ABSTRACT
Pectin extracted from jackfruit waste was dried using different drying techniques, and the chemical composition and structure, flowability, appearance, solubility, and flow behaviour of the dried materials were determined. Yield, galacturonic acid content, and the degree of esterification were found within the ranges of 7–11, 62–68, and 59–66%, respectively, for freeze-dried, spray-dried, vacuum oven-dried, and oven-dried pectin samples from jackfruit waste. Fourier transform infrared spectral analysis showed no major structural differences in the pectin samples produced by the various drying techniques, and their surface structures were comparable to those of analytical and food pectin. The drying methods did not significantly affect the chemical composition (p > 0.05) but did significantly affect the physical appearance, viscosity, and solubility of jackfruit waste pectin (p < 0.05).
Introduction
The physicochemical properties and structural entities of pectin depend on their source and extraction method. The steps followed for commercial pectin production are extraction with hot dilute acid while controlling pH and temperature to obtain pectin with the desired levels of esterification, centrifugation of extract, filtration, precipitation with alcohol, washing with dilute alcohol, pressing to remove excess water, and finally gentle drying.[Citation1] Pectin is a valuable functional food ingredient mainly used for applications, such as a gelling agent,[Citation2] emulsifier,[Citation3,Citation4] thickener,[Citation5] drug-delivery carrier,[Citation6] and binder.[Citation7] The physicochemical and functional properties of this natural plant-based biopolymer are extensively influenced by many factors, such as the chemical composition and the molecular structure of the biopolymer. On the other hand, extraction, purification, drying, and further modification processes can significantly affect the chemical composition and molecular structure, thereby influencing the functional properties of biopolymers.[Citation8,Citation9] The drying process is a critical operation in food because it may induce undesirable changes in colour,[Citation10] texture, density, porosity, sorption characteristics, and overall quality of the dehydrated products.[Citation11] Since the drying process removes a large portion of moisture from food, the final characteristics of the dried product are extensively influenced by the type and the conditions of the drying process.[Citation12] The same raw material may end up as a completely different product depending on the drying conditions. Commonly used drying techniques for processing of pectin are oven drying,[Citation13] vacuum drying,[Citation2] spray drying,[Citation14] and freeze drying.[Citation15] It is obvious that a drying operation at high temperature and for a long time would result in the degradation of flavour compounds, colour, and nutrients of the dehydrated product, thus reducing the quality and overall acceptability of the final product.[Citation11,Citation16]
The jackfruit-processing industry and vendors have disposed of rinds and cores as wastes. Approximately 60% of the whole jackfruit is considered as waste. The disposal of these wastes may have negative environmental effects. Nevertheless, proper utilisation of jackfruit waste could turn it into a valuable commodity and reduce the cost of waste disposal. To reduce the wastes and their negative impact to the environment, beneficial compounds, such as pectin, in jackfruit wastes can be extracted. Koh et al.[Citation17] extracted pectin from jackfruit rind by using acid extraction and alcohol precipitation. Suhaila and Zahariah[Citation18] subsequently optimised the extraction by different extraction (acid and chelating agent) and precipitation (alcohol and acetone) media for the yield and purity of jackfruit waste pectin. In all these cases, low temperature oven-drying was shown to be favourable in getting good-quality pectin powder. A previous study described the effect of the drying process on soy hull pectin and compared it with commercial and analytical grade pectin.[Citation9] However, there are hardly any reports available on the effect of drying methods on the composition and functional properties of jackfruit waste pectin, as ascertained by a literature search. Hence, this study was undertaken to evaluate and compare the chemical and functional properties of freeze-dried, spray-dried, vacuum-oven dried, and oven-dried jackfruit waste pectin.
Materials and methods
Materials
Jackfruit was collected from the Germ Plasm Centre, Bangladesh Agricultural University, Mymensingh, Bangladesh. The samples were analysed in the Unit Operation Lab, Department of Process and Food Engineering, Malaysia. Analytical-grade citrus pectin was purchased from Across Organics Co. Ltd. (New Jersey, USA). Commercial food grade pectin was obtained from Yantai Andre Pectin Co. Ltd. (Yantai, China).
Preparation of jackfruit waste powder Alcohol Insoluble Solids (AISs)
Jackfruit waste was washed until all adhering substances were removed, cut into small pieces, and dried in a Cabinet Drier at 60°C. The dried waste was ground to powder (particle size of 350 micron mesh) using a mechanical grinder (Buhlar Gold, USA). The AISs were prepared following the methods reported by Kuobala et al.[Citation2] with slight modifications. The ground jackfruit waste powder was suspended in 85% (v/v) ethanol at 70°C for 30 min in a shaking water bath. The resulting AIS were washed four times with the same concentration of ethanol and air-dried at 50°C to a constant weight.
Extraction of jackfruit waste pectin
Pectin was extracted from jackfruit waste AISs (100 g) by using 4.0 L of dilute mineral acid adjusted to pH 1.5 using 0.1 N H2SO4 and heated at 90°C for 90 min with constant stirring.[Citation19] The hot extract was filtered through four folds of nylon cloth, cooled to room temperature, and centrifuged at 11000 rpm at 4°C for 10 min. The supernatant was then dispersed in an equal volume of 95% ethanol and allowed to settle for 1 h. The prepared precipitate was washed 3–4 times (until the decant water became colourless) with 70% ethanol followed by washing with 96% ethanol to minimise impurities. The purified pectin was pressed and then dried using one of the selected drying methods.
Pectin drying
Four different drying methods (oven, vacuum oven, spray, and freeze drying) were employed to dry the pectin precipitates. For air-oven drying, the product was dried at 40°C in an air oven to constant weight.[Citation7] For vacuum-oven drying, the precipitates were dried at 40°C under vacuum (24̋ Hg) in a vacuum oven (Fisher Scientific, Hampton, NH, USA) to constant weight.[Citation19] For spray drying, the precipitates were dispersed in deionised water and spray-dried in a lab plant SD-05 (West Yorkshire, UK) spray drier at 150°C inlet temperature and at 100°C outlet temperature.[Citation20] The pectin precipitate was dispersed in deionised water and freeze dried by using a Genesis freeze dryer (The Virtis Company, Gardiner, NY, USA).[Citation21] The dried pectin samples were stored for further analysis. The yield of pectin from each drying method was calculated by the following formula:
Physicochemical properties of pectin
Moisture and galacturonic acid content
The moisture content was determined by drying the pectin powder in the oven at 105°C for 24 h.[Citation22] The anhydro galacturonic acid (GalA) content of pectin was estimated using a colorimetric assay[Citation23] with slight modification.[Citation24] Samples (1 mg/mL) of 400 μL were taken in 15 ml glass tubes. A total of 40 μL of 4 M sulfamic acid-potassium sulfamate solution with a pH of 1.6 was added to all glass tubes and mixed thoroughly by vortex mixture. Following that, 2.4 mL of 75 mM sodium tetraborate in the presence of analytical grade (96.4%) H2SO4 was added, and the solution was stirred vigorously, again using vortex mixture. The solution was placed in a 100°C (boiling) water bath for 20 min and then cooled by plunging tubes into an ice bath for 10 min. After cooling, 80 μL of 0.15% (w/v) m-hydroxydiphenyl in 0.5% (w/v) NaOH was added to the glass tubes containing samples while 80 μL 0.5% (w/v) NaOH was added to glass tubes containing the control. The mixture was stirred vigorously with the vortex. The absorbance of the samples was read at 525 nm against the control. Galacturonic acid content was determined by using a standard curve of galacturonic acid.
Colour values of jackfruit waste pectin
Hunter colour values of jackfruit waste pectin produced by various drying methods were measured by using a colour reader (Minolta Model DP 301, Minolta, Japan). Before each measurement, the colour reader was standardised with the reference white plates provided with the equipment.[Citation9]
Mass-volume-surface area related properties
The material properties, including bulk density, tapped density, true density, and particle size of jackfruit pectin powders, obtained using the different drying methods were determined by standard methods.[Citation25] The true density of the pectin powder was determined by a gas pycnometer (Accupyc II 1340; Micromeritics, Norcross, GA, USA). A 10 ml measuring cylinder was used to measure the bulk density. The weight (W) and volume (V) of the powder were taken inside the measuring cylinder, and the bulk density of the powder was determined by the formula (2). After recording the initial volume, the cylinder was tapped mechanically upwards to reach a constant volume (VT). The tap density was calculated by formula (3). The Hausner ratio[Citation26] and Carr index[Citation27] were calculated by formulas (4) and (5), respectively. The mean particle size and surface area of the extracted pectins and commercial pectin were measured using a particle size analyser (Malvern Mastersizer 2000, Malvern Instrument Ltd, Worcestershire, U.K.).
Structural properties analysis
Surface structure analysis and degree of esterification
Diffuse reflectance Fourier transform infrared (FTIR) spectra of jackfruit waste pectin samples were collected using a Perkin Elmer spectrum 100 (USA) with wavenumber ranging from 400 to 4000 cm−Citation1. FTIR spectra were analysed for surface chemical functional groups by OMNIC software and compared with those of both commercial pectin and analytical grade pectin. The degree of esterification (DE) of the pectin was determined using the peak area relation of the free carboxyl groups (1650 cm−Citation1) and esterified groups (1750 cm−Citation1)[Citation28] as mentioned in the following equation (6).
Scanning electron microscopy (SEM)
The powder morphology (size and surface area) was measured using a scanning electron microscope (JSM-6400, JEOL, Japan) following the method reported in the literature.[Citation29] A small amount of powder was mounted on gold-coated SEM stubs. The SEM analysis was carried out at an accelerating voltage of 5 kV, and the micrographs were captured at magnifications of ×100, ×300, and ×600.
Functional properties analysis
Pectin solubility
The water solubility of dried jackfruit waste pectin samples was determined gravimetrically.[Citation9] Pectin samples (3 g) were dispersed in 100 ml deionised water and adjusted to pH 2, 4, 6, 8, or 10 with either 0.1 M HCl or 0.1 M NaOH. The insoluble pectin portions were determined after centrifugation (2700xg, 15 min, Eppendorf AG, Germany) and were used to determine pectin solubility.
Rheology of pectin solution
The rheology of 3% pectin solution in deionised water (w/v) was determined with a Controlled Stress Rheometer (AR G2, UK) with a cone-plate measuring system (cone dia. 60 mm truncation 30 micrometer).[Citation9] All rheological experiments were carried out at constant temperature (20°C) immediately after preparation of the pectin solution. The shear rate was increased from 0 to 1000 s−Citation1 by the instrument-control software, and strain measurement was recorded. Viscosity was plotted against shear rate.
Statistical analysis
Results of three replicates were used for statistical analysis. The values were expressed as the mean ± standard deviation. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) (version 21) for Windows. The Duncan test was performed to evaluate the significant differences among the mean values. The confidence limits used in this study were based on 95% (p < 0.05).
Results and discussion
Yield and physicochemical properties of pectin
The pectin yield, moisture content, galacturonic acid content, and the degree of esterification of jackfruit waste pectin produced by various drying methods are presented in . The yield, moisture content, galacturonic acid content, and the degree of esterification for freeze-dried, spray-dried, vacuum-oven-dried, and oven-dried jackfruit waste pectin samples ranged between 7.3–11.7, 7.3–9.2, 62.7–68.8, and 59.4–66.0%, respectively. There was no significant difference in yield for oven-dried, vacuum-oven-dried, and freeze-dried jackfruit waste pectin. The yield for spray-dried jackfruit waste pectin was significantly lower (7.3%) than oven-dried (11.3%), vacuum-oven-dried (11.7%), and freeze-dried (11.2%) jackfruit waste pectin. The low yield of pectin using spray drying is attributed to the high loss during drying, which might due to the small operating volume for drying in this study.[Citation9] The jackfruit waste pectin yield reported in an earlier study was 14–17%.[Citation17] This may be due to variations in the pectin content, varieties of jackfruit, and methods used for extraction. The moisture content of all jackfruit waste pectin produced by the different drying techniques was less than 12%, which is in agreement with the requirement of the Food Chemical Codex.[Citation30] There was no significant difference in the galacturonic acid content of jackfruit waste pectin produced by the various drying methods, commercial pectin, and analytical pectin. The galacturonic acid contents in this study were comparable to that of commercial citrus pectin, which contained 70% galacturonic acid.[Citation31] Jackfruit waste pectin could be categorised as a high methoxy pectin as the degree of esterification values for jackfruit waste pectin produced by the various drying methods were observed to be higher than 50%. No significant difference was observed in the DE value of pectin produced by the different drying processes. The effect of the drying techniques was also reported as insignificant on the galacturonic acid content and degree of esterification of pectin extracted from soy hull.[Citation9]
Table 1. Pectin yield, moisture content, galacturonic acid content, and degree of esterification of freeze dried, spray dried, vacuum dried, and oven dried jackfruit waste pectin, commercial and analytical grade pectin.
Colour
The colour of pectin is an important parameter as it affects the appearance of the final product. Colour values of the dried pectin using different drying methods and the analytical and food grade pectin samples are presented in . The Hunter ‘L’, ‘a’, and ‘b’ values for freeze-dried, spray-dried, vacuum-dried and oven-dried jackfruit waste pectin samples ranged between 43.8–71.0, 4.3–10.4, and 18.3–22.8, respectively. Significant differences were observed among the Hunter colour values of the analysed pectin samples. The spray-dried pectin had the highest colour values for lightness (L-value), which exhibited values similar to that of the analytical grade pectin. Oven-dried pectin followed by vacuum-dried pectin had the lowest Hunter values for lightness. Pectin obtained by freeze drying, spray drying, and vacuum drying did not affect the Hunter values for yellowness, and hence values were not significantly different (p < 0.05) from analytical grade pectin. Results indicated that the vacuum-oven-drying and oven-drying methods had the most negative effect on appearance among the drying treatments assessed in this study. Depending on the food application, a drying method may be chosen to obtain pectin with the desired appearance. The lightness (L*) values of the present study are lower than that of guava[Citation32] pectin but higher than that of soy hull pectin.[Citation9]
Table 2. Effect of drying methods on colour of jackfruit waste pectin.
Mass-volume-surface area properties of jackfruit waste pectin powder
The quality of powder is affected by different physical properties such as bulk density, tapped density, true density, inter-particle porosity, particle size, shape, and distribution. presents the material properties of jackfruit waste pectin powder obtained by the different drying processes, commercial and analytical grade pectin. Density is the critical parameter that affects the functional properties of powder. The bulk and tapped densities provide a standpoint from the packing and arrangement of the particles and the compaction profile of a material.[Citation33] The drying process significantly (p < 0.05) influences the bulk density and tapped density of pectin powder. In the current study, the bulk density and tapped density of jackfruit waste pectin powder ranged from 111.3 to 702.5 kg/mCitation3 and 144 to 826.5 kg/mCitation3, respectively, depending on the drying techniques used. The bulk density and tapped density of analytical grade (623 kg/mCitation3) and commercial grade pectin (623 kg/mCitation3) were closer to the density of oven-dried and vacuum-oven-dried pectin. The bulk density values are lower than those previously found for guava[Citation32] and mango peel pectin.[Citation34] The results revealed that spray drying followed by freeze drying significantly reduced the bulk density and tapped density. The density depends on the attractive interparticle force, particle size, shape, and number of contact positions.[Citation35] The reduced particle size of spray-dried and freeze-dried pectin might be the reason for the significant change in bulk density. A similar drying effect was reported in the case of durian seed gum.[Citation36]
Table 3. Effect of drying techniques on the mass-volume-surface area properties of pectin.
Porosity was found to be reversely and similarly related to bulk density and tap density, respectively ([1-(bulk density/tap density)] x100).[Citation37] Hence, the bulk density and the tap density results indicate that the spray-dried and freeze-dried pectin are more porous than others. It is well documented that in the freeze-drying technique, the material is first frozen allowing it to maintain its structure following sublimation of ice under high vacuum.[Citation38] Since there is no liquid phase present in the material during this process, there is no transfer of liquid water to the surface, but instead the ice changes to vapour below the collapse temperature.[Citation39] In consequence, the collapse and the shrinkage of the product is prevented, thereby resulting in a porous dried material.[Citation40] The higher porosity (data are not presented) or lower bulk density in spray-dried pectin powder might be due to hot air coming in contact with the liquid product during spray drying and forming a skin which cause the accumulation and trapping of air inside the particle; consequently, it becomes less dense and porous.[Citation41] On the other hand, the porosity and the bulk density of vacuum-oven-dried and oven-dried pectin were significantly lower and higher than the freeze-dried and spray-dried product, respectively, as these techniques give rise to a compact non-porous structure.[Citation42]
The true density of jackfruit waste pectin ranges from 1157.8 to 1500.4 kg/mCitation3. Results revealed that spray drying and freeze drying reduced the true density. Commercial and analytical grade pectins were also found similar to oven-dried pectin in the sense of true density. Average particle size and specific surface area of jackfruit waste pectin, analytical and commercial grade pectin are presented in . Spray-dried pectin powder showed significantly lower particle size and higher specific surface area than the other samples. Particle size is the material property that influences the powder compaction, flowability, segregation, and other factors.[Citation43] Powder with small particles exhibits poor flow properties and causes handling problems due to caking.[Citation43] The average particle size of jackfruit waste pectin powder prepared by spray drying was significantly lower than all others (). The flowability characteristics of powder can be calculated by the Hausner ratio[Citation26] and the Carr index.[Citation27,Citation43] A lower Hausner ratio (1.00–1.11) and Carr index (0–10) indicate excellent flowability. In contrast, a higher Hausner ratio (1.46–1.59) and Carr index (32–37) indicate very poor flowability. Jackfruit waste pectin produced by freeze drying, spray drying, vacuum-oven drying, and oven drying exhibited Hausner ratios within the range of 1.18–1.29 and Carr index within 15–22.7 which indicated good to passable flowability whereas commercial pectin showed fair flowability and analytical grade pectin observed good flowability. Powder particle size has a great impact on powder flowability.[Citation44] Smaller particles reduce the powder flowability.[Citation43] In this study, spray-dried and freeze-dried pectin showed lower particle size which might cause poor flowability.
Structural properties
Scanning electron micrograph
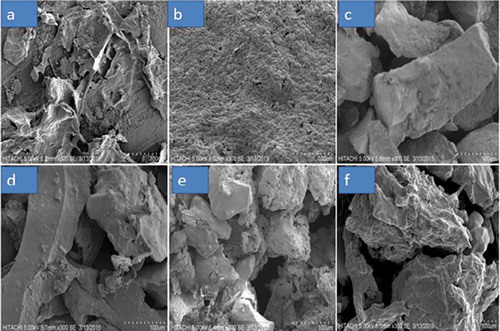
SEM of jackfruit waste pectin powder presented the morphology and the surface appearance of jackfruit waste pectin powder influenced by drying methods and of commercial grade and analytical grade pectin powder. The micrographs are shown in . The micrographs show that all pectin particles had irregular granular powder shapes and had a rough surface. A previous study on apple pomace pectin and mango pectin also found irregular and rough surfaces.[Citation45] An image of spray-dried pectin showed indistinct particle and form clots. This might be due to the low particle size of spray-dried powder and the agglomeration of pectin. Commercial pectin also showed similar results due to small particle size.
Surface structure analysis
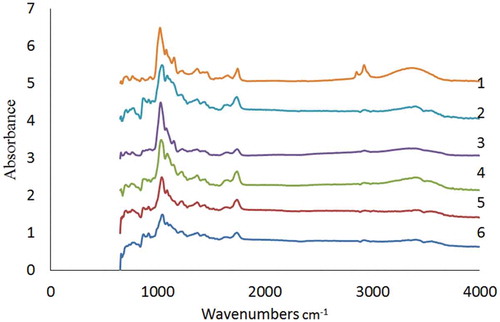
The infrared (diffuse reflectance FTIR) spectra (400–4000 cm−Citation1) of jackfruit waste pectin produced by freeze drying, spray drying, vacuum-oven drying, oven drying, analytical-grade, and commercial-grade pectin samples are presented in . The FTIR analysis was normally used to assess the conformation of pectin bands in the standard region usually between 1000 and 2000 cm−Citation1 for the major chemical and functional groups.[Citation46,Citation47] Bands around 1650 cm−Citation1 and 1750 cm−Citation1 represented the free and esterified carboxyl groups, respectively. It was observed that the esterified carboxyl groups showed an increasing trend in their intensities and band areas as the DE values increased. FTIR spectra in the present study showed no major structural differences in pectin samples produced by various drying treatments. The jackfruit waste pectin structures were comparable to those of analytical and food grade pectin samples. In the figure, the region between 1000 and 1140 cm−Citation1 corresponds to the stretching vibrations of the (C–OH) side groups and the (C–O–C) glycosidic bond vibration. The absorption bands between 1100 and 1200 cm−Citation1 were from the ether (R–O–R) and cyclic C–C bonds in the ring structure of pectin molecules. The absorption band at 1500 cm−Citation1 was due to the OH bending vibration. The band around 1540–1560 cm−Citation1 corresponds to the protein amide in the pectin molecules. The regions between 1590 and 1600 cm−Citation1 indicate the aromatic ring stretching. The broader band from 2400 to 3600 cm−Citation1 was from stretching of the hydroxyl (O–H) groups due to moisture in the pectin samples. The FTIR structural analysis showed that the drying methods had no detrimental effect on the structure of jackfruit waste pectin.
Functional properties
Solubility
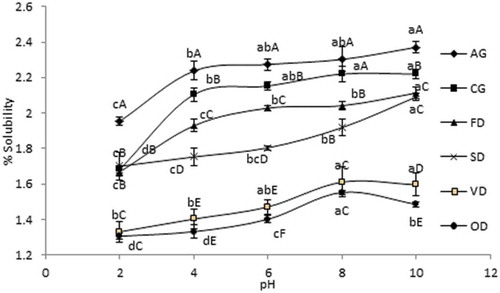
The solubility of jackfruit waste pectin samples produced by freeze drying (FD), spray drying (SD), vacuum-oven drying (VD), oven drying (OD) and commercial grade (CG) and analytical grade (AG) pectin samples are presented in . The present study showed that the drying process significantly (p < 0.05) influenced the solubility of jackfruit waste pectin. Among the pectin samples, the lowest solubility was observed in case of oven-dried pectin whereas analytical pectin showed the highest solubility. The solubility of jackfruit waste pectin samples produced by the freeze-drying and spray-drying methods were comparable to that of commercial food-grade pectin. The high solubility of freeze-dried and spray-dried pectin could be due to their high porosity, smaller particle size, and higher surface area. Freeze-drying and spray-drying techniques significantly reduced the particle size which facilitated pectin particles to become more soluble and amorphous. Between the FD and SD pectin, the SD pectin showed lower solubility than FD pectin even though it had a higher surface area. This was due to abundant smaller particles produced by the high-speed atomisation, which tends to produce a high temperature at the surface[Citation48] and therefore is more sensitive to thermal damage and reduced solubility. The pH values (2 to 10) also have a significant effect on the solubility of the pectin samples. All pectin samples examined in this study are least soluble at pH 2, and the solubility was increased at pH values higher than 4. The stability and hydration rate of high methoxy pectin are significantly affected by the variation in pH.[Citation49] A previous study had reported the poor water solubility of pectin at pH< 4.0 but its high solubility at pH 7.0 or above.[Citation50] Oakenfull[Citation51] revealed that the number of negatively charged free carboxyl groups in high methoxyl pectin was increased as the pH increased, resulting in an increase in the repulsive force and the formation of a weaker gel. Similarly, the weaker pectin at higher pH solution could be readily dissolved.
Figure 3. Solubility of jackfruit waste pectin produced by oven drying (OD), vacuum oven drying (VD), freeze drying (FD), spray drying (SD) methods; commercial (CG) and analytical grade (AG) pectin. The same letter for line graph means that there is no significant difference (p < 0.05). Letter a,b,c,d and A, B, C, D, E represent pH and pectin sample, respectively.
Rheology of pectin solution
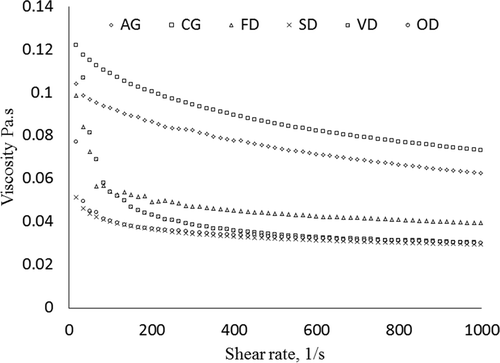
The viscosity properties of 3% (w/w) jackfruit waste pectin produced by freeze drying, spray drying, vacuum-oven drying, and oven drying as well as commercial pectin and analytical grade pectin were evaluated. The apparent viscosity as a function of shear rate for the jackfruit waste pectin, commercial pectin, and analytical-grade pectin are presented in . In general, all pectin samples demonstrated shear-thinning behaviour (the viscosity decreased with an increase in shear rate), which is a typical behaviour of pectin solution. This is due to the polymer chain of pectin being stretched out and elongated in the direction of flow, therefore producing less resistance to flow.[Citation52] The changes in viscosity for jackfruit waste pectin samples produced by various drying methods were greater than the commercial food grade pectin and the analytical grade pectin. This indicated that the viscosities of jackfruit waste pectin produced by the various drying methods were lower than those of the commercial food grade pectin and the analytical grade pectin. The viscosity as a function of shear rate also indicated that the pectin produced by freeze drying followed by spray-drying methods showed higher viscosity than by vacuum-drying and oven-drying methods. This may be due to the natures of freeze-dried and spray-dried pectin in that they could disperse uniformly and hence increase the viscosity of the pectin solution. However, the drying techniques did not affect the viscosity of soy hull pectin in a previous study.[Citation9]
Conclusion
Drying is an important process for food preservation, and different drying techniques so far developed have a remarkable influence on the quality of the finished products. Jackfruit waste pectin obtained by freeze-, spray-, vacuum oven-, and oven-drying showed significant variations in terms of physical appearance, viscosity, and solubility as well as the mass, volume, and surface area properties. Freeze-dried samples had higher solubility and viscosity, with brighter colour than those obtained by other drying methods. Smaller particle size, greater surface area, and lower flowability were observed in the spray-dried samples. On the other hand, the oven-dried samples were similar to the vacuum-dried samples with regard to colour, solubility, viscosity, and flowability. However, no significant effect of drying methods was observed on the composition and structure of the pectin. In conclusion, depending on the food application or end-product quality, a suitable drying method may be chosen to obtain pectin with the desired functional properties and appearance.
Funding
The authors would like to acknowledge the Ministry of Education, Bangladesh (project code: 2000/99/MoE), the Organization for Women in Science for the Developing World (OWSD) for their financial support, and the Ministry of Education Malaysia for granting the Fundamental Research Grant Scheme (FRGS) with project code 03-02-13-1289FR. This paper was first presented at the Second International Conference on Food Properties (iCFP 2) held in Bangkok, Thailand on 31 May–2 June, 2016, and it received recognition as one of the best papers
Additional information
Funding
References
- Herbstreith, Fox, Corporate Group. Pectin a product of nature, 2005, Available in http://www.herbstreith-fox.de/pdf/ehfnatur.
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.J.; Thibault, J.F.; Ralet, M.C. Effect of Extraction Conditions on Some Physicochemical Characteristics of Pectins from “Améliorée” and “Mango” Mango Peels. Food Hydrocolloids 2008, 22(7), 1345–1351.
- Coma, V. Polysaccharide-Based Biomaterials with Antimicrobial and Antioxidant Properties. Polímeros Ciência E Tecnologia 2010, 20(2), 1–12.
- Kartal, C.; Unal, M.K.; Otles, S. Production and Stabilization of a Flaxseed Oil Multi-Layer Emulsion Containing Sodium Caseinate and Pectin. International Journal of Food Properties 2016, 19 (6), 1–12.
- Bagherian, H.; Zokaee, A.F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chemical Engineering and Process Intensification 2011, 50(11–12), 1237–1243.
- Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A Novel Pectin Material: Extraction, Characterization and Gelling Properties. International Journal of Molecular Science 2010, 11(10), 3686–3695.
- Khule, N.R.; Mahale, N.B.; Shelar, D.S.; Rokade, M.M.; Chaudhari, S.R. Extraction of Pectin from Citrus Fruit Peel and Use as Natural Binder in Paracetamol Tablet. Der Pharmacia Lettre 2012, 4(2), 558–564.
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between Different Techniques for Water-Based Extraction of Pectin from Orange Peels. Desalination 2008, 218(1–3), 229–237.
- Monsoor, M.A. Effect of Drying Methods on the Functional Properties of Soy Hull Pectin. Carbohydrate Polymer 2005, 61(3), 362–367.
- Katekhong, W.; Charoenrein, S. Color and Gelling Properties of Dried Egg White: Effect of Drying Methods and Storage Conditions. International Journal of Food Properties 2016, Published online: 19 Oct 2016, 1–12.
- Drouzas, A.E.; Tsami, E.; Saravacos, G.D. Microwave/Vacuum Drying of Model Fruit Gels. Journal of Food Engineering 1999, 39(2), 117–122.
- Sundaram, J.; Durance, T.D. Water Sorption and Physical Properties of Locust Bean Gum-Pectin-Starch Composite Gel Dried Using Different Drying Methods. Food Hydrocolloids 2008, 22(7), 1352–1361.
- Wang, Y.; Li, D.; Wang, L.J.; Li, S.J.; Adhikari, B. Effects of Drying Methods on the Functional Properties of Flaxseed Gum Powders. Carbohydrate Polymer 2010, 81(1), 128–133.
- Carvajal-Millan, E.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Micard, V.; Leon, N.P.; De, Gardea, A. Maize Bran Gum: Extraction, Characterization and Functional Properties. Carbohydrate Polymer 2007, 69(2), 280–285.
- Barresi, A.A.; Pisano, R.; Fissore, D.; Rasetto, V.; Velardi, S.A.; Vallan, A.; Parvis, M.; Galan, M. Monitoring of the Primary Drying of a Lyophilization Process in Vials. Chemical Engineering and Processing: Process Intensification 2009, 48(1), 408–423.
- Jaya, S.; Das, H. A Vacuum Drying Model for Mango Pulp. Drying Technology 2003, 21(7), 1215–1234.
- Koh, P.C.; Leong, C.M.; Noranizan, M.A. Microwave-Assisted Extraction of Pectin from Jackfruit Rinds Using Different Power Levels. International Food Research Journal 2014, 21(5), 2091–2097.
- Suhail, M.; Zahariah, H. Extraction and Characterization of Pectin from Various Tropical Agrowastes. ASEAN Food Journal 1995, 10(2), 43–50.
- Rehman, Z.U.; Salariya, A.M.; Habib, F.; Shah, W.H.. Utilization of Mango Peels as a Source of Pectin. Journal of Chemical Society of Pakistan 2004, 26(1), 73–76.
- Monsoor, M.A.; Proctor, A. Preparation and Functional Properties of Soy Hull Pectin. Journal of the American Oil Chemists’ Society 2001, 78(7), 709–713.
- Kalapathy, U.; Proctor, A. Effect of Acid Extraction and Alcohol Precipitation Conditions on the Yield and Purity of Soy Hull Pectin. Food Chemistry 2001, 73(4), 393–396.
- AOAC. Official Methods of Analysis 13th edition, Association of Official Analytical Chemistry, Washington, DC., UK, 1984, 1018 p.
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of Uranic Acids without from Neutral Sugars. Analytical Biochemistry 1991, 197, 157–162.
- Ibarz, A.; Pagán, A.; Tribaldo, F.; Pagán, J. Improvement in the Measurement of Spectrophotometric Data in the M-Hydroxydiphenyl Pectin Determination Methods. Food Control 2006, 17(11), 890–893.
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; et al. Physicochemical and Nutritional Properties of Spray-Dried Pitaya Fruit Powder as Natural Colorant. Food Science Biotechnology 2012, 21(3), 675–682.
- Hausner, H.H. Friction Conditions in a Mass of Metal Powder. International Journal of Powder Metallurgy 1967, 3, 7–13.
- Carr, R.L. Evaluating Flow Properties of Powders. Chemical Engineering 1965, 72, 116–124.
- Gnanasambandam, R.; Proctor, A. Determination of Pectin Degree of Esterification by Diffuse Reflectance Fourier Transform Infrared Spectroscopy. Food Chemistry 2000, 68(3), 327–332.
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of Maltodextrin Concentration and Inlet Temperature during Spray Drying on Physicochemical and Antioxidant Properties of Amla (Emblica officinalis) Juice Powder. Food and Bioproduct Processing 2014, 92(3), 252–258.
- FCC. Food Chemical Codex. 3rd edition, National Academy of Science, Washington, DC., UK, 1981, 518 p.
- Kravtchenko, T.P.; Voragen, A.G.J.; Pilnik, W. Analytical Comparison of Three Industrial Pectin Preparations. Carbohydrate Polymer 1992, 18(1), 17–25.
- Thongsombat, W.; Sirichote, A.; Chanthachum, S. The Production of Guava Juice Fortified with Dietary Fiber. Songklanakarin Journal of Science and Technology 2007, 29(1), 187–196.
- Singh, A.K.; Selvam, R.P.; Sivakumar, T. Isolation, Characterisation and Formulation Properties of a New Plant Gum Obtained from Mangifera indica. International Journal of Biomedical Research 2010, 1(2), 35–41.
- Malviya, R.; Kulkarni, G.T.. Extraction and Characterization of Mango Peel Pectin as Pharmaceutical Excipient. Polymer in Medicine 2012, 42(3–4), 185–190.
- Peleg, M.L.; Bagley, E.B. Physical Properties of Foods; AVI Publishers Co.: Westport, CN, 1983.
- Mirhosseini, H.; Amid, B.T. Effect of Different Drying Techniques on Flowability Characteristics and Chemical Properties of Natural Carbohydrate-Protein Gum from Durian Fruit Seed. Chemistry Central Journal 2013, 7(1), 1.
- Benković, M.; Bauman, I. Flow Properties of Commercial Infant Formula Powders. World Academy of Science, Engineering and Technology 2009, 54(6), 495–499.
- Oetjen, G.W.; Haseley, P. Freeze-Drying, 2nd edition, Wiley-VCH Weinheim, Germany; 2004.
- Krokida, M.K.; Maroulis, Z.B. Effect of Drying Method on Shrinkage and Porosity. Drying Technology 1997, 15(10), 2441–2458.
- Karel, M. Freeze Dehydration of Foods In Principles of Food Science, Part II: Physical principles of food preservation; Karel, M.; Fennema, O.R.; Lund, D.B.; Eds.; Marcel Dekker, Inc.: New York, USA, 1975; 359–395.
- Goula, A.M.; Adamopoulus, K.G. Effect of Maltodextrin Addition during Spray Drying of Tomato Pulp in Dehumidified Air: II. Powder Properties. Drying Technology. 2008, 26, 726–737.
- Kang, O.L.; Yong, P.F.; Maaruf, A.G.; Osman, H.; Nazaruddin, R. Physicochemical and Antioxidant Studies on Oven-Dried, Freeze-Dried and Spray-Dried Agaro-Oligosaccharide Powders. International Food Research Journal 2014, 21(6), 2363–2367.
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of Instant Soymilk Powders by Ultrafiltration, Spray Drying and Fluidized Bed Agglomeration. Journal Food Engineering 2008, 84(2), 194–205.
- Fitzpatrick, J.J. Particle Properties and the Design of Solid Food Particle Processing Operations. Food and Bioproducts Processing 2007, 85(4), 308–314.
- Jiang, Y.; Du, Y.; Zhu, X.; Xiong, H.; Woo, M.W.; Hu, J. Physicochemical and Comparative Properties of Pectins Extracted from Akebia Trifoliata Var. Australis Peel. Carbohydrate Polymer 2012, 7(2), 1663–1669.
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydrate Polymer 2000, 43(2), 195–203.
- Walter, R.H. The Chemistry and Technology of Pectin Vol. 24; Academic Press: New York, USA, 1991; 197 p.
- Perez-Munoz, F.; Flores, R.A. Particle Size of Spray-Dried Soymilk. Applied Engineering in Agriculture 1997, 13, 647–652.
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-Based Systems for Colon-Specific Drug Delivery via Oral Route. Biomaterials 2003, 24, 3333–3343.
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.K.; Jain, S.K. Metronidazole Loaded Pectin Microspheres for Colon Targeting. Journal of Pharmaceutical Science 2009, 98(11), 4229–4236.
- Oakenfull, D.G. The Chemistry of High-Methoxy Pectins. In R.H. Walter (Ed.), The Chemistry and Technology of Pectin; Academic Press: New York, USA, 1991; 88–108.
- Dickinson, E. An Introduction to Food Colloids; Oxford University Press: Oxford, UK, 1992.