ABSTRACT
Bitterness in dried jujube fruit can adversely influence its palatability and consumer acceptability. In the present investigation, sensory-guided fractionation with techniques of liquid–liquid extraction, macroporous resin separation, and LC-MS/QTof was applied to identify the bitter compounds in dried jujube fruit (Z. jujuba cv. junzao). The results showed that bitter substances can be successfully extracted from dried jujube fruit using ethanol followed by n-butanol solution. The extract could then be further separated into five fractions by utilizing the macroporous resin separation technique. From this, three bitter fractions were obtained where 35 compounds, including 7 nitrogen compounds, 14 flavonoids, 12 saponins, and 2 fatty acid oxides,were tentatively identified. Based on the structures of these compounds, bitterness generation is likely to result from endogenous phytochemicals of jujube fruit and Maillard reactions during drying processing and also storage.
Introduction
Jujube (Z. jujuba Mill.) is generally recognised as the most important zizyphus species for fruit production in the buckthorn family Rhamnacea.[Citation1] Jujube is native to China and widely distributed in the subtropical and temperate areas of the northern hemisphere, especially the north of China,[Citation2] where it has been commonly used as an edible or medicinal plant for more than 3000 years. As the largest jujube producer, China has more than 90% share of the total world productions, and its production reached 5.88 million tons in 2012 (Chinese Statistic Yearbook of 2013). In recent years, a series of phytochemicals, including alkaloid,[Citation3] polyphenol[Citation4] polysaccharide,[Citation5,Citation6] and saponin,[Citation7,Citation8] have been found to be widely distributed in the tissues of zizyphus species. While much attention has been focused on the isolation, identification, quantification, and functions of these phytochemicals of jujube fruit, there has been lack of studies related to the taste and aroma of jujube fruits, which are key factors for understanding of their food value and developing methods for jujube processing and storage.
In the past, jujube bitterness was not regarded as important, partially because its high sugar content could mask the bitter flavour, or due to the fruit generally being consumed in the form of food additives and traditional Chinese medicine, in which the added amount of jujube was quite low. Currently, with the increase of output, the bitterness of jujube fruit and its products is gradually drawing attention. The majority of available papers related to jujube bitterness have been published in Chinese. The fruit of Z. jujuba cv. Grande de Albatera tasted bitter, and the sensory score of bitter intensity reached 0.5 using caffeine solutions as sensory standard solutions (the sensory score of 0.5 g/L caffeine solution is 1.5).[Citation9] The fruits of four Spanish jujube cultivars were also reported to be bitter, but the intensity was relatively low.[Citation10] After extraction with ethanol, the taste flavour of jujube juice was obviously improved and bitterness was no longer perceive.[Citation11] The bitter intensity of the dried fruit of Z. jujuba cv. hami was associated with the ratio of sugar and organic acid, drying conditions, and soluble protein. After analysing by GC-MS, the bitterness of jujube wine was ascribed to the combined effects of phenethyl alcohol, isoamylol, ketone, phenols, and aldehyde.[Citation12] In a more recent paper, it was found that the bitter intensity was correlated with the increase of bitter amino acids in jujube fruit.[Citation13] These studies have indicated that bitterness could be perceived in jujube fruit and its products, however there is limited knowledge on the molecules imparting bitter taste to jujube fruit and its products, as well as little understanding of the formation mechanism involved in the formation of these compounds.
Although there are, up to now, among 700 jujube cultivars in China, Z. jujuba cv. junzao has the most common cultivar due to its superior properties of saline-alkali tolerance, cold and heat tolerance, drought-resistant, barren resistance, and high productivity. Therefore, this cultivar was used in this investigation. The techniques of sensory-guided fraction and LC-MS/QTof were applied to investigate the bitter chemical profiles. Defining the chemical profile of bitter substances will provide insight into the origin of bitterness in fruits of Z. jujuba cv. junzao, and facilitate the development of strategies for jujube processing, de-bittering and variety breeding.
Materials and methods
Chemicals
A D101 macroporous resin was obtained from Shanghai Mosu Scientific Equipment Co., Ltd (Shanghai, China). Methanol (HPLC grade) was purchased from Fisher (Fair Lawn, NJ, USA). All other chemical reagents were of analytical grade, and all solutions were prepared using distilled/deionised water.
Sample preparation
After harvest, the samples were washed with distilled water, and dried by hot air at 50°C for 30 h, resulting in 18.32% of moisture content of the dried fruit; these were then packed in polyethylene bags, and stored at ambient temperature for 3 months. Finally, dried fruit was chopped into halves for removing seeds, and cut into pieces (about 2 mm) and storage at −18°C for use.
Analytical scheme
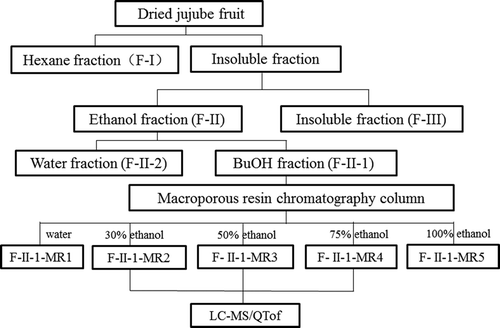
Most known bitter substances from plant tissues have been successfully extracted with methanol or ethanol solution,[Citation14,Citation15] and found to be hydrophobic compounds. Therefore, ethanol solution was applied to extract bitter substances from dried jujube fruit, and the sequentially extracted procedures of bitter fraction of jujube fruit were made as follows ().
Extraction of dried jujube fruit
The jujube pieces (100 g) were extracted with hexane (2 × 500 mL) at room, temperature for 12 h. After filtration the hexane fractions were combined and concentrated in a rotary evaporator to produce the hexane soluble portion (F-I). The residue was then extracted with 95% ethanol solution (2 × 1 L) at room temperature for 24 h. After filtration, the ethanolic fractions were combined and concentrated at 45°C (F-II). In addition, the insoluble ethanol fractions were dried (F-III). The yields and sensory bitter taste intensities of each fraction were evaluated ().
Table 1. Yields and bitter intensities of dried jujube fruit extracts.
Isolation of bitter compounds
The F-II was dispersed in 150 mL deionised water and extracted with water-saturated butanol (3 × 150 mL). The butanol layers were combined and concentrated in a rotary evaporator to obtain a butanol soluble portion (F-II-1). The water layer was concentrated in a rotary evaporator (F-II-2).
The F-II-1 fraction was dispersed in 20 mL deionised water and loaded onto a glass chromatographic column (30 mm × 300 mm), which was wet-packed with 150 mL pretreated D101 resin. Then, it was eluted sequentially with water and ethanol solution (at the concentration of 30%, 50%, 75%, and 100%, respectively) at 2 BV/h flow rate (five times of resin volume). Five fractions, F-II-1-MR1, F-II-1-MR2, F-II-1-MR3, F-II-1-MR4, and F-II-1-MR5, were obtained by freeze-drying. Their yields and sensory bitter intensities of individual fractions obtained were evaluated, as shown in .
HPLC analysis of bitter fractions
Before HPLC injections, the bitter fractions (F-II-1-MR2, F-II-1-MR3, and F-II-1-MR4) were dissolved in 5 mL methanol, and filtered through a 0.22 μm pore size membrane filter. HPLC analysis of bitter fractions was performed on a Waters 2695 HPLC coupled to a Waters 2998 diode array detector(Waters Corp., Milford, MA, USA). The column used was a Dikma ODS C18, 4.6 × 250 mm, 5 μm particle size. The column was maintained at a temperature of 40 °C. UV absorption of the HPLC eluates were recorded at wavelengths of 254, 280, and 360 nm for real-time monitoring of the peak intensity, and full spectra (200–400 nm) were continuously recorded for each HPLC fraction. The mobile phase was 0.5% (v/v) formic acid in water (eluant A) and methanol (eluant B), and the flow rate was 0.7 mL/min and the injection volume was 10 μL. The gradient program of Fraction II-1-MR2 was as follows: 0–30 min, 10–40% of B, 30–40 min, 40–100% of B, 40–45 min, 100% of B, 45–50 min, 100–10% of B, 50–60 min, 10% of B. The gradient program of Fraction II-1-MR3 was as follows: 0–30 min, 30–80% of B, 30–35 min, 80–100% of B, 35–40 min, 100% of B, 40–45 min, 100–30% of B, 45–60 min, 30% of B. The gradient program of Fraction II-1-MR4 was as follows: 0–40 min, 50–100% of B, 40–45 min, 100% of B, 45–50 min, 100–50% of B, 50–60 min, 50% of B.
LC-MS/QToF analysis of bitter fractions
LC-MS/QTof analysis of bitter fractions of Z. jujuba cv. junzao was performed on an AB Triple TOF 5600plus (AB SCIEX, Framingham, MA, USA) mass spectrometer coupled to a Waters UPLC (Waters Corp., Milford, MA, USA) equipped with UV–vis detector. The MS conditions were described as follows: the scan range was set at m/z 100–2000, the source voltage was −4.5 kV and the source temperature was 500°C in negative ionisation mode, the pressure of gas (N2) was set to 50 psi, and the curtain gas was set to 30 psi. For MS/MS, collision energy was −35 V, collision energy spread was 10 V, declustering potential was −100 V. The injection volume was 10 μL. The eluent was split and approximately 0.7 mL/min was introduced into the mass detector. Analyst® TF 1.6 software (AB-Sciex), which was installed with the software of ChemSpider, was used for data acquisition and processing.
Sensory analysis
The sensory panel was composed of seven panelists (four female and three males) between the ages of 22 − 36 years, who had given informed consent to participate in the sensory tests of the present investigation and had no history of known taste disorders. In order to reduce potential bias, the basic information of the study was provided to all panelists, and they were trained to evaluate bitter intensity using quinine hydrochloride references solutions. Three reference levels were provided to panelists (2.0, 5.0, 10.0 mg.L−Citation1 quinine hydrochloride solution), corresponding to bitterness intensity ratings of 3, 6, and 10, respectively.[Citation16] For each sample, all panelists were asked to thoroughly rinse their mouths with purified water before and after evaluation, and tasted the sample by swirling it around in mouth for several seconds, and then spit it out, and each sample was evaluated twice.
Results and discussion
Sequential solvent extraction
Initially, the sample was extracted with hexane to obtain fraction F-I after solvent evaporation under reduced pressure. The residual material was then extracted with 95% ethanol to produce F-II after solvent rotary evaporation. The final insoluble part (F-III) had the highest yield, accounting for approximately 66% of the dry mass of jujube fruits. The yields for fractions I and II were 3.25% and 32.43%, respectively. Fraction II was perceived bitter as well as sweet, whereas fraction I had a fatty mouth-feel, as expected for triglycerides, and fraction III had negligible taste attributes. F-II was then extracted with water-saturated butanol to get fraction F-II-1 and F-II-2, with yields for F-II-1 and F-II-2 were 1.62% and 25.35%, respectively. F-II-1 was strongly bitter, whereas sweet was the only flavour perceived in the F-II-2, due to high content of sugars in F-II-2. The yields and bitter attributes of these fractions are shown in .
Macroporous resins fractionation
Macroporous resins have been used in chemical and medicinal industries widely, especially for extraction, separation and purification of biochemical product.[Citation17] Therefore, in order to screen out most of the non-bitter substances, a portion of F-II-1 was further separated by using D101 macroporous resins.
The portion of F-II-1-MR1, eluted with deionised water, accounted for approximately 51% of weight of the water-saturated butanol extract and exhibited sweet attributes. The portion of F-II-1-MR2 was a brown powder and exhibited strongly bitter as well as lightly sour attributes. F-II-1-MR3 was also a brown powder and exhibited bitter as well as lightly astringent properties. F-II-1-MR4 was a light brown powder and exhibited pure bitter properties. However, F-II-1-MR5, whose yields were lowest among all eluted portions, was a canary yellow amorphous powder and tasteless.
Through separation by macroporous resins, most of non-bitter substances were not only successfully screened out, but also astringent and sour flavours were occasionally perceived in the fractions. To our knowledge, the astringent flavour was perceived in jujube fruit for the first time. According to the flavour theory of mixture suppression, central cognitive effects can occur when different qualities of taste stimuli are mixed together and the perceived intensity of one or more of the components is diminished by the perception of the other.[Citation18] Therefore, after removing sugars, the flavours of sour, astringent and bitter could be perceived.
Identification of bitter compounds
From the bitter fractions, a total of 35 compounds, including 7 nitrogen compounds, 14 flavonoids, 12 saponins, and 2 fatty oxides, were identified by LC-MS/QTof in the negative ion mode (). All compounds were tentatively identified by comparison their MS spectral data with those generated by the Peakview software to produce empirical formula whose chemical structures and names were obtained from the online database of Chemspider, and which were further verified base on m/z value, retention time, elution order, and the UV adsorption value compared with the published data.
Table 2. Spectrometric data of components found in of Ziziphus jujuba cv. junzao.
Nitrogen compounds
According to the MS fragment behaviours, the compounds of C1-C7 match well with those in the databases, and were identified as 5-(4-hydroxy-3-methoxyphenyl)-2,4-imidazolidinedione, corydalmine, methyl4-{methyl[(3,4,5-triacetoxy-6-azidotetrahydro-2 H-pyran-2-yl)carbony]amino]benzoate, 5-allyl-1-(2,3,4,-tris-O-benzoylpentofuranosyl)-2,4(1 H,3 H)-pyrimidinedione, N,N’-[(2-methoxyphenyl)methylene]bis(4-nitrobenzamide), 2-[amino(hydroxyl)methylene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5a,6,12a-tetrahydro-1,3,12(2 H,4 H,5 H)-tetrocenetrione, 2-[(6-amino-2,4-dioxo-1-propyl-1,2,3,4-tetrahydro-5-pyrimidinyl)(butyl)amino]-2-oxoethyl2-hydroxy-3-methylbenzoate, respectively. Comparing these results with the published literature, the seven compounds were reported for the first time in jujube fruit.
In complex food system, nitrogen compounds, including peptides,[Citation19] bitter amino acids such as L-tryptopha,[Citation14] and Maillard reaction product,[Citation16] have been generally reported as bitter constituents. In the present study, peptides and bitter amino acids were not discovered in the bitter fraction; this might due to differences of jujube variety, minute amounts of these compounds in jujube fruit, or losses during extraction and separation of bitter fractions. Nevertheless, the amino, pyran and furan moieties of C3-C7 indicated that these five compounds were likely to belong to the products of Maillard reaction. If so, the formation mechanism of the bitterness of jujube fruit might be partially explained to occur during processing and storage. Maillard reaction[Citation20] have been generally considered as a vital reaction for formation of jujube flavour and brown flesh colour, but there is no evidence to date whether Maillard reactions resulted in the bitterness of jujube fruits during processing and storage. So, future studies should pay attention to the Maillard reaction’s effect on jujube flavour.
Flavonoids
Compounds C8-C21 were discovered in the fractions of F-II-1-MR2 and F-II-1-MR3, and were identified or tentatively identified as flavonoids, including six quercetin derivatives, one kaempferol derivative, four flavone C-glycosides and three other flavones.
Compounds C8, C11, C12, C16, C18, and C21 shared the same fragment ions at m/z 301 and 300, in full-scan mode in the triple quadrupole system, which are usually considered as key fragment ions of quercetin derivatives.[Citation21,Citation22] These compounds, except for the compound C21, were identified and shown in the . Among these seven quercetin derivatives, the peak of C11, identified as rutin, was the highest in the LC and MS spectrum. In jujube fruit, rutin has been reported as the most abundant flavonoi,[Citation23,Citation24] and is a known bitter chemical. So, rutin might be considered as a bitterness marker in jujube fruit.
Compound C13 had fragment ions at m/z 284 and 285 that were just less 16 (one oxygen atom) than these characteristic ions of quercetin derivatives. It could be deduced that C13 belonged to kaempferol derivatives, because ions at m/z 284 and 285 are the key fragment ions of kaempferol derivatives. Additionally, the formula, fragment ions and UV absorption value could match those of kaempferol-3-O-robinobioside which is a known flavonoid discovered in Z. jujuba. So, C13 could be identified as kaempferol-3-O-robinobioside. Similarly, compounds of C9, C10 and C20 were identified as eriocitren, phloretin-3ʹ,5ʹ-di-glucoside,[Citation4] and ramnazin-3-O-rutinoside, respectively.
Through observing the fragmentation behaviour of compounds C14, C15, C17 and C19, it was found that most of them produced fragment ions at m/z [M-H-90]−, [M-H-120]−, [M-H-150]− and [M-H-162]−, which were equal to ions of [M-H-C3H6O3]−, [M-H-C4H8O4]−, [M-H-C5H5O5]−, and [M-H-Hexose]−. In mass spectrometry, it has been reported that in flavone C-glycosides, cross-ring cleavage of sugar residues produced fragment ions by losses of 60, 90, and 120 am.[Citation25] Therefore, compounds C14, C15, C17, and C19 should be classified into flavone C-glycosides. After further analysis, these four compounds couldn’t match well with those recommended in the Chemspider database, probably due to minute amount of flavone C-glycosides in plant kingdom. Additionally, comparing with molecular mass, these four compounds were also different from known flavone C-glycosides identified from the seeds of Z. jujub.[Citation26] So, further separation and analysis techniques should be applied to elucidate the structure of these compounds.
Although, flavonoids, up to now, have not been reported as bitter compounds in jujube fruit, much of previous literature mentioned phenolic substances are widely distributed in the plant kingdom and responsible for the bitterness and astringency in food and beverage.[Citation27] So, these flavonoids, identified from the bitter fractions, are likely to partially explain the bitterness of jujube fruit.
Saponins
Compounds C22-C32 and C34, discovered in the fraction of F-II-1-MR4, were also monitored by LC-UV, but UV absorption was not observed among these compounds in the spectrum due to lack of chromophoric groups. By further analysis of their mass spectrum, they were tentatively identified as saponins, and three of them have already been identified in Z. jujub.[Citation28]
Compounds C27-C31 shared the same basic skeleton, and their fragmentation behaviours were quite similar, with exception of the difference of molecular mass, fragment ions at m/z 911 and 749 were produced in these compounds. Although the Peakview software gave empirical formulas, high match rate compounds were not found in the database of Chemspider. Gu[Citation28] investigated the phytochemicals of the leaves from two zizyphus species by LC-PDA-MS/ELSD, and reported that the ions at m/z 911, 749, and 603 were the fragment ions of Zizyphus saponin I and Zizyphus saponin II, which were identified by NMR. Because the molecular mass of Zizyphus saponin I was the same as Zizyphus saponin I,[Citation28] C30 might be either Zizyphus saponin I or Zizyphus saponin II. For the compound C27, the ion at m/z 1043 was its molecular ion, which completely matched with Jujuboside .[Citation29] Similarly, C28 was identified as jujuboside I, which was previously separated from Ziziphi Spinosae Seme.[Citation30] Although the compounds C29 and C31 also produced the same fragment ions with C27, C28, and C30, there were not corresponded saponins reported in the previous literature.
For compound C22, its molecular ion at m/z 1077 exhibited losses of 132 u and 162 u, yielding to fragment ions at m/z 945 and 783 characteristic of a pentose and a hexose, respectively. Its fragment ions were all less 34 amu than those of C27-C31. So, this indicated that C22 was likely to belong to saponins. Adopting similar strategies, it can be concluded that compounds C23-C26 also belonged to saponins.
Compounds C32 and C34, they had long retention times and low molecular weights, and were identified as 24-dihydrocucurbitacin D and quillaja sapogen, respectively. In the portion of F-II-1-MR4, a total of 12 compounds were putatively identified as saponins, and nine of them were reported for the first time in zizyphus species. An earlier published study reported that jujubasaponins I-III, isolated from the fresh leaves of Z. jujuba, showed anti-sweet activity. In recent studies, a series of steroidal saponins were identified as the key contributors to the typical bitter taste of white asparagus spear.[Citation31] So, we inferred that these twelve saponins might be the main contributors to the bitter taste of F-II-1-MR4.
Fatty acid oxides
Compounds C33 and C35, they were identified as 3-oxohexadecanoic acid and 3-oxooctadecanoic acid, respectively. According to their structure, they were likely to originate from oxidation reactions of fatty acids.
Bitterness of compounds might be predicted by the theory that the ratio of the number of C atoms to the number of OH groups, or the R value (nC/nOH), of a molecule could give an indication of the bitterness of these compounds. Sweet compounds yield R values of 1.00–1.99, bitter compounds 2.00–6.99, and non-bitter compounds have values above 7.0.[Citation32] If this theory is applied to judge the bitterness of compounds in this study, compounds C33 and C35 could be grouped into non-bitter substances, because their R values (nC/nOH) were far beyond 7.00. The flavonoids and saponins (C8-C31) could be grouped into bitter chemicals, because sugar moieties increased their OH groups and lowered their R values (nC/nOH) to the scope of bitter compounds. For compounds C1-C7, their R values were beyond 7.00, and these seven compounds seemed not to be classified into bitter substances. However, in this theory, there was a supplementary specification, for nitrogen compounds, one nitrogen atom could be counted as one OH groups to calculate the R value. Therefore these nitrogen compounds could be also considered as bitter substances.
Based on the current results, nitrogen compounds, flavonoids and saponins were the main contributors to the bitterness of jujube fruit. Nitrogen compounds were likely to originate from Mailard reactions, which is vital for jujube flavour formation during drying processing. It is generally known that improper processing and storage will increase the content of Mailard reaction products, therefore the flavour quality of jujube fruit and its products might be improved by controlling Mailard reactions. Because flavonoids and saponins were endogenous substances of jujube fruit and relatively stable during processing and storage, from a manufacturing standpoint, variety breeding, application of glycoside hydrolase and solvent extraction may be solution for altering the bitterness of jujube fruit and its products.
In summary, although the techniques of high performance preparative liquid chromatography and nuclear magnetic resonance weren’t applied in this experiment, the chemical profile of bitterness in jujube fruits was basically evaluated by sensory-guided fractionation combined with LC-MS/QTof due to powerful online retrieval function of Chemspider database and available phytochemical data of zizyphus species. The future work should pay attention to variations of these compounds during processing and storage, and development of processing strategies to optimise palatability of jujube products, thus improving the product quality.
Acknowledgements
We are grateful to Prof. Andrew Sinclair for English modifying, and Dr Xiaodan Wu for MS data analysis.
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 31460396).
Additional information
Funding
References
- Gao, Q.H.; Wu, C.S.; Wang, M. The Jujube (Ziziphus jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. Journal of Agriculture and Food Chemistry 2013, 61, 3351–3363.
- Elaloui, M.; Laamouri, A.; Fabre, J.; Mathieu, C.; Vilarem, G.; Hasnaoui, B. Distribution of Free Amino Acids, Polyphenols and Sugars of Ziziphus jujuba Pulps Harvested from Plants Grown in Tunisia. Natral Product Research 2015, 29, 94–97.
- Kang, K.B.; Ming, G.; Kim, G.J.; Ha, T.K.; Choi, H.; Oh, W.K.; Sung, S.H. Jubanines F-J, Cyclopeptide Alkaloids from the Roots of Ziziphus jujuba. Phytochemistry 2015, 119, 90–95.
- Wojdylo, A.; Carbonell-Barrachina, A.A.; Legua, P.; Hernandez, F. Phenolic Composition, Ascorbic Acid Content, and Antioxidant Capacity of Spanish Jujube (Ziziphus jujube Mill.) Fruits. Food Chemistry 2016, 201, 307–314.
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H.; Zhao, Z. Hepatoprotective Effects of Polysaccharides Extracted from Zizyphus Jujube Cv. Huanghetanzao. International Journal of Biological Macromolecules 2015, 76, 169–175.
- Li, J.; Ai, L.; Hang, F.; Ding, S.; Liu, Y. Composition and Antioxidant Activity of Polysaccharides from Jujuba by Classical and Ultrasound Extraction. International Journal of Biological Macromolecules 2014, 63, 150–153.
- Guo, S.; Duan, J.A.; Zhang, Y.; Qian, D.; Tang, Y.; Zhu, Z.; Wang, H. Contents Changes of Triterpenic Acids, Nucleosides, Nucleobases, and Saccharides in Jujube (Ziziphus jujuba) Fruit during the Drying and Steaming Process. Molecules 2015, 20, 22329–22340.
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-Quantitative Determination of Triterpenic Acids of Ziziphus jujuba Fruits and Evaluation of Their Capability to Interfere in Macrophages Activation Inhibiting NO Release and Inos Expression. Food Research International 2015, 77, 109–117.
- Galindo, A.; Noguera-Artiaga, L.; Cruz, Z.N.; Burló, F.; Hernández, F.; Torrecillas, A.; Carbonell-Barrachina, Á.A. Sensory and Physico-Chemical Quality Attributes of Jujube Fruits as Affected by Crop Load. LWT - Food Science and Technology 2015, 63, 899–905.
- Hernandez, F.; Noguera-Artiaga, L.; Burlo, F.; Wojdylo, A.; Carbonell-Barrachina, A.A.; Legua, P. Physico-Chemical, Nutritional, and Volatile Composition and Sensory Profile of Spanish Jujube (Ziziphus jujuba Mill.) Fruits. Journal of the Science of Food and Agriculture 2016, 96, 2682–2691.
- Liu, X.H.;. Study on Debitter of the Extracted Fluid of Jujube. Food Science and Technology 2006, 9, 84–87. ( Chinese journal).
- Li, A.P.; Ding, Y.P.; Chen, J.H.; Zeng, J.X. The Bitter Substances Origin and Composition Analysis in Jujube Wine. Journal of Chinese Institute of Food Science and Technology 2013, 7, 236–241. ( Chinese journal).
- Jiang, X.; Li, H.R.; Wang, W.; Li, Q.; Hong, J.Y. Relationship between Amine Acid and Bitter Taste of Xingjiang Jujube. Food Science and Technology 2016, 7, 87–91. ( Chinese journal).
- Bin, Q.; Peterson, D.G. Identification of Bitter Compounds in Whole Wheat Bread Crumb. Food Chemistry 2016, 203, 8–15.
- Dawid, C.; Hofmann, T. Quantitation and Bitter Taste Contribution of Saponins in Fresh and Cooked White Asparagus (Asparagus officinalis L.). Food Chemistry 2014, 145, 427–436.
- Jiang, D.; Peterson, D.G. Identification of Bitter Compounds in Whole Wheat Bread. Food Chemistry 2013, 141, 1345–1353.
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and Desorption Behaviour of the Flavonoids from Glycyrrhiza Glabra L. Leaf on Macroporous Adsorption Resins. Food Chemistry 2015, 168, 538–545.
- Keast, R.S.J.;. Modification of the Bitterness of Caffeine. Food Quality and Preference 2008, 19, 465–472.
- Karametsi, K.; Kokkinidou, S.; Ronningen, I.; Peterson, D.G. Identification of Bitter Peptides in Aged Cheddar Cheese. Journal of Agriculture and Food Chemistry 2014, 62, 8034–8041.
- Fang, S.; Wang, Z.; Hu, X.; Chen, F.; Zhao, G.; Liao, X.; Wu, J.; Zhang, Y.A.N. Energy Requirement and Quality Aspects of Chinese Jujube (Zizyphus jujuba Miller) in Hot Air Drying Followed by Microwave Drying. Journal of Food Process Engineering 2011, 34, 491–510.
- Pawlowska, A.M.; Camangi, F.; Bader, A.; Braca, A. Flavonoids of Zizyphus Jujuba L. and Zizyphus Spina-Christi (L.) Willd (Rhamnaceae) Fruits. Food Chemistry 2009, 112, 858–862.
- Kelebek, H.;. LC-DAD-ESI-MS/MS Characterization of Phenolic Constituents in Turkish Black Tea: Effect of Infusion Time and Temperature. Food Chemistry 2016, 204, 227–238.
- Lam, C.T.; Chan, P.H.; Lee, P.S.; Lau, K.M.; Kong, A.Y.; Gong, A.G.; Xu, M.L.; Lam, K.Y.; Dong, T.T.; Lin, H.; Tsim, K.W. Chemical and Biological Assessment of Jujube (Ziziphus jujuba)-Containing Herbal Decoctions: Induction of Erythropoietin Expression in Cultures. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Science 2016, 1026, 254–262.
- San, B.; Yildirim, A.N. Phenolic, Alpha-Tocopherol, Beta-Carotene and Fatty Acid Composition of Four Promising Jujube (Ziziphus jujuba Miller) Selections. Journal of Food Composition and Analysis 2010, 23, 706–710.
- Renata Colombo, J.H.Y.; Emerson Ferreira, Q.; Karine, N.; Kurt, H. LC-MS/MS Analysis of Sugarcane Extracts and Differentition of Monnosaccharides Moieties of Flavonone C-Glycosides. Journal of Liquid Chromatography & Related Technologies 2013, 36, 239–248.
- Wu, Y.; Zhang, J.; Chen, M.; Yu, B.; Wang, D.; Liu, J.-G.; Hu, Y. C-Glucosyl Flavones from Ziziphus jujuba Var. Spinosa. Chemistry of Natural Compounds 2015, 51, 247–251.
- Gomez-Carneros, A.;. Bitter Taste, Phytonutrients, and the Consumer: A Review. The American Journal of Clinical Nutrition 2000, 72, 1424–1435.
- Guo, S.; Duan, J.A.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous Qualitative and Quantitative Analysis of Triterpenic Acids, Saponins and Flavonoids in the Leaves of Two Ziziphus Species by HPLC-PDA-MS/ELSD. Journal of Pharmaceutical and Biomedical Analysis 2011, 56, 264–270.
- Zhao, J.; Li, S.P.; Yang, F.Q.; Li, P.; Wang, Y.T. Simultaneous Determination of Saponins and Fatty Acids in Ziziphus jujuba (Suanzaoren) by High Performance Liquid Chromatography-Evaporative Light Scattering Detection and Pressurized Liquid Extraction. Journal of Chromatography A 2006, 1108, 188–194.
- Wang, Y.; Ding, B.; Luo, D.; Chen, L.Y.; Hou, Y.L.; Dai, Y.; Yao, X.S. New Triterpene Glycosides from Ziziphi Spinosae Semen. Fitoterapia 2013, 90, 185–191.
- Dawid, C.; Hofmann, T. Structural and Sensory Characterization of Bitter Tasting Steroidal Saponins from Asparagus Spears (Asparagus Officinalis L.). Journal of Agriculture and Food Chemistry 2012, 60, 11889–11900.
- Owen, R.F.;. Food Chemistry, 3rd edn; Marcel Dekker, Inc.: New York, USA. 1996.