ABSTRACT
The physicochemical characteristics, phenolic contents, and antioxidant capacities of the juices, seeds, and peels of six pomegranate cultivars from Shandong and Xinjiang provinces of China were compared. Results showed that high variability occurred in the physicochemical properties of the fruits, including fruit size, 100-aril weight, juice yield, titratable acidity (TA), total reducing sugar (TS), total soluble solids (TSS), and TS/TA. Comparison of the distribution of the phenolic contents and antioxidant activities in the peels, juices, and seeds revealed that the pomegranate peels possessed much more phenolic components and stronger antioxidant activities than the seeds and juices. The phenolic components of pomegranate peels were structurally identified and quantified by ultra-performance liquid chromatography coupled with mass spectrum (UPLC–DAD–ESI/MSn), and punicalagin (α- and β-isomers) was found as the predominant phenolic component. Correlation analysis revealed that the antioxidant activities were highly positively correlated with the total phenolics and flavonoids. The above results indicated that the pomegranate peel was the most valuable part because of abundant total phenolics and high antioxidant activities, and the best cultivar for fresh consumption, juice extraction, and bioactive components preparation was “SD-QP.”
Introduction
Pomegranate (Punica granatum L.) is known as one of the oldest fruit crops, which is native to a region of Iran, and has been distributed throughout the Mediterranean region, South Asia, and Middle East.[Citation1,Citation2] Pomegranate fruit is commercially valued for its juice-containing arils and is consumed widely as whole fresh fruit or marketed as dietary supplements such as extracted juice, jam, and wine.[Citation3]
Traditionally, the consumer preference as whole fresh fruit for pomegranate depends on the physicochemical characteristics, such as exocarp and aril colour, hardness of seed, maturity, juice content, and acidity and sweetness.[Citation4] Besides being consumed as a fruit, the peel of pomegranate has been used in medications for treatment of diarrhoea, metrorrhagia, and stomach ache for thousands of years in China.[Citation1] Recent studies also revealed that the pomegranate fruit has potent health benefits including efficacy against infectious diseases, atherosclerosis, coronary heart disease, and cancer because of the abundant phenolic compounds, such as ellagic acid, punicalin, galloylglucose, and punicalagin in its juice and peel.[Citation5,Citation6] Epidemiological studies have shown that reduced cancer mortality and cerebrovascular and cardiodisease are associated with consumption of phenolic-rich fruits.[Citation7,Citation8] Therefore, increased popularity of pomegranate products as functional foods is expected in the future.[Citation9–Citation12] However, the quality and amount of bioactive components in pomegranate fruit is affected by seasonal differences, geographic origin, and cultivar.[Citation13–Citation16] In terms of physicochemical quality and phenolic contents, the cultivar and geographic region difference is more influential than the seasonal difference.[Citation14–Citation17]
Pomegranate has been cultivated in China for more than 2000 years. It is widespread, with more than 200 cultivars, and it is especially common in Shandong, Xinjiang, Yunnan, and Shaanxi provinces.[Citation18] Many studies have been carried out in recent years concerning the physicochemical quality of fresh pomegranate fruit from different cultivars, such as fruit size, weight, and flavour.[Citation4,Citation19–Citation21] However, systemic profiles of the physicochemical characteristics, bioactive components, and antioxidant activity of these cultivars have been scarcely reported. Furthermore, few studies have examined the distribution profiles of the bioactive components in fruit peel, juice, and seeds.
This study aimed to compare the physicochemical characteristics, phenolic content and composition, and antioxidant capacities of six local and popular commercial pomegranate cultivars grown in Shandong and Xinjiang regions of China and to demonstrate the common phenolic distribution profiles and their relationships. The results of this study will provide clues for further breeding efforts and will contribute to cultivar and raw material selection for the development of new pomegranate functional foods.
Materials and methods
Standards and reagents
Folin–Ciocalteu reagent (FCR), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2ˊ-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ellagic acid, gallic acid, punicalin, punicalagin (α- and β-isomers), rutin, and catechinic were offered by Spring & Autumn Co. (Nanjing, China). Acetonitrile, methanol, formic acid, and other solvents used for UPLC (ultra-performance liquid chromatography) analysis were purchased from Merck (Darmstadt, Germany). Distilled water was prepared with Millipore Milli-Q apparatus (Milford, CT, USA). All other analytical grade reagents were purchased from Beijing Chemical Co. (Beijing, China).
Samples and pretreatment
The pomegranate fruits of Bolicui (SD-BLC), Damaya (SD-DMY), and Qingpi (SD-QP) were collected in Zaozhuang, Shandong province, and Kashgar Suan (XJ-KS), Hotan CeLe1# (XJ-HT), and Piyaman (XJ-PYM) were collected in Kashgar and Hotan, Xinjiang Province of China.
All fruits were freshly collected in the 2013 harvesting season. After collection, the fresh fruit weights and diameters were measured (n = 10). According to the methods in literature,[Citation17,Citation22] the fresh arils and peel (including the leathery exocarp and mesocarp) of each fruit cultivar were manually separated and weighed, then the arils of six fruit cultivars were manually squeezed, filtered through three layers of gauze, and stored at –20°C. The pomegranate seeds were cleaned, lyophilized, ground, and then defatted with petroleum ether in a water bath (80°C, 2 × 2 h). Finally, the defatted seed residues and peels were lyophilized, ground, and extracted with 80% methanol–water solution using a Soxhlet apparatus at 80°C (3 × 2 h). The slurries were combined, filtered, evaporated at 60°C using a rotary evaporator, and lyophilized to obtain peel and seed extracts for further use.
Determination of total phenolics, total flavonoids, and proanthocyanidins
Total phenolics in peel, seeds, and juice were estimated as gallic acid equivalent (GAE) using a modified Folin–Ciocalteu method described previously.[Citation23] The total phenolic contents in dried seeds and peel were expressed as mg GAE g−Citation1 DW (dried weight). The content in juice was expressed as mg GAE g−Citation1 FW (fresh weight). Total flavonoids were determined as described by Saikia et al.[Citation24] and expressed as mg rutin equivalent (RE) g−Citation1 DW in peel and seeds and mg RE g−Citation1 FW in juice. Total proanthocyanidins were quantified using the vanillin assay following the protocol reported by Min et al.[Citation25] The contents of total proanthocyanidins were expressed as mg catechinic equivalent (CE) g−Citation1 DW in peel and seeds and mg CE g−Citation1 FW in juice.
Analysis of phenolic compounds in pomegranate peel
UPLC-DAD/ESI-MSn analyses were carried out using an Agilent 1260 Infinity II LC system coupled with a QTRAP® 5500 system (AB, USA). Analytical separation was carried out at 30°C on a ZORBAX RRHD SB-C18 column (2.1 mm id × 50 mm, 1.8 μm), at a flow rate of 0.6 mL min−Citation1. Linear gradient elution was performed with 0.2% formic acid water solution (eluent A) and acetonitrile (eluent B), and the elution program was as follows: 0–30 min, B: 10%; 30–35 min, B: 10→20%; 35–40 min, B: 20→25%; 40–55 min, B: 25%; 55–60 min, B: 25→60%.
The MS was obtained by scanning ions with mass to charge (m/z) ratio ranged 100–1500 Da in negative electrospray ionization (ESI) mode using an AB SciexQTrap 5500 mass spectrometry. Under the model of information dependent acquisition (IDA), the main parameters were as follows: scan type, Q3 MS (Q3); ion spray voltage (IS), −4500 V; curtain gas (CUR), 25 psi; source temperature (TEM), 550°C; declustering potential (DP), −60 V; auxiliary gas (N2), 50 psi; entrance potential (EP), −10 V; collision cell exit potential (CXP), −22 V; collision energy (CE), −42 V; under the enhanced product ion (EPI) scan model, the main parameters are as follows: IS, −4500 V; CUR, 25 psi; collision-activated dissociation (CAD), high; TEM, 550°C; DP, −60 V; CE, −60 V; EP, −10 V. Mass data were acquired and processed using SCIEX Analyst® software (version 1.6.1).
To identify and quantify the individual phenolic compound, mixed stock solutions of gallic acid, punicalin, α/β-punicalagin, and ellagic acid reference materials were serially diluted to 0.5–200 mg L−Citation1. Both the mixed reference solutions and samples were analysed. The extracted ion chromatogram (EIC) (Fig. 3C) was used to identify the peaks for integration. The concentration of each phenolic component was calculated based on the calibration curves of each reference substance plotted as the peak area at λmax as a function of the series of concentrations.[Citation26] The λmax used to quantify gallic acid, punicalin, punicalagin, and ellagic acid were 274, 380, 380, and 254 nm, respectively. The peaks of punicalagin (α- and β-isomers) were assigned according to references,[Citation26] and the summary concentration of punicalagin was calculated as the total of α and β. The linearity of the instrument was confirmed by performing a linear regression on the collected data (rCitation2 = 0.9999) ranged 0.5–200 mg L−Citation1. The limits of detection (LOD) and limits of quantification (LOQ) were 0.015, 0.05, 0.07, 0.02 and 0.05, 0.15, 0.20, 0.10 mg L−Citation1 for gallic acid, punicalin, α/β-punicalagin, and ellagic acid, respectively.
Antioxidant capacities
The antioxidant activities of pomegranate extracts, including DPPH, ABTS radical scavenging capacity, and ferric reducing antioxidant power (FRAP) were estimated as described.[Citation23,Citation27] All extracts were reconstituted with 80% methanol aqueous solution to a concentration of 0.1 g L−Citation1. For DPPH radical scavenging capacity assay, 0.1 mL of extract was mixed with 2.5 mL of freshly prepared DPPH methanol solution (0.1 mM) and measured at 517 nm after incubation for 20 min in the dark. For ABTS assay, 0.1 mL of extract solution was mixed with 1.5 mL of ABTS•+ working solution, and the absorbance at 734 nm was recorded after 6 min. In the test of FRAP, the FRAP reagent was composed of 300 mM acetate buffer (25 mL, pH = 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl (2.5 mL), and 40 mM FeCl3 6H2O (2.5 mL). An aqueous of extract solution (0.1 mL) was mixed with 3.0 mL FRAP agent and incubated for 5 min at room temperature before recording the absorbance at 593 nm” conveys the intended meaning>An aqueous of extract solution (0.1 mL) was mixed with 3.0 mL FRAP agent and incubated for 5 min at room temperature before recording the absorbance at 593 nm. In all assays, Trolox at a series of concentrations were used to plot the calibration curves and the antioxidant activities were expressed as mg TEAC (Trolox equivalent antioxidant capacity) g−Citation1 DW in peel or seeds and mg TEAC g−Citation1 FW in juices.
Statistical analysis
All of the results were expressed as mean ± standard deviation (SD) for at least triplicate analyses of each sample. The analysis of variance (ANOVA) and Duncan’s multiple range test were performed by IBM SPSS statistical 20.0 software (SPSS, Inc., Chicago, IL, USA). The principal component analysis (PCA) and Pearson correlation coefficient were also evaluated by 20.0 SPSS statistical software.
Results and discussion
Physicochemical characteristics of pomegranate fruit
The physicochemical properties such as exocarp and aril colour, fruit size and weight, aril weight, and juice yields are important commercial quality factors. As shown in , the colours of the exocarp, aril, and juice varied significantly among the six cultivars. SD-BLC had the lightest exocarp, aril, and juice colour, and XJ-KS had the darkest colour. The single fruit weight also varied significantly (), but, the variability in fruit size (fruit diameter) was not as great as that of the single fruit weight. The 100-aril weight ranged from 32 to 74 g, with the highest in SD-BLC and the lowest in SD-QP. The aril yield in all of these cultivars was more than 50%, which is consistent with that of cultivars in Croatia (35.7–62.1%) and Morocco (53.4–61.2%).[Citation21,Citation28] Juice yield ranged significantly from 36 to 50%, which is much lower than that reported for cultivars in Croatia (67.4–76.9%), but higher than Iran (26.95–46.55%).[Citation14,Citation21] These differences might stem from the methods of juice extraction as well as the cultivar, as mechanical pressing generally yields less juice than blender extraction does.[Citation29]
Table 1. Fruit weight, diameter, 100-aril weight, aril yield, and juice yield of six Chinese pomegranate cultivars.
Figure 1. Pomegranate fruits, arils, seeds, and juices of six cultivars from China. A, fruits; B, arils; C, seeds; D, fruit juices.

For most fruit species, the titratable acidity (TA) and total reducing sugar (TS) are important indicators of tartness and sweetness, and the TS/TA ratio is a valuable indicator of flavour and taste quality reflecting the balance between tartness and sweetness. The typical values of the TS and TA were 68.2–217.2 (TS) and 2.7–36.6 mg g−Citation1 FW (TA).[Citation14,Citation16] According to the results of TS, TA, and TS/TA () and the classification proposed by Melgarejo et al.,[Citation13] SD-BLC, SD-DMY, SD-QP, and XJ-PYM belong to the sour-sweet cultivars, which are tastier and more suitable for fresh consumption, and XJ-KS and XJ-HT belong to the sour group. Total soluble solid (TSS) content varied from 13.71% (SD-BLC) to 18.35% (XJ-HT), similar with the values of other reported pomegranate cultivars.[Citation14–Citation19] These data provided evidence that the physicochemical characteristics of pomegranate fruit varied greatly by cultivar.
Table 2. Total soluble solids (TSS), total reducing sugar (TS), titratable acid (TA), pH, and TS/TA of the juice of six Chinese pomegranate cultivars.
Correlation analysis was carried out to assess influences on the dependent variables. No highly positive correlation between fruit size and fruit weight was observed (γ = 0.504). For example, there was no significant difference in fruit size among SD-QP, SD-DMY, and XJ-PYM, although their individual fruit weights varied significantly. Both fruit weight and fruit diameter were negatively correlated with TA (γ =—–0.517 and –0.807) and positively with pH and TS/TA. However, the fruit weight and fruit diameter were not correlated with the 100-aril weight, in contrast to the correlations reported for Croatian cultivars.[Citation21] The 100-aril weight was positively correlated with aril yield and juice yield, but highly negatively correlated with TSS and TS. Therefore, increased aril weight resulted in increased aril and juice yield, but not in increased TSS or TS yields. TA and TS were both positively correlated with the TSS, and the TS/TA ratio was highly correlated with fruit diameter and weight. These data indicated that the fruit weight and diameter and aril weight are import physical properties, and TSS and TA are notable chemical factors of pomegranate fruit.
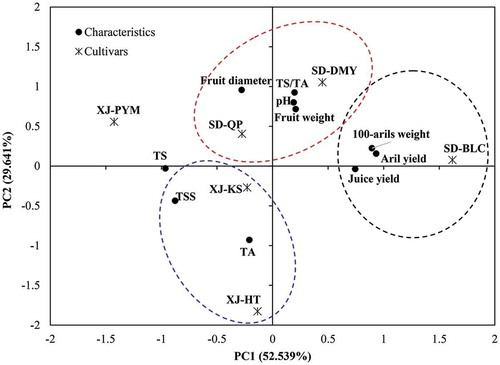
PCA was carried out to show the dispersion of the six pomegranate cultivars based on the different parameters of physicochemical characteristics (). PCA was constructed with two principal components (PC1 and PC2) explaining 82.18% of the total variance in the data, which have been chosen on the basis of their eigenvalues (>1). According to the PCA biplot, six pomegranate cultivars were discriminated successfully. Aril yield, 100-aril weight, and juice yield were contributed most to separating SD-BLC from other cultivars, while SD-DMY were characterized by fruit weight, TS/TA, and pH. SD-QP was characterized by fruit diameter, XJ-HT was characterized by TA, while XJ-KS was characterized by TSS and TS. In addition, XJ-PYM was characterized by TS and well separated from other cultivars.
Total phenolics, flavonoids, and proanthocyanidins in peel, juice, and seeds of pomegranate
The total phenolics, flavonoids, and proanthocyanidins in fruit peel, juice, and seeds of the six cultivars are shown in . The majority of the total phenolics (92.24–98.09%), flavonoids (92.61–98.32%), and proanthocyanidins (84.87–97.25%) in the six cultivars were found in the pomegranate peel, and only a small amount of these compounds was present in the fruit juice and seeds. The total phenolics, flavonoids, and proanthocyanidins observed in pomegranate peel among these six cultivars were significantly different (p < 0.05). The total phenolic and flavonoid contents in SD-QP and SD-DMY were much higher than those in the other four cultivars in this study and higher than those in the southern Tunisia cultivar “Gabsi” reported by Elfalleh et al.,[Citation30] which had only 85.60 mg GAE g−Citation1 phenolics and 51.52 mg RE g−Citation1 flavonoids. In the present study, the total phenolic and flavonoid contents were the lowest for SD-BLC peel, whereas the proanthocyanidin content of this cultivar was much higher than those of XJ-HT, XJ-KS, and XJ-PYM. As for juice, the highest levels of total phenolics and flavonoids were found in XJ-KS (1.39 mg GAE g−Citation1 FW and 0.68 mg RE g−Citation1 FW), and the lowest were found in SD-BLC (0.39 mg GAE g−Citation1 FW and 0.19 mg RE g−Citation1 FW). The total phenolic contents of the juices of XJ-KS, SD-QP, and XJ-HT were much higher than those of 10 Moroccan pomegranate varieties (0.41–0.83 mg GAE g−Citation1) reported by Legua et al.,[Citation15] but lower than Turkish commercial pomegranate juices (2.602–10.086 mg GAE mL−Citation1) reported by Tezcan et al. [Citation31] and 10 pomegranate cultivars (3.151–7.429 mg GAE mL−Citation1) reported by Li et al. [Citation16] The flavonoid content of juices of these cultivars was similar to the data (0.46–0.72 mg g−Citation1) reported by Fawole et al. [Citation20] The total proanthocyanidin content of juices in the present study ranged from 0.09 to 0.14 mg CE g−Citation1 FW, with the highest found for SD-QP. The total proanthocyanidin content found in this study was lower than those (0.53 mg CE g−Citation1) reported by Li et al. [Citation32] The pomegranate seeds of the six cultivars showed less significant differences in the total phenolics, flavonoids, and proanthocyanidins. The above results indicated that the pomegranate peel was the most valuable part because it had the highest levels of total phenolic, proanthocyanidin, and flavonoid content. The best cultivar for total phenolics was SD-QP.
Table 3. Total phenolic, flavonoid, and proanthocyanidin contents of the juice, peel, and seeds of six Chinese pomegranate cultivars.
Antioxidant activities of six pomegranate cultivars
The antioxidant capabilities of the various pomegranate juices, peels, and seeds were assessed using DPPH and ABTS radical scavenging and FRAP. For ease of comparison, the values were expressed as mg TEAC g−Citation1 DW in the peel and seeds and as mg TEAC g−Citation1 FW in the juice. Results of the DPPH, ABTS, and FRAP (as shown in ) clearly indicate that the pomegranate peel showed the strongest antioxidant activity. Differences in the antioxidant activity of the peel among the six cultivars were significantly different (p < 0.05). The highest and the lowest antioxidant activities were found for cultivars SD-QP and SD-BLC, respectively. shows that the DPPH, ABTS, and FRAP of pomegranate peel were highly positively correlated with the total phenolic (γ = 0.954, 0.989, and 0.988, respectively) and flavonoid (γ = 0.983, 0.931, and 0.905, respectively) contents, in agreement with previous reports.[Citation33] The antioxidant activities of pomegranate juice and seeds were far lower than that of the peel and were also associated with the total phenolics. However, Gil et al.[Citation33] and Tezcan et al.[Citation31] reported that commercial pomegranate fruit juices have higher antioxidant capacities than do laboratory hand-pressed juices because pomegranate peels are mixed into the fruit juice. Thus, the variations in antioxidant activities of pomegranate fruits reflected the effects of the fruit parts, the cultivar, and sample extraction methods.[Citation6,Citation30]
Table 4. DPPH, ABTS radical scavenging activity, and FRAP of pomegranate juice, peel, and seeds.
Table 5. Correlation matrix of the total phenolics (TP), total flavonoids (TF), total proanthocyanidins (TPC) and antioxidant activities of the pomegranate juice, peel, and seeds.
Identification of phenolic components in pomegranate peel
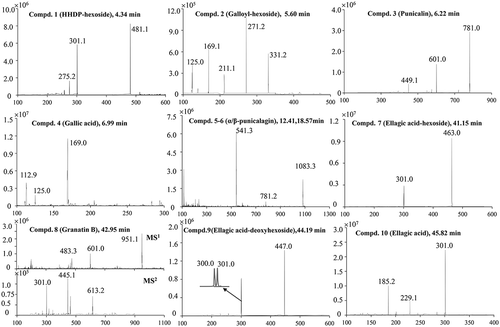
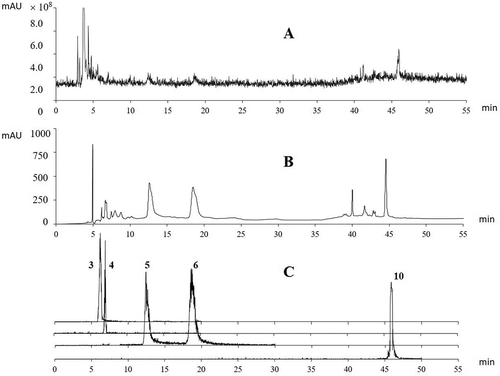
The UPLC-total ion chromatogram (TIC) and ultraviolet (UV) chromatograms of the pomegranate peel extracts at 254 nm and EIC mass spectra (m/z) of punicalin, gallic acid, punicalagin (α- and β-isomers), and ellagic acid are shown in . The retention times (Rt), UV/Vis (λmax), mass spectra (m/z), and peak assignments for the individual phenolic components are specified in . Ten phenolic compounds were structurally identified in the pomegranate peel extracts of the six tested cultivars based on the MS profiles (). Compound 1, which eluted after 4.34 min, yielded a negative molecular ion at m/z 481 and produced a characteristic hexahydroxydiphenoyl (HHDP) residue (m/z 301). The fragmentation pattern and UV/Vis spectra were identical to those of HHDP-hexoside, which has been previously reported in pomegranate juice and peel.[Citation6,Citation34] Compound 2, with a Rt at 5.60 min, displayed a molecular ion at m/z 331 and fragmented to ions at m/z 169 (indicating the loss of a typical hexose moiety) and m/z 125 (characteristic fragment with the loss of carboxylic moiety from gallic acid). Hence, this compound was assigned as galloylhexoside, which has been identified in pomegranate juice previously.[Citation6,Citation33] Compound 3 displayed an [M–H]– ion at m/z 781 and produced typical fragment ions of gallagic acid at m/z 601, with the loss of a glucose moiety and m/z 449 (with further loss of a galloyl moiety). By comparing the Rt, λmax, and mass spectra with those of controlled substance of punicalin (4,6-gallagyl-glucoside), the compound was identified as punicalin, which has already been well characterized in pomegranate juice, bark, peel, and leaves.[Citation6,Citation35] Compound 4, identified as gallic acid by comparison of the Rt, λmax, and mass spectra with the controlled substances, released an [M–H]– ion at m/z 169 and produced fragment ions at m/z 125 (MSCitation2). The peaks eluted after 12.41 and 18.57 min displayed identical mass spectra, with an [M–H]– ion at m/z 1083.3, a typical [M–2 H]Citation2– ion at m/z 541, and a fragment ion at m/z 781, equivalent to the [M–H]– ion of punicalin (compounds 5 and 6). The characteristic mass spectra and λmax (at 380 and 258 nm) were in agreement with the two isomers of punicalagin, which has been identified in many studies as the major phenolic compound in pomegranate.[Citation6,Citation33,Citation36,Citation37] By comparing the Rts of the two isomeric forms (α- and β-anomers), the peak at 12.4 min was assigned as α-punicalagin, and the other peak at 18.5 min as β-punicalagin. Lin et al. [Citation38] reported that punicalin and punicalagin had potent antioxidant activities and hepatoprotective effects on acetaminophen-induced liver damage in rats. Compound 7 revealed a [M–H]– ion at m/z 463 and characteristic fragment ions at m/z 301, with the loss of a hexoside moiety. The maximum absorption bands of the UV/Vis spectra occurred at 252 and 360 nm. Both the mass and UV/Vis spectra were in accordance with ellagic acid-hexoside, which has previously been found in pomegranate husk.[Citation34–Citation39] Compound 8, which eluted after 42.95 min, exhibited an [M–H]– ion at m/z 951 and produced a characteristic gallagyl fragment at m/z 601 in the MSCitation1 spectra. In the MSCitation2 spectra, typical fragments pointing to ellagic acid were observed at m/z 301 and 613, as a result of additional losses of water and ellagic acid moiety from the prominent fragment. Based on these characteristic ion fragments, it was identified as granatin B (galloyl-HHDP-DHHDP-hexoside), which was reported in pomegranate juice by Mena et al.[Citation34] Compound 9 revealed an [M–H]– ion at m/z 447.3, and it produced a typical moiety of ellagic acid at m/z 301 and a distonic radical at m/z 300. Therefore, compound 9 was identified as ellagic acid-deoxyhexoside, which is the deoxyhexoside of ellagic acid-hexoside (compound 7). This compound has been previously detected in pomegranate husk.[Citation39] Compound 10 was identified as ellagic acid, with a typical molecular ion of m/z 301 and characteristic fragment ions at m/z 229 (from the loss of four H2O) and 185 (with further loss of CO2). Ellagic acid is present in the juice, seeds, and leaves of pomegranate and also has been reported in some other fruits, nuts, and vegetables.[Citation6,Citation15,Citation23]
Table 6. Retention times, UV/Vis spectra, and characteristic ions of phenolic components in pomegranate peel.
Figure 3. The UPLC-DAD-ESI/MSn chromatograms of pomegranate peel extracts. A, total ion chromatogram (TIC); B, UV chromatogram at 254 nm; C, extracted ion chromatogram (EIC) of punicalin (3, at m/z 781/601), gallic acid (4, at m/z 169/125), α/β-punicalagin (5, 6, at m/z 1083/541), and ellagic acid (10, at m/z 301/229).
Overall, these phenolic components in pomegranate peel included eight ellagitannins (ellagic acid, HHDP-hexoside, ellagic acid-hexoside, granatin B, punicalin, α/β-punicalagin, and ellagic acid-deoxyhexoside), one gallotannin (galloyl-hexoside), and one hydroxybenzoic acid (gallic acid). Previously, Fischer et al.[Citation6] identified 48 compounds from Peruvian pomegranate fruit (including peel, mesocarp, aril, and juice), and Cristofori et al.[Citation19] reported nine components in pomegranate peel from Italy. These varied results may be affected by many factors, such as cultivar source, growing and climatic conditions, extraction processes, and identification methods. The phenolic compounds noted above could be important contributors to the antioxidant capacity of pomegranate fruit. Although 10 phenolic compounds were identified in the pomegranate peel extracts of the six cultivars, the type of linkage of the different fragments in the ellagic acid-hexoside, HHDP-hexoside, ellagic acid-deoxyhexoside, and galloylhexoside was still ambiguous. Further assignment of these compounds was impossible using the present UPLC-DAD/ESI-MSn data.
Quantification of phenolic components in pomegranate peel
The identified phenolic components were quantified with respect to reference substances to compare the content of the major individual phenolic components in pomegranate peel from different cultivars. The typical chromatogram of TIC, UPLC-DAD at 254 nm and the EIC of gallic acid, punicalin, punicalagin, and ellagic acid are shown in . lists the contents of these compounds, calculated from their individual calibration curves, plotted as peak area at λmax as a function of the concentrations of reference substances in the six pomegranate cultivars.
Table 7. Phenolic contents of the peels of six pomegranate cultivars.
The total contents of these four phenolic compounds in the peels of the six cultivars ranged from 19.05 to 124.42 mg g−Citation1 DW and represented 33.04–79.82% of the total phenolics in the pomegranate peel extracts. Among these phenolic compounds, α/β-punicalagin was the dominant one, accounting for more than 90% of the phenolics in all of the tested cultivars (range, 17.8–114.5 mg g−Citation1 DW), followed by punicalin, ellagic acid, and gallic acid. The punicalagin level was in accordance with literature reports indicating punicalagin contents of 39.8–121.5 mg g−Citation1 for 16 pomegranate cultivars.[Citation37] However, the punicalin, ellagic acid, and gallic acid contents were very small, with ranges of 0.75–5.42, 0.39–3.04, and 0.12–1.44 mg g−Citation1 DW, respectively. Similarly, Seeram et al.[Citation39] reported that pomegranate fruit husk contains major ellagitannins in the whole fruit, such as punicalagin (80–85% w/w) and ellagic acid (1.3% w/w), as well as an unquantified amount of punicalin.
Among the six cultivars, SD-QP possessed the highest contents of gallic acid, punicalin, α/β-punicalagin, and ellagic acid, and SD-BLC possessed the lowest. Meanwhile, changes in the global amount of the four individual phenolics in the six pomegranate cultivars coincided with the trend in the total phenolic content determined by the Folin–Ciocalteu method. Additionally, the total phenolic content in the pomegranate peel was positively correlated with antioxidant activity. According to the above findings, the SD-QP peel may be an ideal material for phenolic preparation (especially for α/β-punicalagin), and α/β-punicalagin could be the critical contributor to the antioxidant activity of pomegranate fruit.
Conclusion
In the present study, we demonstrated that the physicochemical characteristics, antioxidant capacities, and phenolic profiles of pomegranate fruit were significantly affected by the cultivar. Among the six cultivars studied, SD-QP showed the highest content of phenolics and flavonoids, the highest TS/TA ratio, and the best antioxidant activities, indicating that SD-QP would be the best cultivar for the pomegranate phenolic industry. Notably, this study indicated that the pomegranate juice traditionally consumed only contains a minor portion of the total phenolics, flavonoids, and proanthocyanidins of the pomegranate fruit, and the majority of the phenolics and flavonoids are present in the pomegranate peel. These phenolic compounds consist of eight ellagitannins, one gallotannin, and one hydroxybenzoic acid, among which α/β-punicalagin was the most abundant. Therefore, pomegranate peel, rather than the juice and seeds, is a better choice for the pomegranate phenolic preparation industry. This study increased our knowledge concerning variations in the physicochemical characteristics and phenolic profiles, as well as antioxidant activities of pomegranate fruit, and provided clues for further breeding efforts. The research will aid in the selection of the optimal cultivar and raw materials for the development of new nutritional supplements.
Funding
The study was financially supported by Beijing Higher Education Young Elite Teacher Project (YETP0757), the Fundamental Research Funds for the Central Universities, China (YX2015-13), Special Found for Beijing Common Construction Project (2014GJ01), and National Natural Science Foundation of China (J1103516).
Additional information
Funding
References
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate Biology and Biotechnology: A Review. Scientia Horticulturae 2013, 160, 85–107.
- Mayuoni-Kirshinbaum, L.; Porat, R. The Flavor of Pomegranate Fruit: A Review. Journal of the Science of Food and Agriculture 2014, 94(1), 21–27.
- Wetzstein, H.Y.; Zhang, Z.; Ravid, N.; Wetzstein, M.E. Characterization of Attributes Related to Fruit Size in Pomegranate. HortScience 2011, 46(6), 908–912.
- Al-Said, F.A.; Opara, L.A.; Al-Yahyai, R.A. Physico-Chemical and Textural Quality Attributes of Pomegranate Cultivars (Punica granatum L.) Grown in the Sultanate of Oman. Journal of Food Engineering 2009, 90(1), 129–134.
- Lansky, E.P.; Newman, R.A. Punica granatum (Pomegranate) and Its Potential for Prevention and Treatment of Inflammation and Cancer. Journal of Ethnopharmacology 2007, 109(2), 177–206.
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/Msn. Food Chemistry 2011, 127(2), 807–821.
- Hertog, M.G.; Sweetnam, P.M.; Fehily, A.M.; Elwood, P.C.; Kromhout, D. Antioxidant Flavonols and Ischemic Heart Disease in a Welsh Population of Men: The Caerphilly Study. The American Journal of Clinical Nutrition 1997, 65(5), 1489–1494.
- Kulkarni, A.P.; Aradhya, S.M.; Divakar, S. Isolation and Identification of a Radical Scavenging Antioxidant–Punicalagin from Pith and Carpellary Membrane of Pomegranate Fruit. Food Chemistry 2004, 87(4), 551–557.
- Jurenka, J. Therapeutic Applications of Pomegranate (Punica granatum L.): A Review. Alternative Medicine Review 2008, 13(2), 128.
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and Its Many Functional Components as Related to Human Health: A Review. Comprehensive Reviews in Food Science and Food Safety 2010, 9(6), 635–654.
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a Functional Food and Nutraceutical Source. Annual Review of Food Science and Technology 2011, 2, 181–201.
- Medjakovic, S.; Jungbauer, A. Pomegranate: A Fruit that Ameliorates Metabolic Syndrome. Food & Function 2013, 4(1), 19–39.
- Melgarejo, P.; Salazar, D.M.; Artes, F. Organic Acids and Sugars Composition of Harvested Pomegranate Fruits. European Food Research and Technology 2000, 211(3), 185–190.
- Tehranifar, A.; Zarei, M.; Nemati, Z.; Esfandiyari, B.; Vazifeshenas, M.R. Investigation of Physico-Chemical Properties and Antioxidant Activity of Twenty Iranian Pomegranate (Punica granatum L.) Cultivars. Scientia Horticulturae 2010, 126(2), 180–185.
- Legua, P.; Melgarejo, P.; Abdelmajid, H.; Martínez, J.J.; Martínez, R.; Ilham, H.; Hafida, H.; Hernández, F. Total Phenols and Antioxidant Capacity in 10 Moroccan Pomegranate Varieties. Journal of Food Science 2012, 77(1), C115–C120.
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical Characteristics, Polyphenol Compositions and Antioxidant Potential of Pomegranate Juices from 10 Chinese Cultivars and the Environmental Factors Analysis. Food Chemistry 2015, 175, 575–584.
- Gundogdu, M.; Yilmaz, H. Organic Acid, Phenolic Profile and Antioxidant Capacities of Pomegranate (Punica granatum L.) Cultivars and Selected Genotypes. Scientia Horticulturae 2012, 143, 38–42.
- He, L.; Xu, H.; Liu, X.; He, W.; Yuan, F.; Hou, Z.; Gao, Y. Identification of Phenolic Compounds from Pomegranate (Punica granatum L.) Seed Residues and Investigation into Their Antioxidant Capacities by HPLC–ABTS+ Assay. Food Research International 2011, 44(5), 1161–1167.
- Cristofori, V.; Caruso, D.; Latini, G.; Dell’Agli, M.; Cammilli, C.; Rugini, E.; Bignami, C.; Muleo, R. Fruit Quality of Italian Pomegranate (Punica granatum L.) Autochthonous Varieties. European Food Research and Technology 2011, 232(3), 397–403.
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and Phytochemical Properties and Antioxidant Activities of Three Pomegranate Cultivars Grown in South Africa. Food and Bioprocess Technology 2012, 5(7), 2934–2940.
- Radunić, M.; Špika, M.J.; Ban, S.G.; Gadže, J.; Díaz-Pérez, J.C.; MacLean, D. Physical and Chemical Properties of Pomegranate Fruit Accessions from Croatia. Food Chemistry 2015, 177, 53–60.
- Elfalleh, W.; Nasri, N.; Marzougui, N.; Thabti, I.; M’rabet, A.; Yahya, Y.; Lachiheb, B.; Guasmi, F.; Ferchichi, A. Physico-Chemical Properties and DPPH-ABTS Scavenging Activity of Some Local Pomegranate (Punica granatum) Ecotypes. International Journal of Food Sciences and Nutrition 2009, 60(Sup2), 197–210.
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of Extraction and Enrichment of Phenolics from Pomegranate (Punica granatum L.) Leaves. Industrial Crops and Products 2013, 42, 587–594.
- Saikia, S.; Dutta, H.; Saikia, D.; Mahanta, C.L. Quality Characterisation and Estimation of Phytochemicals Content and Antioxidant Capacity of Aromatic Pigmented and Non-Pigmented Rice Varieties. Food Research International 2012, 46(1), 334–340.
- Min, B.; Gu, L.; McClung, A.M.; Bergman, C.J.; Chen, M.H. Free and Bound Total Phenolic Concentrations, Antioxidant Capacities, and Profiles of Proanthocyanidins and Anthocyanins in Whole Grain Rice (Oryza sativa L.) of Different Bran Colours. Food Chemistry 2012, 133(3), 715–722.
- Qu, W.; Breksa III, A.P.; Pan, Z.; Ma, H. Quantitative Determination of Major Polyphenol Constituents in Pomegranate Products. Food Chemistry 2012, 132(3), 1585–1591.
- Zhao, S.; Liu, J.Y.; Chen, S.Y.; Shi, L.L.; Liu, Y.J.; Ma, C. Antioxidant Potential of Polyphenols and Tannins from Burs of Castanea mollissima Blume. Molecules 2011, 16(10), 8590–8600.
- Martínez, J.J.; Hernández, F.; Abdelmajid, H.; Legua, P.; Martínez, R.; El Amine, A.; Melgarejo, P. Physico-Chemical Characterization of Six Pomegranate Cultivars from Morocco: Processing and Fresh Market Aptitudes. Scientia Horticulturae 2012, 140, 100–106.
- Rajasekar, D.; Akoh, C.C.; Martino, K.G.; MacLean, D.D. Physico-Chemical Characteristics of Juice Extracted by Blender and Mechanical Press from Pomegranate Cultivars Grown in Georgia. Food Chemistry 2012, 133(4), 1383–1393.
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total Phenolic Contents and Antioxidant Activities of Pomegranate Peel, Seed, Leaf and Flower. Journal of Medicinal Plants Research 2012, 6(32), 4724–4730.
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant Activity and Total Phenolic, Organic Acid and Sugar Content in Commercial Pomegranate Juices. Food Chemistry 2009, 115(3), 873–877.
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chemistry 2006, 96(2), 254–260.
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. Journal of Agricultural and Food Chemistry 2000, 48(10), 4581–4589.
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly) Phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC–Msn. Molecules 2012, 17(12), 14821–14840.
- Tanaka, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XL. Revision of the Structures of Punicalin and Punicalagin, and Isolation and Characterization of 2-O-Galloylpunicalin from the Bark of Punica granatum L. Chemical and Pharmaceutical Bulletin 1986, 34, 650–655.
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC–Msn. Phytochemistry 2003, 64(2), 617–624.
- Lu, J.; Ding, K.; Yuan, Q. Determination of Punicalagin Isomers in Pomegranate Husk. Chromatographia 2008, 68(3–4), 303–306.
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, H.Y. Antioxidant and Hepatoprotective Effects of Punicalagin and Punicalin on Acetaminophen-Induced Liver Damage in Rats. Phytotherapy Research 2001, 15(3), 206–212.
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid Large Scale Purification of Ellagitannins from Pomegranate Husk, A By-Product of the Commercial Juice Industry. Separation and Purification Technology 2005, 41(1), 49–55.