuations in the relative amount of MbO2 than of MetMb.[Citation24] Therefore, the color of meat was affected by the amount of myoglobin and the proportions of its forms, including MbO2, Mb, and MetMb.
Table 1. L*, a*, and b* value of non-oxidized and oxidized Sheldrake breast muscle.
WHC and shear force of Sheldrake breast meat
Moisture in muscle can be divided into free water and bound water. About 85% of the water is found in muscle cell (myofibrils); meanwhile, only a small part of the water is stored in muscle cell gap.[Citation25] shows the WHC of the non-oxidized and oxidized Sheldrake breast meat. At low degree of oxidation, WHC of Sheldrake meat increased slightly (p < 0.05). However, when the concentration of H2O2 increased from 15 to 30 mM, the WHC of Sheldrake meat decreased significantly from 61.08% to 49.81% (p < 0.05). Our results were consistent with previously reported findings by Delles and Xiong,[Citation26] who observed that the ability of fresh muscle to retain endogenous water decreased when myofibrils were exposed to oxidizing agents. This might be due to the oxidation and subsequent aggregation of myosin and enlargement of the intercellular space appeared to contribute to the reduced WHC.
Figure 1. WHC (a), shear force (b), and TBARS value (c) of non-oxidized and oxidized Sheldrake breast muscle.
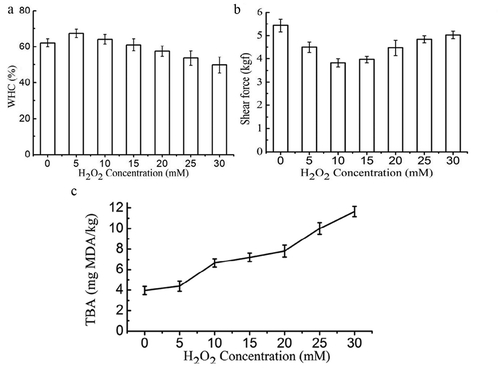
shows the shear force of non-oxidized and oxidized Sheldrake breast meat. Increase in H2O2 concentration from 0 to 10 mM resulted in decrease of shear force from 5.43 to 3.82 kgf suggesting that mild oxidation might improve meat tenderness. It is reported that a high amount of intra-myofibrillar water and a low amount of extra-myofibrillar water may be associated with more tender meat. Hence, decreased shear force may be related to water distribution and mobility in meat, increased WHC could lead to the tendency of tenderness improvement.[Citation27] Nevertheless, shear force increased with further increase in H2O2 concentrations from 10 to 30 mM. This is consistent with previously reported findings by Bao et al.,[Citation28] who found that increased oxygen concentration of modified atmosphere packaging (MAP) resulted in higher shear force in pork meat. Clausen et al.[Citation29] showed reduced tenderness of beef in high-oxygen MAP accompanied by increased lipid and protein oxidation. Protein cross-linking has previously been suggested as a potential mechanism for oxygen-induced meat toughening.[Citation30] Lagerstedt et al.[Citation31] reported that high-oxygen MAP negatively influences shear force. Results from Bao et al.[Citation28] suggested that the mechanism of oxygen-induced toughening of meat is through protein oxidation leading to cross-linking of structural proteins. Protein oxidation induces formation of cross-linking in structural proteins which resulted in increase of the mechanical strength and toughness of the meat.
Lipid oxidation
TBARS value increased significantly (p < 0.05) with concentration of H2O2 (Fig. 1c). This is not surprising as oxidation of the protein muscle resulted in formation of secondary oxidation products such as low-molecular-weight aldehydes, ketones, and fatty acids[Citation32]. It is reported that oxidative reactions could be transferred between lipids and proteins in a reciprocal manner.[Citation33] Zhang et al.[Citation34] also suggested oxidizing lipids could promote protein oxidation, and consumption of oxidized oil was related to higher protein carbonyl content in breast meat of broiler chickens. Burcham and Kuhan[Citation35] showed that incubation of model proteins with the lipid peroxidation product malondialdehyde resulted in a time- and concentration-dependent increase in carbonyl contents. Bao et al.[Citation28] suggested that oxidizing lipids likely served as a pool of reactive species (such as lipid radicals, hydroperoxides, and aldehydes) that may have spread and expanded oxidation damage in the protein fraction.
Study 2
Effects of oxidation on chemical properties of MP
Carbonyls are formed in proteins through four mechanisms: (1) direct oxidation of the side chains of amino acids; (2) covalent bonding to non-protein carbonyl compounds; (3) reaction of reducing sugar; and (4) cleavage of the peptide backbone.[Citation36] shows protein carbonyl values increased significantly (p < 0.05) with concentration of H2O2. Carbonyl content is one of the most reliable measures of protein oxidation. The most sensitive amino acids toward oxidation are heterocyclic amino acids. In addition, amino and phenolic groups of amino acids are susceptible to oxidation. Not only tryptophan, histidine, and proline but also lysine, cysteine, methionine, and tyrosine are prone to oxidation, where a hydrogen atom is abstracted from OH-, S-, or N-containing groups.[Citation37] However, compared with other studies,[Citation38,Citation39] the level of protein carbonyl values in our research was lower. The probable reason may be that Sheldrake meat protein has a lower content of heterocyclic amino acids, amino and phenolic groups of amino acids, or other sensitive amino acids, which are prone to oxidation. It is reported that formation of protein carbonyls was H2O2 dose-dependent with low FeCl3 (0.01 mM) concentration and the carbonyl contents in samples treated with high FeCl3 (0.1 mM) concentration were approximately twofold higher than those treated with a low dose of FeCl3 (0.01 mM) across the entire H2O2 concentration range.[Citation40] Therefore, the presence of high FeCl3 would activate the generation of protein carbonyls.
Figure 2. Protein carbonyls (a), sulfhydryl group contents (b), and the surface hydrophobicity (c) of non-oxidized and oxidized MP.
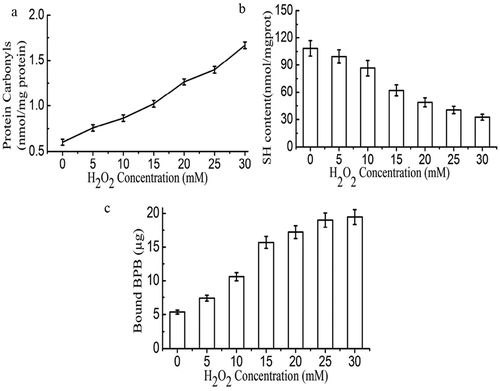
During oxidation, sulfhydryl groups form disulfide bonds which are one of the indicators of protein oxidation. shows that total sulfur content decreased significantly (p < 0.01) with increased H2O2. Sulfhydryl value of fresh myofibril protein was 108.54 nmol/mg which was reduced to 32.48 nmol/mg after being treated in HRGS with 30 mM H2O2. A sharp decrease of the total SH content in the gel samples usually indicates the formation of S–S bond.[Citation41] Therefore, the results demonstrated that the S–S bond plays a vital role in the oxidation of protein. Protein oxidation leads to exposure of some of the hydrophobic groups which enhanced protein surface hydrophobicity. Similarly, our findings showed that increased hydrogen peroxide content leads to increased protein surface hydrophobicity ().
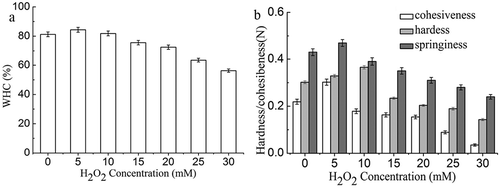
Effects of oxidation on textural properties and water holding capacity of MP gel
shows the WHC and textural properties of MP gels. WHC increased gradually and then decreased with H2O2 concentration (p < 0.05), suggesting mild oxidation might improve the WHC of MP gel (). WHC of MP gels treated in HRGS with 5–10 mmol/L H2O2 showed no significant difference. Meanwhile, MP gels treated in HRGS with 25–30 mmol/L showed a significant decrease in WHC (p < 0.05) in comparison with non-oxidized gels.
The hardness of MP gels increased gradually and then decreased with H2O2 concentration (, p < 0.05). The integrity of MPs is a key factor affecting gel properties.[Citation41] The decrease in gel strength can be attributed to deterioration of the protein structure in MP gel. Similar findings were reported by Wang et al.,[Citation42] who found heat exposure decreased the gel strength of MP gels. Higher degree of protein denaturation resulted in higher exposure of the functional groups and subsequently hardness of the MP gels. Tabarestani et al.[Citation43] suggested that weakened gel structures seen as lower hardness might be caused by the reduction of the formation of linkages between aggregated helices. Hardness is related directly to the strength of the gel structure under compression. On the other hand, springiness and cohesiveness of MP gels decreased with increased H2O2 concentration (p < 0.05).
Effects of oxidation on protein structure of myofibrillar protein gel (MP gel)
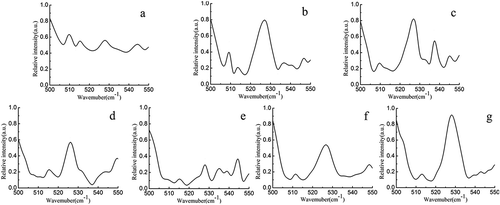
shows the Raman spectra of the non-oxidized and oxidized MP gels in the 500–550 cm−1 region. Bands in this region are assigned to disulfide and SH bonds. Non-oxidized MP gel showed a major band near 510 cm–1 and minor bands at the 516–530 and 535–545 cm–1 regions (). Band located near 510 cm−1, which is the all-gauche conformation, can be assigned to cysteine-containing protein.[Citation44] Intensity of the band at 510 cm−1 decreased gradually with increased H2O2 concentration which almost disappeared at H2O2 concentration of 15 mM. Meanwhile, bands located at 516–530 and 535–545 cm–1 have been assigned to disulfide bonds in the gauche–gauche–trans and trans–gauche–trans conformations, respectively.[Citation44] Intensity of the band assigned to gauche–gauche–trans conformations increased with H2O2 concentration (<15 mM). Meanwhile, intensity of the band assigned to trans–gauche–trans conformations remained unchanged with H2O2 concentration. Our findings are consistent with that of Bouraoui,[Citation45] who also observed increment in the relative intensity of band near 530 cm−1 in cooked codfish. It is concluded that heat treatment during cooking caused changes in disulfide bond stretching or aliphatic chain vibrations and formation of gauche–gauche–trans disulfide bonds.
Figure 4. Raman spectra (500–550 cm–1 region) of non-oxidized (a) and oxidized MP gels treated with different HRGS (5 mM H2O2 (b); 10 mM H2O2 (c); 15 mM H2O2 (d); 20 mM H2O2 (e); 25 mM H2O2 (f); 30 mM H2O2 (g)).
shows the deconvoluted and curve-fitted Raman bands of amide I in oxidized and non-oxidized MP gels. In non-oxidized MP gel, amide I band was centered at 1654 cm–1 () with a relative content of α-helix and β-sheet structures of 48.27% and 27.21%, respectively (). Protein oxidation induced formation of β-sheet structures, which was confirmed by shift of the major band to 1657 cm–1 (). Increase in H2O2 concentration up to 30 mM resulted in only 26.59% of α-helix structure indicating changes in protein secondary structures upon oxidation. Decreased α-helix structure coupled with increased β-sheet, β-turn, and random coils structures indicated protein unfolding upon oxidation. Decreased α-helix suggested the aggregation of MPs,[Citation46] while increased α-helix content at low degree of oxidation indicated reorganization of protein structures.
Figure 5. Deconvoluted and curve-fitted Raman bands of amide I of (a) and oxidized MP gels treated with different HRGS (5 mM H2O2 (b); 10 mM H2O2 (c); 15 mM H2O2 (d); 20 mM H2O2 (e); 25 mM H2O2 (f); 30 mM H2O2 (g)).
Figure 6. Relative content of the protein secondary structures (a) and normalized bands intensity (760 and 1340 cm–1) and I850/I830 intensity ratio (b) of non-oxidized and oxidized MP gel.
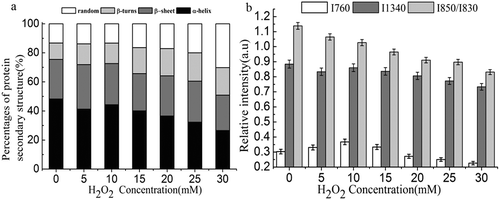
shows the normalized band intensity (760 and 1340 cm–1) and I850/I830 intensity ratio of oxidized and non-oxidized MP gels. Normalized band intensity at 760 and 1340 cm–1 decreased with increased H2O2 concentration indicating exposed tryptophan residues.[Citation47] This is consistent with aforementioned findings on increased protein surface hydrophobicity with H2O2 concentration. The doublet bands located near 830 and 850 cm−1 are assigned to the Fermi resonance ring of tyrosine residues. Ratios of I850/I830 are useful in monitoring the microenvironment around tyrosyl residues. I850/I830 values of 0.7–1.0 indicate tyrosine residues are buried within the protein network. Meanwhile, I850/I830 value of greater than 1.0 indicated exposed tyrosine residues.[Citation48] Non-oxidized MP gel had an I850/I830 value of 1.17 indicating exposed tyrosine residues. I850/I830 value decreased with increased H2O2 concentration indicating buried tyrosine residues upon oxidation.
Statistical test was done to analyze the correlation between H2O2 concentration with protein secondary structures and between protein secondary structures with physical properties of MP gels. H2O2 concentration showed negative and highly significant correlation with α-helix and β-sheet structures (p < 0.01). Moderate amount of H2O2 led to α-helix unfolding and changes of protein secondary structures to β-sheet structure. Significant positive correlation was found between β-sheet structure and textural properties of MP gel. Positive and highly significant correlation was found between the content of β-turn and random coil structures with H2O2 concentration. Excessive amount of H2O2 may destroy the β-sheet conformation forming β-turn and random coil structures. A negative correlation was found between random coil structure and WHC of gels.
Conclusion
Oxidation of Sheldrake meat resulted in changes in protein secondary structures. Upon oxidation, Sheldrake meat and MP gel demonstrated decreased WHC and increased meat toughness. Furthermore, it darkened the color of Sheldrake breast meat. Raman spectra showed protein oxidation resulted in protein unfolding, decreased α-helix structure, and increased β-sheets, β-turns, and random coils structures. In addition, protein oxidation also caused changes in physical and functional properties of Sheldrake meat. Proper methods should be developed to control protein oxidation of Sheldrake breast meat in order to preserve the good organoleptic properties.
LJFP_A_1258573_Supplementary_Grapgic.zip
Download Zip (651.9 KB)Supplemental data
Supplemental data for this article can be accessed on the publisher’s website
Funding
We acknowledge funding support from Science and Technology Program of Ningbo (2012B82017), Ministry of Science and Technology Program of China (2014GB2C220153), Modern Agricultural Technical Foundation of China (CARS-43-17), and K. C. Wong Magna Fund in Ningbo University.
Additional information
Funding
References
- Liu, C.L.; Pan, D.D.; Ye, Y.F.; Cao, J.X. 1 H NMR and Multivariate Data Analysis of the Relationship Between the Age and Quality of Duck Meat. Food Chemistry 2013, 141, 1281–1286.
- Armenteros, M.; Heinonen, M.; Ollilainen, V.; Toldrá, F.; Estévez, M. Analysis of Protein Carbonyls in Meat Products by using the DNPH-method, Fluorescence Spectroscopy and Liquid Chromatography-electrospray Ionisation-mass Spectrometry (LC–ESI–MS). Meat Science 2009, 83, 104–112.
- Estévez, M.; Ollilainen, V.; Heinonen, M. Analysis of Protein Oxidation Markers α-aminoadipic and γ-glutamic Semialdehydes in Food Proteins using Liquid Chromatography (LC)− Electrospray Ionization (ESI)− Multistage Tandem Mass Spectrometry (MS). Journal of Agricultural and FOOD Chemistry 2009, 57, 3901–3910.
- Fuentes, V.; Ventanas, J.; Morcuende, D.; Estévez, M.; Ventanas, S. Lipid and Protein Oxidation and Sensory Properties of Vacuum-packaged Dry-cured Ham Subjected to High Hydrostatic Pressure. Meat Science 2010, 85, 506–514.
- Estévez, M.; Cava, R. Lipid and Protein Oxidation, Release of Iron from Heme Molecule and Colour Deterioration during Refrigerated Storage of Liver Pâté. Meat Science 2004, 68, 551–558.
- Xiong, Y.L.; Blanchard, S.P.;Ooizumi, T.; Ma, Y. Hydroxyl Radical and Ferryl-Generating Systems Promote Gel Network Formation of myofibrillar Protein. Journal of Food Science 2010, 75, C215–C221.
- Estévez, M. Protein carbonyls in Meat Systems: A Review. Meat Science 2011, 89, 259–279.
- Herrero, A.M. Raman Spectroscopy a Promising Technique for Quality Assessment of Meat and Fish: A Review. Food Chemistry 2008, 107, 1642–1651.10.
- Zhang, D.M.; Jiang, D.P.; Yanney, M.; Zou, S.; Sygula, A. Ratiometric Raman Spectroscopy for Quantification of Protein Oxidative Damage. Analytical Biochemistry 2009, 391, 121–126.
- AOAC. Official Methods of Analysis AOAC. Gaithersburg, MD, 2000.
- Ali, S.; Zhang, W.G.; Rajput, N.; Khan, M.A.; Li C.B.; Zhou, G.H. Effect of Multiple Freeze–thaw Cycles on the Quality of Chicken Breast Meat. Food Chemistry 2015, 173, 808–814.
- Fan W.J.; Zhang Y.K.; Chen Y.C., Sun J.X.; Yi Y.W. TBARS Predictive Models of Pork Sausages Stored at Different Temperatures. Meat Science 2014, 96, 1–4.
- Sun, W.Z.; Zhao, Q.Z.; Zhao, M.M.;Yang, B.; Cui, C.; Ren, J.Y. Structural Evaluation of myofibrillar Proteins during Processing of Cantonese Sausage by Raman Spectroscopy. Journal of Agricultural and Food Chemistry 2011, 59, 11070–11077.
- Levine, R.L.; Williams, J.A.; Stadtman, E. R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology 1994, 233, 346–57.
- Cui, C.; Zhou, X.S.; Zhao, M.M.; Yang, B. Effect of Thermal Treatment on the Enzymatic Hydrolysis of Chicken Proteins. Innovative Food Science & Emerging Technologies 2009, 10, 37–41.
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical Note: A Simplified Procedure for Myofibril Hydrophobicity Determination. Meat Science 2006, 74, 681–683.
- Li, K.; Kang, Z.L.; Zou, Y.F.; Xu, X.L.; Zhou, G.H. Effect of Ultrasound Treatment on Functional Properties of Reduced-salt Chicken Breast Meat Batter. Journal of Food Science and Technology 2015, 52, 2622–2633.
- Dondero, M.; Figueroa, V.; Morales, X.; Curotto, E. Transglutaminase Effects on Gelation Capacity of Thermally Induced Beef Protein Gels. Food Chemistry 2006, 99, 546–554.
- Chen, H.Y.; Han, M.Y. Raman Spectroscopic Study of the Effects of Microbial Transglutaminase on Heat-induced Gelation of Pork myofibrillar Proteins and its Relationship with Textural Characteristics. Food Research International 2011, 44, 1514–1520.
- Xu, X.L.; Han, M.Y.; Fei, Y.; Zhou, G.H. Raman Spectroscopic Study of Heat-induced Gelation of Pork myofibrillar Proteins and its Relationship with Textural Characteristic. Meat Science 2011, 87, 159–164.
- Quevedo, R.;Valencia, E.; Cuevas, G.; Ronceros, B.; Pedreschi, F.; Bastias, J. M. Color Changes in the Surface of Fresh Cut Meat: A Fractal Kinetic Application. Food Research International 2013, 54, 1430–1436.
- Mancini, R.A.; Hunt, M. Current Research in Meat Color. Meat Science 2005, 71, 100–121.
- Zhang, J.; Wang, Y.; Pan, D. D.; Cao, J. X.; Shao, X. F.; Chen, Y. J.; Sun, Y. Y.; Ou, C. R. Effect of Black Pepper Essential Oil on the Quality of Fresh Pork During Storage. Meat Science, 2016, 117, 130–136.
- Karamucki, T.; Jakubowska, M.; Rybarczyk, A.; Gardzielewska, J. The Influence of Myoglobin on the Colour of Minced Pork Loin. Meat Science 2013, 94, 234–238.
- Liu, Z.; Xiong, Y.L.; Chen, J. Morphological Examinations of Oxidatively Stressed Pork Muscle and Myofibrils upon Salt Marination and Cooking to Elucidate the Water-binding Potential. Journal of Agricultural and Food Chemistry 2011, 59, 13026–13034.
- Delles, R.M.; Xiong, Y.L. The Effect of Protein Oxidation on Hydration and Water-binding in Pork Packaged in an Oxygen-enriched Atmosphere. Meat Science 2014, 97, 181–188.
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water Distribution and Mobility in Meat during the Conversion of Muscle to Meat and Ageing and the Impacts on Fresh Meat Quality Attributes — A Review. Meat Science 2011, 89, 111–124.
- Bao, Y.L.; Ertbjerg, P. Relationship Between Oxygen Concentration, Shear Force and Protein Oxidation in Modified Atmosphere Packaged Pork. Meat Science 2015, 110, 174–179.
- Clausen, I.; Jakobsen, M.; Ertbjerg, P.; Madsen, N.T. Modified Atmosphere Packaging Affects Lipid Oxidation, myofibrillar Fragmentation Index and Eating Quality of Beef. Packaging Technology & Science 2009, 22, 85–96.
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen Packaging Atmosphere Influences Protein Oxidation and Tenderness of Porcine longissimus dorsi During Chill Storage. Meat Science 2007, 77, 295–303.
- Lagerstedt, Å.; Lundström, K.; Lindahl, G. Influence of Vacuum or High-oxygen Modified Atmosphere Packaging on Quality of Beef M. longissimus Dorsi Steaks after Different Ageing Times. Meat Science 2011, 87, 101–106.
- Soyer A., Ozalp B., Dalmi§, U.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chemistry 2010, 120,1025–1030.
- Zhang, W.G.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Critical Reviews in Food Science and Nutrition 2013, 53, 1191–1201.
- Zhang, W.G.; Xiao, S.; Lee, E.J.; Ahn, D.U. Consumption of Oxidized oil Increases Oxidative Stress in Broilers and Affects the Quality of Breast Meat. Journal of Agricultural and Food Chemistry 2010, 59, 969–974.
- Burcham, P.C.; Kuhan, Y.T. Introduction of Carbonyl Groups into Proteins by the Lipid Peroxidation Product, Malondialdehyde. Biochemical and Biophysical Research Communications 1996, 220, 996–1001.
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein Oxidation in Muscle Foods: A Review. Molecular Nutrition & Food Research 2011, 55, 83–95.
- Doorn, J.A.; Petersen, D.R. Covalent Modification of Amino Acid Nucleophiles by the Lipid Peroxidation Products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chemical Research in Toxicology 2002, 15, 1445–1450.
- Aewsiria, T.; Benjakula, S.; Visessanguan, W. Functional Properties of Gelatin from Cuttlefish (Sepia pharaonis) Skin as Affected by Bleaching using Hydrogen Peroxide. Food Chemistry 2009, 115, 243–249.
- Xue, M.; Huang, F.; Huang, M.; Zhou, G.H. Influence of Oxidation on myofibrillar Proteins Degradation from Bovine via μ-calpain. Food Chemistry 2012, 134, 106–112.
- Park, D.; Xiong, Y.L.; Alderton, A.L. Concentration Effects of Hydroxyl Radical Oxidizing Systems on Biochemical Properties of Porcine Muscle myofibrillar Protein. Food Chemistry 2007, 101, 1239–1246.
- Chang, T.; Wang, C.J.; Yang, H.; Xiong, S.B.; Liu, Y.M.; Liu R. Effects of the Acid- and Alkali-Aided Processes on Bighead Carp (Aristichthys nobilis) Muscle Proteins. International Journal of Food Properties 2016, 19, 1863–1873.
- Wang, R.R.; Pan, X.J.; Peng, Z.Q. Effects of Heat Exposure on Muscle Oxidation and Protein Functionalities of Pectoralis Majors in Broilers. Poultry Science 2009, 88, 1078–1084.
- Tabarestani, H.S.; Sedaghat, N.; Jahanshahi, M.; Motamedzadegan A.; Mohebbi M. Physicochemical and Rheological Properties of White-Cheek Shark (Carcharhinus dussumieri) Skin Gelatin. International Journal of Food Properties 2016, 19, 2788–2804.
- Li-Chan, E.C.Y. The Applications of Raman Spectroscopy in Food Science. Trends in Food Science & Technology 1996, 71, 361–370.
- Bouraoui, M.; Nakai, S.; Li-Chan, E. In Situ Investigation of Protein Structure in Pacific Whiting Surimi and Gels using Raman Spectroscopy. Food Research International 1997, 30, 65–72.
- Dihort-García, G.; Tolano-Villaverde, I.J.; Ezquerra-Brauer, J.M.; OcañoHiguera, V.M.; Ramírez de León, J.A.; Torres-Arreola W.; Marquez-Rios, E. Effects of pH and Sodium Chloride on the Gelling Properties of a Protein Concentrate Obtained from Jumbo Squid Mantle (Dosidicus Gigas). International Journal of Food Properties 2016, 19, 314–325.
- Tu, A. T. Use of Raman Spectroscopy in Biological Compounds. Journal of Chinese Chemical Society 2003, 50, 1–10.
- Badii, F.; Howell, N.K. Fish Gelatin: Structure, Gelling Properties and Interaction with Egg Albumen proteins. Food Hydrocolloids 2006, 20, 630–640.