ABSTRACT
Real beef soup samples were separately supplemented with 5-nucleotides extracted from mushroom before and after extraction of soluble sugars (NM and NMs, respectively) and yeast extract. Headspace volatiles and flavour palatability of each sample were compared with beef soup supplemented with 5-IMP and control sample. Each investigated enhancer revealed significant release of thiol-containing compounds. 3-Mercapto-2-butanone was the major identified compound in the headspace volatiles of all samples. However, the sample supplemented with 5-IMP + NM showed the highest release of this compound followed by S-NM compared with other samples. The high palatability of sample S-NM may be correlated to the high content of 2-methyl-3-furanthiol.
Introduction
Flavour is the main factor affecting the acceptability of meat by consumers. Flavour of the cooked meat is predominantly derived from the well-known Maillard reaction and the degradation and/or oxidation of lipid.[Citation1] However, only limited compounds are considered as potent odourants of meat aroma depending on their odour threshold and concentration in the food.[Citation2] Perception of aroma depends not only on nature and concentration of volatile compounds but also on their availability that can be modulated by physicochemical interaction with other food components.[Citation3] Therefore, the variations in the composition or constituents of the food product affect the volatility of aroma compounds.
Umami substances such as monosodium glutamate (MSG) and 5-nucleotides are effective enhancers and positive flavour modulators in food products.[Citation4] Therefore, they are used worldwide as additives to impart umami taste to the food processed in industrial scale as well as to home and restaurant-made food.[Citation5,Citation6] Umami effect is resulted by joined stimulation of added and natural umami substances.[Citation6] Synergy of umami substances has been widely studied and confirmed.[Citation7] Flavour nucleotides, 5-disodium inosinate (5-IPM) and 5-disodium guanylate (5-GMP), are disodium salts containing compounds. They impart slight salty taste and possess broth-like and meaty notes.[Citation7]
Most of the reported studies, dealing with the effect of umami compounds on food flavour, had been concerned with their effect on palatability and pleasantness of simple food model systems.[Citation8] The effect of rising the added amount of umami compounds on the palatability of various food products had been evaluated.[Citation6] Ventanas et al.[Citation4] designed simple model systems including meat broth and two meat odour active compounds (1-octen-3-ol and 2, 6 dimethylpyrazine). The authors evaluated the effect of added umami compounds on the perceived odour and flavour in the model systems and also their effect on the perception of the odour active compounds. However, these previous studies used pure chemical umami compounds.
Umami compounds with a pleasant savoury taste produced by glutamate, ribonucleotides, and chemicals occur naturally in many foods including mushroom, meat, fish, cheese, and yeast.[Citation5,Citation9] Edible mushroom, with its umami taste, is considered a good source of umami enhancers and was reported as a promising potential for application in food spices industry.[Citation10] Agaricus bisporus (A. bisporus) was reported amongst the most widely cultivated edible mushroom species.[Citation11] The taste components such as soluble sugars, free amino acids, and 5—nucleotides of A. bisporus had been reported.[Citation9,Citation12] On the other hand, the economic production of yeast compared with other organisms has made it the organism of choice for the production of extracts rich in 5—nucleotides. Yeast extracts are available in commercial scale and extensively used by food industries as flavouring agents.[Citation13]
Meat products are complex systems including non-volatile compounds such as protein, fat, and carbohydrates in addition to endogenous umami compounds. During processing, several interactions can take place between these compounds and the generated endogenous volatile compounds. These interactions affect the perception of the volatile compounds in beef products. Therefore, the results of simple beef model system do not give accurate indication for the release and perception of volatile compounds from a real beef product.[Citation14]
Evaluation of the effect of umami compounds, such as 5-nucleotides, on palatability of foods is usually carried out by using soups.[Citation15] Therefore, the main objective of the present study was to evaluate the enhancement efficiency of edible sources rich in flavour 5-nucleotides, such as mushroom and yeast extracts, to be used as natural enhancers in real beef soup. To achieve this objective, flavour 5-nucleotides in each extract were quantified. The effect of supplementation of beef soup with each extract on its palatability and headspace volatiles was evaluated compared with unsupplemented soup (control sample) and soup supplemented with pure chemical 5-IMP.
Materials and methods
Materials
Mushroom (Agaricus bisporus) was purchased from Poloshum Mushroom Co. (Dokki, Egypt). Yeast extract powder was purchased from Lab M Limited (Topley House, 52 Wash Lane, Bury, Lancashire BL96AS, United Kingdom). Beef meat was purchased from local market on the day of slaughter. Flavour 5-nucleotides; 5-inosine monophosphate (5-IMP), 5-guanosine monophosphate (5-GMP), and 5-xanthosine monophosphate (5-XMP) were purchased from Sigma-Aldrich Chemical Co. s (St. Louis, Mo, USA). Authentic volatile compounds and standard n-paraffin (C6—C22) were purchased from Sigma-Aldrich Co. s (St. Louis, Mo, USA). All other chemicals were at least of analytical grade.
Extraction of 5ʹ-nucleotides
5ʹ-nucleotides were extracted and analysed as described by Taylor et al.[Citation16] Air-dried mushroom powder (20 g) was extracted with 500 ml of deionised water. This suspension was heated to boiling for 1 min, cooled, and then centrifuged at 4500 rpm for 15 min. The extraction was repeated once with 300 ml of deionised water. The combined filtrate containing 5-nucleotides with soluble sugar (NMs) was rotary evapourated to a final volume of 100 ml and filtered.[Citation12] For preparation of 5-nucleotids extract free from soluble sugar (NM), air-dried mushroom (Agaricus bisporus) powder (30 g) was extracted with 500 ml of 80% aqueous ethanol. This suspension was shaken for 45 min at room temperature and filtered through a Whatman No. 4 filter paper. The residue was washed five times with additional 250 ml portions of 80% ethanol. The precipitate was dried again and used to extract the nucleotides as mentioned before. From each extract, 100 μl were taken out to be analysed using a high performance liquid chromatography (HPLC). The reminder of each extract was subjected to freeze drier (Snijders Scientific b.v. Model L45 Fm-Ro, Tilburg-Holand).
High Performance Liquid Chromatography (HPLC) analysis
The HPLC system was consisted of Agilent 1100 series (Waldborn, Germany), quaternary pump (G1311A), Degasser (G1322A), Thermostated Auto Sampler (G1329A), variable wave length detector (G1314A), and Zorbax 300SB C18 column (Agilent Technologies, USA) (250 × 4. 8 × 5 um). Injection (20 µl) was carried out at wave length 254 nm. The mobile phase was 0.5 M KH2PO4/H3PO4 (pH 4.0, Adwa Instruments Kft Co., Romania) at a flow rate of 1 ml/min (Yang, Lin, & Mau, 2001). Each 5-nucleotide was quantified by the calibration curves of the authentic flavour 5- nucleotides.
Preparation of beef soup
One kg minced beef (1% fat) was mixed with sodium chloride (10 g) and onion (50 g). No other ingredients were added to avoid the possible uncontrolled variability which might affect the palatability of soup samples. The soup was cooked (40 min. in 3 L boiling water), cooled down, filtered twice in order to remove meat solids, and stored at 4°C (overnight) until preparation of the samples supplemented with enhancers. Before addition of the investigated enhancers, the soup was let at room to research room temperature (30°C) and then divided into six equal samples, with the first considered as control (SC, unsupplemented with enhancer) sample. The investigated umami compounds 5-IMP, NM, NMs, yeast extract (YE), and mixture of 5-IMP + NM (ratio, 1:1) were added (0.015% w/w) separately to the other five beef soup samples and stirred with magnetic stir. All samples were subjected to sensory analysis as well as isolation and gas chromatography–mass spectrometry analysis (GC–MS) of the headspace volatiles.
Isolation of meat soup volatiles
Each sample (100 ml) under investigation with 9.72 µg 3- heptanol (internal standard) was placed in a 100 mL headspace glass vial sealed with a PTFE faced silicon septum (Supelco, Bellefonte, PA, USA). The volatile compounds in headspace of the samples were extracted by solid phase microextraction (SPME) (Supleco, 57348-U, Bellefonte, PA, USA), with coated fibre of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS)(coating thickness:50/30 µm). The SPME fibre was exposed to the headspace of each sample for 1 h at 65 ºC, and then it was inserted into the GC injection port for desorption (260ºC/10 min in splitless mode). Before use, the fibre was conditioned in the injection port of GC (270ºC/1 h) as recommended by manufacture.
GC–MS analysis
GC–MS analysis was performed by using a gas chromatography (Hewlett–Packard model 5890) coupled to a mass spectrometer (Hewlett–Packard-MS (5970). A fused silica capillary column DB5 (60 m × 0.32 mm i.d. × 0.25 µm film thickness) was used. The oven temperature was maintained initially at 50°C for 5 min and then programmed to rise to 250°C at a rate of 4°C/min. Helium was used as the carrier gas at flow rate of 1.1 ml/min. The injection was conducted in the splitless mode for 5 min at 260°C. Mass spectra in the electron impact mode (EI) were obtained at 70 eV and scan m/z range from 39 to 400 amu. The volatile compounds were identified using both the mass spectra of National Institute of Standard and Technology (NIST) database and comparison with those of authentic compounds and published data.[Citation17–Citation19] Retention indices of the separated volatile compounds were determined using homologous series of normal n-alkanes,C6-C22. The relative areas of the volatile components identified were determined by comparing their peak areas to that of 3-heptanol, an internal standard compound, on total ion chromatograms of GC–MS.
Sensory analysis
Odour sensory analysis was conducted to evaluate the palatability of the investigated soup samples supplemented with the enhancers as well as control sample (unsupplemented with enhancers). All samples were warmed up to 70°C before evaluation, put (20 ml each) in coded warmed glass bakers, covered by petri dishes and kept warm until tested.[Citation6] The evaluation was carried out by 20 members (12 female, 8 male) drawn from Food Industry and Nutrition Division, National Research Centre, Cairo, Egypt. The panelists participated occasionally in hedonic tests. They were asked to rank the six samples according to the degree of liking on bar diagrams expressed in the range of 1 (least like)–9 (most like). The sensory evaluation was carried out according to ISO 8589.[Citation20] Each sample was prepared in triplicate.
Statistical analysis
Data were analysed using one-way analysis of variance (ANOVA), and least significant difference (LSD) was performed to determine any significant difference amongst various treatments that were used to compare the means. Differences were considered to be significant at p < 0.05.
Results and discussion
Content of flavour 5-nucleotides in mushroom (Agaricus bisporus) and yeast extract
5-Inosine monophophate (5-IMP), 5-guanosine monophosphate (5-GMP), and 5-xanthosine monophosphate (5-XMP) are the flavour 5-nucleotides.[Citation9] As shown in , 5-XMP was detected only in the 5-nucleotides (NMs and NM) extracted from mushroom. Total content of 5-nucleotides was higher in NM extract (2.73 mg/g) than in NMs (1.71 mg/g) and YE (1.59 mg/g). However, in all samples, 5-IMP comprised higher concentration compared with 5-GMP, and this finding is in agreement with previous studies.[Citation10] The total content of 5-IMP and 5-GMP in sample NM was approximately in the same range as that reported in a previous study,[Citation12] while, in the same study, the content of 5-XMP was higher than that found in the present study. This difference may be attributed to many factors such as conditions of growth, post-harvest conditions, and maturity.
Table 1. Content of flavour 5-nucletiodes in mushroom and yeast extract.
Volatile compounds in beef soup samples
The main volatile compounds identified in the headspace volatiles of beef soup samples (supplemented with different investigated enhancers) and control sample (unsupplemented with enhancers) are cited in . A total of 66 volatile compounds were positively identified in the investigated samples, and most of them have been previously reported as key active compounds of cooked meat.[Citation18,Citation21] Generally, flavour of beef products are derived from the complex interaction of flavour precursors such as amino acids, peptides, sugars, thiamine, nucleotides, and products of lipid oxidation.[Citation21–Citation23]
Table 2. Volatile compounds in headspace of beef soup samples supplemented and unsupplemented (SC) with enhancers (5-nucleotides).
Control sample
Concerning the volatile compounds in the control sample, only 34 compounds could be identified, most of them are lipid-derived compounds. Methanthiol (1), dimethyl sulfide (2), 2-methyl-3-thiophenthiol (37), 2-acetyl—2, 5- dimethylthiophene (49), Bis- (2-methyl-3-furyl) disulfide (63), 1- (2-furylmethyldithio) propanone (64) and 2- (2- furylmethyldithio) −2-butanone (65) are the identified sulphur containing compounds in headspace of control sample. Sulphur-containing compounds are important in cooked beef flavour since some heterocyclic sulphur-containing compounds were described as possessing meat-like aromas.[Citation25] These compounds could be generated from either thermal degradation of cysteine or cystine[Citation26] through the interaction between carbonyl compounds and sulphur-containing amino acids[Citation27] or as a result of thermal decomposition of thiamine.[Citation28]
Methanthiol (1), which smelt like cooked cabbage,[Citation29] was identified in considerable concentration (). This compound was found in cooked ground beef[Citation30] and boiled beef[Citation29] and reported as one of the character impact odour of stewed beef juice.[Citation31] It can be perceived at very low concentration due to its low threshold (0.2 μg /kg).
3-Mercapto-2-butanone (14) was the major identified compound in the volatiles of control sample (). It was described to have cooked rise meat aroma note and considered as the impact compound of process flavourings prepared from enzymatic hydrolyzed beef protein,[Citation18] hydrolyzed soybean protein[Citation24,Citation32–Citation34] with cysteine, thiamine, and taurine. Mercaptoketones that produce important meat-like volatiles can be prepared by the reaction of dicarbonyls, 2, 3-butandione (3) and 2, 3- pentandione (9) (sugar degradation products) with hydrogen sulfide (Strecker degradation products of cysteine).[Citation35]
Acetyl thiazole (33) and benzo thiazole (47) were found in the aroma of the control sample (). These compounds were reported amongst the dominant sulphur-containing compounds in cooked beef meat.[Citation36] They are thermal degradation products of cysteine either alone or in the presence of reducing sugar such as ribose or xylose[Citation37] and can be formed by heat degradation of thiamine.[Citation38]
The two furans, 2- furfural (16) and 4- hydroxy −5- methyl −3- (2 H) furanone (20), were detected in small concentration in the headspace of SC sample (). These compounds are considered as the secondary intermediates for production of the thiol containing volatile compounds.[Citation39] Compound 20 could be generated from the degradation of ribonucleotides such as ribose −5- phosphate or from sugar degradation.[Citation29]
As shown in , the lipid degradation products identified in the present study were including 16 aldehydes, 7 ketons, 7 alcohols, and 4 alkyl furans. These compounds are mostly generated from degradation and/or thermal oxidation of unsaturated and saturated fatty acids[Citation40] in food materials. Hexanal (13), benzaldehyde (28), nonanal (41), decanal (45), 2, 4—nonadienal (46), (E) −2- decanal (48), undecanal (51), (E) −2- undecenal (55), and tridecanal (61) were the aldehydes found in the headspace of control sample (). Most of these compounds were identified in boiled beef[Citation29] and cooked beef.[Citation36] Aldehydes are believed to not only contribute to the aroma of food including beef[Citation41] but also react with other compounds to produce flavour through amino carbonyl reaction. 3- Hydroxy −2- butanone (10), 1- nonene −3- one (39), and decanone (44) were the only ketones found in headspace volatiles of control beef soup. These compounds have been implicated in the buttery aroma of cooked meats.[Citation42] 1- Octene −3- ol (29), 2- ethyl-1-hexanol (34), decanol (50), undecanol (56), and 1- dodecanol (58) were the identified alcohols in headspace of control sample (). Compounds 29 and 34 were found in headspace of cooked beef.[Citation23] Compound 34 was the predominant alcohol in cooked meat.[Citation36] The straight chain primary alcohol has been reported to have greenish, woody, and fatty floral.[Citation43] Pentyl furan, which had been identified in the volatiles of different beef products,[Citation23,Citation29,Citation30] was found in the volatiles of the control sample ().
The three disulfide compounds, bis (2- methyl-3- furyl) disulfide (63), 1- (2- furylmethyldithio) propanone (64), and 2- (2- furylmethylditio) 2- butanone (65), were detected in the volatiles of control sample. These compounds possess potency savoury aromatics, and they are generally produced from dehydrogenation amongst furanthiols, thiophenethiols, and α- mercaptoketones.[Citation44]
Beef soup samples supplemented with 5-nucleotides
Addition of 5-nucleotides; 5-IMP, NMs, NM, YE, and 5-IMP + NM separately to the beef soup revealed a significant release of most of the identified compounds (). The release of the total volatiles of the investigated samples was in the order of S-NM > S-5-IMP + NM > S-NMs > S-5-IMP > S-YE > SC. However, some compounds such as hexanal (13), 2- furfural (16), benzaldehyde (28), 1- nonene −3- one (39), 2, 4- nonadienal (46), benzo thiazole (47), and benzyl furan (62) showed opposite trend.
The ability of nucleotides to release selected volatile compounds (salting-out phenomenon) had been confirmed early.[Citation45] Salting-out phenomenon has been well stablished,[Citation46] when salt concentration increases water molecules are bound by salt ions, decreasing the ‘free water’ available for volatile compounds solubilisation which enhances flavour release.[Citation47] In the present study, NaCl was added at constant concentration to all investigated samples. Salting-out phenomenon of NaCl has been demonstrated using sensory techniques for model system containing odour active compounds typically present in meat products.[Citation4] Previous studies showed that MSG, IMP, and GMP enhance the perception of other taste active compounds including NaCl.[Citation48] In contrast, Ventanas et al.[Citation4] reported that the combination of NaCl with umami compounds revealed modulator effect rather than potentiator effect.
The effect of the investigated enhancers on the release or retention of some selected key aromatic compounds in headspace of beef soup is discussed as follows to clarify their potentiator properties.
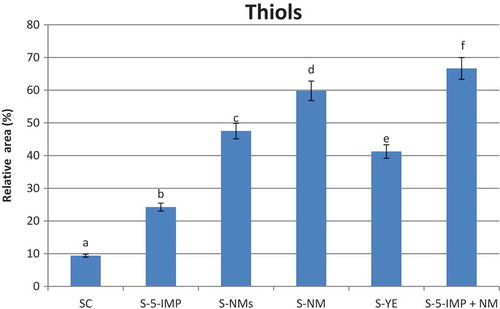
Thiols
Thiols are very important volatile compounds, as many of them are considered as key odourant of beef aroma.[Citation18,Citation21,Citation22] As shown in , all thiol-containing compounds showed variable release by addition of different investigated enhancers (5-IMP, NMs, NM, YE, and 5-IMP + NM). The release of volatile compounds depends on their physicochemical characteristics,[Citation3] and therefore, the release of the thiol-containing compounds (hydrophilic, more soluble in water compared with other compounds) from the matrix was higher. Compound 14, the major identified compound in control sample (SC), showed more than two fold higher releases in sample S-5-IMP than its concentration in SC sample. As shown in , the highest release of this compound was found in sample S-5-IMP + NM followed by S-NM, S- NMs, S-YE, S-5-IMP, and SC. The total yield of furan thiols, 2-methyl-3-furanthiol (19) and 2- furan methanthiol (24), the most potent odourants of boiled beef aroma,[Citation21] showed the highest value in sample S-NM followed by S-5-IMP + NM () compared with other investigated samples. These two compounds were absent in control sample.
The higher release of the thiol compounds from sample S-NM compared with S-5-IMP may be correlated to the synergy effect of 5-GMP and 5-XMP that comprised 0.27 mg/g and 0.21 mg/g, respectively, of the nucleotide (NM) extracted from mushroom (). The enhancement effect of 5-GMP was found to be stronger than MSG.[Citation30] On the other hand, the lower content of 5-IMP, 5-GMP, and 5-XMP in the nucleotide extract NMs () gave rise to a decrease in the released thiols in this sample compared with S-NM. However, the salting-out effect of the soluble sugar[Citation49] present in NMs may compensate some of this decrease, while simple sugar interaction with water increases the concentration of flavour compounds in remaining ‘free water’.
The decrease in thiol-containing compounds in headspace of sample S-YE () may be attributed to the lower content of 5-IMP and 5-GMP and absence of 5-XMP in the yeast extract () or to the presence of yeast micro molecules (polysaccharides). The ability of yeast macromolecules to bind aroma compounds was mainly investigated in model solutions.[Citation50] However, no literatures were found regarding the more complex food matrices such as beef products. Effect of commercial yeast autolysate was investigated[Citation51] on a model solution of five volatile compounds, ethyl octanoate, linalool, 2-phenylethanol, β–ionone, and octanoic acid. The results revealed poor effect in headspace concentration of ethyl octanoate and linalool. In contrast, the headspace concentration of phenylethanol, at 20°C and pH 4, showed a significant decrease. It is generally known that colloids, especially polysaccharides, can oppositely affect the perception of aroma substances either reducing or increasing their volatility.[Citation52]
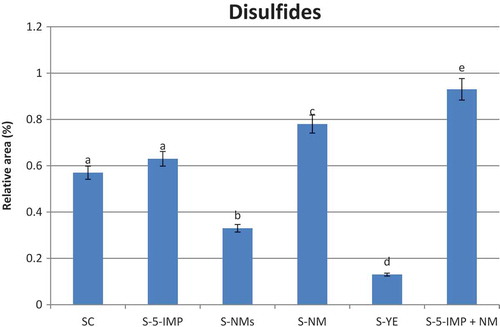
Disulfides
Four disulfides were detected in the present study: dimethyl disulfide (12), bis (2- methyl-3- furyl) disulfide (63), 1 (2- furylmethyldithio) propanone (64), and 2 (2- furylmethyldithio) 2- butanone (65). Compound 12 is a degradation product of methionine,[Citation24] and it is responsible for onion- and cabbage-like odours.[Citation19] Compounds 63–65 possess savoury aromatic note and are generally formed by the dehydrogenation amongst furanthiol, thiophenthiol, and α—mercaptoketones.[Citation44] It is well known that compound 19 is easily oxidised to compound 63. As shown in , supplementation of beef soup with different enhancers revealed variable effect on the total release of the disulfide compounds. Insignificant (p > 0.05) increase of the total sulfide compounds was found in headspace of sample S-5-IPM compared with SC, whereas, the synergy effect between 5-IMP and the flavour nucleotides present in NM extract revealed a significant increase in headspace of sample S-5-IMP + NM. In contrast, the highest retention of the disulfides was found in sample S-YE followed by S-NMs. According to previous studies,[Citation50] the major binding sites for aroma compounds in yeasts are connected with lipidic fraction of yeast walls[Citation52] and mannoproteins,[Citation53] and therefore the intensity of such interactions is generally higher for the most hydrophobic compounds. Our finding confirms those reported by other authors[Citation51,Citation52] who stated that the less polar compounds are mostly involved in the interaction with yeast walls.
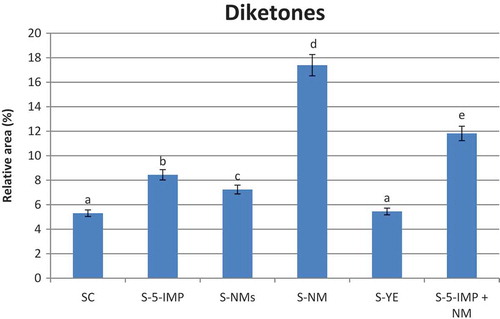
Diketones
The two diketones; 2,3—Butandione (3) and 2,3—pentandione (9), were identified in the present study (). They are degradation products of sugar and considered as important intermediate in the formation of the two mercaptoketone compounds 14 and 21 by the interaction with hydrogen sulfide.[Citation35] These diketones were reported amongst the meat odour active compounds and described to have a buttery note.[Citation1] As shown in , all the investigated enhancers favoured the release of these compounds except for YE. Their high release in the headspace of samples S-NM and S-5-IMP + NM may be attributed to the synergy effect between 5-IMP, 5- GMP, and 5-XMP in the two investigated enhancers NM and 5-IMP + NM.
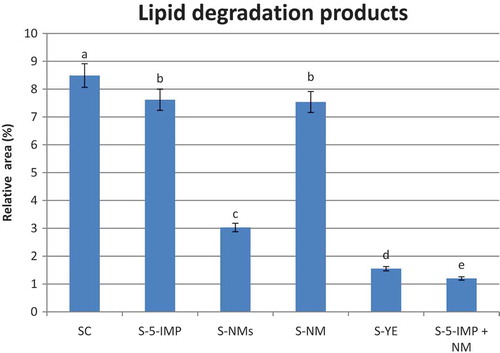
Lipid degradation products
According to the results observed in , it is obvious that supplementation of beef soup with 5-nucleotides gave rise to retention of the lipid degradation volatile compounds. No significant (p > 0.05) differences were found between the behaviour of the two soup samples supplemented with 5-IMP and NM. These two samples showed the least perception of lipid degradation products, compared with other investigated samples. Beef soup supplemented with 5-IMP + NM showed the highest retention of the lipid derived compounds followed by S-YE sample. This unexpected result may be related to the differences amongst the chemical structure of the chemical groups which are consisting the lipid degradation products such as aldehydes, ketones, alcohols, and alkylated furans.
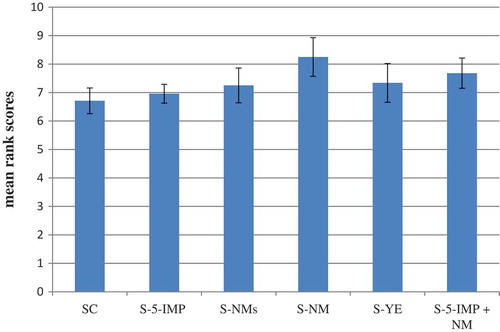
Sensory evaluation
Sensory analyses are very much necessary to evaluate the aforementioned physicochemical interactions and influence of added enhancers on the beef soup flavours. The effect of supplementation of beef soup with mushroom or yeast extracts, as potential sources of natural enhancers, on its palatability was investigated. The palatability of each sample was compared with that of beef soup supplemented with 5-IMP and control sample. As shown in , sample S-5IMP showed insignificant rise (p > 0.05) in palatability response, whereas the other investigated samples showed significant (p < 0.05) increase of hedonic response. Sample S-NM showed the highest degree of liking () compared with other investigated samples. As shown in , the headspace volatiles of sample S-NM comprised the highest content of 2-methyl-3-furanthiol (19), 2-furan methanthiol (24), and 2-methyl −3(methylthio) furan (26), in addition to the high concentration of 3-Mercapto-2-butanone (14). These compounds were reported as active compounds in beef-like aroma.[Citation17] Compounds 19 and 24, the most potent odourants of beef flavour,[Citation21] were reported amongst the key aroma compounds in boiled beef.[Citation29] The high palatability response of sample S-5-IMP + NM may be correlated to the higher content of the disulfide compounds, which possess savoury aromatic note,[Citation44] in its headspace compared with the other samples (), in addition to the presence of the potent odourants of beef flavour ().
Conclusion
The present study highlights the possibility of using edible sources, which contain significant quantities of flavour 5-nucleotides, as potential sources of flavour enhancers instead of the pure chemicals that are expensive and may be less safe. Most reported studies dealing with the enhancing effect of flavour enhancers on volatiles and palatability of food products had been carried out on simple model systems. The present study is the first that evaluated the enhancing effect of natural enhancers on real food product. Results of sensory analysis confirmed the physicochemical interaction between the components of beef soup, including the aroma compounds, and flavour 5-nucleotides extracted from mushroom and yeast. 5-IMP was the major flavour 5-nucleotide identified in the investigated extracted nucleotides. However, the pure 5-IMP showed less enhancing effect compared with the investigated samples S-NM and S-5-IMP+NM, which may be correlated to the synergistic effect between 5-IMP, 5-GMP, and 5-XMP in the extracted nucleotides.
References
- Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas Chromatography–Olfactometry in Food Flavour Analysis – Review. Journal of Chromatography A 2008, 1186, 123–143.
- Schieberle, P.; Grosch, W. Evaluation of the Flavour of Wheat and Rye Bread Crusts by Aroma Extract Dilution Analysis. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung 1987, 185, 111–113.
- Voilley, A.; Etiévant, P. Flavour in Food; Woodhead publishing limited: Cambridge, England, 2006.
- Ventanas, S.; Sari Mustonen, S.; Puolanne, E.; Tuorila, H. Odour and Flavour Perception in Flavoured Model Systems: Influence of Sodium Chloride, Umami Compounds and Serving Temperature. Food Quality and Preference 2010, 21, 453–462.
- Yamaguchi, S. Basic Properties of Umami and Its Effects on Food Flavour. Food Reviews International 1998, 14(2–3), 139–176.
- Baryłko-Pikielna, N.; Kostyra, E. Sensory Interaction of Umami Substances with Model Food Matrices and Its Hedonic Effect. Food Quality Preference 2007, 18, 751–758.
- Ninomiya, K. Umami: A Universal Taste. Food Reviews International 2002, 18(1), 23–38.
- McCabe, C.; Rolls, E.T. Umami: A Delicious Flavour Formed by Convergence of Taste and Olfactory Pathways in the Human Brain. European Journal of Neuroscience 2007, 25, 1855–1864.
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Non-Volatile Taste Components of Several Commercial Mushrooms. Food Chemistry 2001, 72(4), 465–471.
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent Developments on Umami Ingredients of Edible Mushrooms - A Review. Trends in Food Science and Technology 2013, 33, 78–92.
- Chang, S.T. World Production of Cultivated Edible and Medicinal Mushrooms in 1997 with Emphasis on Lentinus Edodes (Berk.) Sing, in China. International Journal of Medicinal Mushrooms 1999, 1(4), 1–7.
- Tseng, Y.H.; Mau, J.L. Contents of Sugars, Free Amino Acids and Free 5ʹ-Nucleotides in Mushrooms, Agaricus bisporus, during Post-Harvest Storage. Journal of the Science of Food and Agriculture 1999, 79, 1519–1523.
- Nagodawithana, T. Yeast-Derived Flavours and Flavour Enhancers and Their Probable Mode of Action. Food Technology 1992, 46, 138–144.
- Fadel, H.H.M.; Taher, M.S.; Gad, A.M.; Kandil, E.M.; Abd El-Aleema, F.S. Flavour Release from a Banana Soft Drink Complex Model System: Evaluation of the Efficiency of Different Adsorbents for Trapping the Released Volatiles during Storage. International Journal of Food Properties 2015, 18, 796–807.
- Roininen, K.; Lahteenmaki, L.; Tuorila, H. Effect of Umami Taste on Pleasantness of Low-Salt Soups during Repeated Testing. Physiology and Behavior 1996, 60, 953–958.
- Taylor, M.W.; Hershey, R.A.; Levine, R.A.; Coy, K.; Olivelle, S. Improved Method of Resolving Nucleotides by Reverse-Phase High Performance Liquid Chromatography. Journal of Chromatography A 1981, 219, 133–139.
- Baek, H.H.; Kim, C.J.; Ahn, B.H.; Nam, H.S.; Cadwallader, K.R. Aromas Extract Dilution Analysis of a Beef-Like Process Flavour from Extruded Enzyme-Hydrolyzed Soybean Protein. Journal of Agricultural and Food Chemistry 2001, 49, 790–793.
- Song, H.; Xia, L. Aroma Extract Dilution Analysis of a Beef Flavour Prepared from Flavour Precursors and Enzymatically Hydrolyzed Beef. Flavour and Fragrance Journal 2008, 23(1), 185–193.
- Wu, C.-M.; Wang, Z. Volatile Compounds in Fresh and Processed Shiitake Mushrooms (Lentinus Edodes Sing). Food Science and Technology Research 2000, 6(3), 166–170.
- ISO 8589. Sensory Analysis-General Guidance for the Desigh of test rooms. International Standardization Organisation, Geneva; 1998.
- Song, S.; Zhang, X.; Hayat, K.; Huang, M.; Liu, P.; Karangwa, E.; Gu, F.; Jia, C.; Xia, S.; Xiao, Z.; Niu, Y. Contribution of Beef Base to Aroma Characteristics of Beef-Like Process Flavour Assessed by Descriptive Sensory Analysis and Gas Chromatography–Mass Spectrometry and Partial Least Squares Regression. Journal of Chromatography A 2010, 1217, 7788–7799.
- Ba, V.H.; Touseef, A.; Inho, H. Significant Influence of Particular Unsaturated Fatty Acids and pH on the Volatile Compounds in Meat-Like Model Systems. Meat Science 2013, 94, 480–488.
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of Headspace Solid Phase Microextraction (HS-SPME) for Gas Chromatography Mass Spectrometry (GC–MS) Analysis of Aroma Compounds in Cooked Beef Using Response Surface Methodology. Microchemical Journal 2013, 111, 16–24.
- Wu, Y.-F.G.; Cadwallader, K.R. Characterization of the Aroma of Meat-Like Process Flavouring from Soybean-Based Enzyme-Hydrolyzed Vegetable Protein. Journal of Agricultural and Food Chemistry 2002, 50, 2900–2907.
- Mottram, D.S. Meat Flavour. In Understanding Natural Flavours; Piggott, J.R.; Paterson, A.; Eds.; Chapman and Hall: Glasgow, 1994; 140–163.
- Shu, C.-K.; Hagedorn, M.L.; Mookherjee, B.D.; Ho, C.-T. Volatile Components of the Thermal Degradation of Cystine in Water. Journal of Agricultural and Food Chemistry 1985, 33, 438–442.
- Zhang, Y.; Ho, C.-T. Volatile Compounds Formed from Thermal Interaction of 2, 4-Decadienal with Cystine and Glutathione. Journal of Agricultural and Food Chemistry 1989, 37, 1016–1020.
- Güntert, M.; Brüning, J.; Emberger, R.; Köpsel, M.; Kuhn, W.; Thielmann, T.; Werkhoff, P. Identification and Formation of Some Selected Sulfur-Containing Flavour Compounds in Various Meat Model Systems. Journal of Agricultural and Food Chemistry 1990, 38, 2027–2041.
- Moon, S.-Y.; Cliff, M.A.; Li-Chan, E.C.Y. Odour-Active Components of Simulated Beef Flavour Analyzed by Solid Phase Microextraction and Gas Chromatography–Mass Spectrometry and –Olfactometry. Food Research International 2006, 39, 294–308.
- MacLeod, G.; Ames, J.M. Capillary Gas Chromatography-Mass Spectrometric Analysis of Cooked Ground Beef Aroma. Journal of Food Science 1986, 51, 1427–1434.
- Guth, H.; Grosch, W. Identification of the Character Impact Odourants of Stewed Beef Juice by Instrumental Analyses and Sensory Studies. Journal of Agricultural and Food Chemistry 1994, 42, 2862–2866.
- Fadel, H.H.M.; Abdel Samad, A.; Kobeasy, M.I.; Abdel Mageed, M.A.; Lotfy, S.N. Evaluation of Aroma Quality and Stability of an Encapsulated Beef-Like Process Flavouring Prepared from Precursors and Enzymatically Hydrolyzed Soybean Protein. International Journal of Food and Nutritional Sciences 2014, 3, 23–31.
- Fadel, H.H.M.; Abdel Samad, A.; Kobeasy, M.I.; Abdel Mageed, M.A.; Lotfy, S.N. Flavour Quality and Stability of an Encapsulated Beef-Like Process Flavouring Prepared from Soybean Based Acid Hydrolyzed Protein. International Journal of Food Processing Technology 2015, 2, 17–25.
- Lotfy, S.N.; Fadel, H.H.M.; El-Ghorab, A.H.; Shaheen, M.S. Stability of Encapsulated Beef-Like Flavourings Prepared from Enzymatically Hydrolysed Mushroom Proteins with Other Precursors under Conventional and Microwave Heating. Food Chemistry 2015, 187, 7–13.
- Madruga, M.S.; Mottram, D.S. The Effect of Ph on the Formation of Volatile Compounds Produced by Heating a Model System Containing 5-IMP and Cysteine. Journal of the Brazilian Chemical Society 1998, 9, 261–271.
- Wettasinghe, M.; Vasanthan, T.; Temelli, F.; Swallow, K. Volatile Flavour Composition of Cooked by - Product Blends of Chicken, Beef and Pork: A Quantitative GC- MS Investigation. Food Research International 2001, 34, 149–158.
- Vernin, G.; Parkanyi, C. Mechanism of Formation of Heterocyclic Compounds in Maillard and Pyrolysis Reaction. In Chemistry of Heterocyclic Compounds in Flavours and Aromas; Vernin, G.; Ed.; Ellis Horwood Limited: Chichester, 1982; 151–207.
- Grosch, W.; Zeiller-Higart, G.; Cerny, C.; Guth, H. Studies on the Formation of Odourants Contributing to Meat Flavour. In Progress in Flavour Precursor Studies; Schreier, P.; Winterhalter, P.; Eds.; Allured: Carol Stream, 1993; 329–342.
- Cerny, C.; Davidek, T. Alpha-Mercaptoketone Formation during the Maillard Reaction of Cysteine and [1-(13) C] Ribose. Journal of Agricultural and Food Chemistry 2004, 52(4), 958–961.
- Elmore, J.S.; Mottram, D.S.; Enser, M. Effect of the Polyunsaturated Fatty Acid Composition of Beef Muscle on the Profile of Aroma Volatiles. Journal of Agricultural Food Chemistry 1999, 47(4), 1619–1625.
- Moody, W.G. Beef Flavour – a Review. Food Technology 1983, 37(5), 227–232.
- Peterson, R.J.; Izzo, H.J.; Jungemann, E.; Chang, S.S. Changes in Volatile Flavour Compounds during the Retorting of Canned Beef Stew. Journal of Food Science 1975, 40, 948–954.
- Chang, S.S.; Peterson, R. Recent Developments in the Flavour of Meat. Journal of Food Science 1977, 42, 298–305.
- Chen, Y.; Ho, C.-T. Effect of Carnosine on Volatile Generation from Maillard Reaction of Ribose and Cysteine. Journal of Agriculture and Food Chemistry 2002, 50(8), 2372–2376.
- Schinneller, D.J.; Dougherty, R.H.; Biggs, R.H. Influence of Selected 5ʹ-Nucleotides on Flavour Threshold of Octanal. Journal of Food Science 1972, 37, 935–937.
- Salles, C. Odour-Taste Interactions in Flavour Perception. In Flavour in Food; Voilley, A.; Etiévant, P.; Eds.; Woodhead publishing in Food Science, Technology and Nutrition: Cambridge, England, 2006; 345–368.
- Rabe, S.; Krings, U.; Berger, R.G. Initial Dynamic Flavour Release from Sodium Chloride Solutions. European Food Research and Technology 2003, 218, 32–39.
- Keast, R.S.J.; Breslin, P.A.S. An Overview of Binary Taste–Taste Interactions. Food Quality and Preference 2002, 14, 111–124.
- King, C.J. Physical and Chemical Properties Governing Volatilization of Flavour and Aroma Components. In Physical Properties of Food; Peleg, M.; Bagley, E.B.; Eds.; Westport, CT, USA: AVI Publishing Company, 1983; 399–421.
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between Aroma Compounds and Whole Mannoprotein Isolated from Saccharomyces Cerevisiae Strains. Food Chemistry 2007, 100, 22–30.
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between Yeast Autolysates and Volatile Compounds in Wine and Model Solution. Food Chemistry 2011, 127, 473–480.
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of Yeasts Walls on the Behavior of Aroma Compounds in a Model Wine. American Journal of Enology and Viticulture 1994, 45, 29–33.
- Lubbers, S.; Voilley, A.; Feuillat, M.; Charpentier, C. Influence of Mannoproteins from Yeast on the Aroma Intensity of a Model Wine. Lebensmittel-Wissenschaft & Technologie 1994, 27, 108–114.