ABSTRACT
As the major commercial goji berry in the world, Ningxia goji (Lycium barbarum L.) berries are generally used as a traditional herbal medicine and functional food. This berry was appreciated by consumers due to its unique aroma. However, the berry’s characteristic aroma compounds are still unknown. Volatiles of Ningxia goji berries (Lycium barbarum L.) were characterized by gas chromatography–mass spectrometry (GC–MS), and their characteristic aroma components were identified by aroma dilution analysis (AEDA) and gas chromatography–olfactometry (GC–O). The developmental changes in these fruits were also monitored. Among 56 volatile compounds identified, hexanal and (E)-2-hexenal predominated in the Ningxia goji berries, which accounted for 70–94% of the total volatiles. Flavor dilution factors (FD) of 17 identified compounds were 27, but only 15 compounds had more than one odor activity value (OAV). The combined results of the GC–O and OAV revealed that hexanal, (E)-2-hexenal, nonanal, isoamylol, 1-hexanol, 1-octen-3-ol, hexyl acetate, methyl salicylate, ethyl octanoate, o-cymene, d-limonene, linalool, β-cyclocitral, β-elemene, and 2-pentylfuran were the characteristic aroma volatiles in the Ningxia goji berries. During fruit development, the content of hexyl acetate, linalool, and 2-pentylfuran increased remarkably. Conversely, β-cyclocitral and β-elemene increased at the early stage, but decreased with other characteristic compounds. We first identified the characteristic aroma volatiles of the Ningxia goji berries and determined the dynamic changes of the volatiles during development.
Introduction
Lycium plants are distributed widely in the world, and their fruits are called goji berries. These berries are generally used as a traditional herbal medicine and functional food in Asian countries.[Citation1,Citation2] Of the dozens of Lycium species around the world, approximately 90% of the commercially available goji berries are produced by Lycium barbarum, which originated from the north central regions of China.[Citation3] L. barbarum (Niangxia goji) is famous for its unique aroma and nutrition quality[Citation4]
As the main commercial source of fresh goji berry fruit and products, the Ningxia goji berry has been widely described by many researchers. Amagase and Farnsworth had reviewed the botanical characteristics, phytochemistry, clinical relevance, efficacy, and safety of Lycium barbarum fruit (goji).[Citation1] Many functional compounds in goji berries were identified and their antioxidant activities were also evaluated.[Citation3] He et al.[Citation5] characterized the antioxidant and antiproliferative acidic polysaccharides from Chinese goji fruits. Inbaraj et al.[Citation6] investigated the phenolic acids and flavonoids in Lycium barbarum. The polysaccharides in Lycium barbarum Linnaeus were determined.[Citation7] Wang et al.[Citation8] isolated the carotenoids, flavonoids, and polysaccharides from Lycium barbarum L. Li et al.[Citation9] also evaluated the antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Up to date, many reports have studied the bioactivities of compounds in Lycium barbarum fruit. Ming et al.[Citation10] evaluated the effect of the Lycium barbarum polysaccharide (LBP) administration on blood lipid metabolism and oxidative stress in mice fed a high-fat diet in vivo. Wang et al.[Citation11] found that LBP inhibits the infectivity of Newcastle disease virus in chicken embryo fibroblasts. Amagase et al.[Citation2] found that Lycium barbarum (goji) juice improves antioxidant biomarkers in the serum of healthy adults. Chang et al.[Citation12] reported the use of Lycium barbarum against aging-associated diseases. Thus, even though there are many studies on Lycium barbarum, these papers have mainly focused on the functional component composition and health-promoting properties of Lycium barbarum. Chen et al.[Citation13] developed a low-temperature headspace-trap gas chromatography with mass spectrometry for the determination of trace volatile compounds from the Lycium barbarum L. fruit. Though smell is an important nutritional clue for human beings, the information about the aroma quality of Lycium barbarum L. is still unknown. The present study aimed to determine the content and composition of aroma volatiles in the Ningxia goji berry, identifies its active aroma characteristic, and describes the effect of fruit development on the aroma compound content. These findings will provide useful information for aroma regulation and quality breeding for the wolfberry.
Materials and methods
Chemicals
Hexanal, (E)-2-hexenal, (E)-2-octenal, nonanal, (E)-2-nonenal, decanal, 4-methylcyclohexanol, isoamylol, 1-pentanol, 1-hexanol, methyl 2-methylbutanoate, hexyl acetate, ethyl octanoate, methyl salicylate, ethyl octanoate, toluene, styrene, o-cymene, naphthalene, (Z)-2-dodecene, hexadecane, d-limonene, (E)-ocimene, linalool, β-elemene, dihydro-β-ionone, β-ionone, β-humulene, 3-octanone, 1,4-cineol, eucalyptol, hexyloxirane, dodecane, nonylcyclopropane, 8-methylheptadecane, 4,6-dimethyldodecane, tetradecane, heneicosane, 2-pentylfuran, 2-hexyltetrahydrofuran, hexanoic acid, guaiacol, and butylated hydroxytoluene were obtained from Sigma (St. Louis, MO, USA). Heptanal, (Z)-2-heptenal, 1-octen-3-ol, (5-decyl) benzene, thujopsene, 1-methyl-1-ethylcyclopentane, β-cyclocitral, camphocean, 1,3,5,7-cyclooctatetraene, 3,5,5-trimethyl-1-hexene, 2,6-di-tert-butyl-4-sec-butylphenol, and β-butoxyethanol were purchased from Alfa Aesar (Ward Hill, MA, USA). A standard series of C7–C30 saturated alkanes from Supelco (Bellefonte, PA, USA) was used for retention index (RI) determination. All other reagents were of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Internal standards of chlorononane and ethyl octanoate were obtained from Sigma Co. Ltd.
Fruit materials
Three Ningxia goji berry (Lycium barbarum) cultivars were grown at the National Goji Germplasm Repository at the Ningxia Academy of Agricultural Sciences in Ningxia, China (). Fruits were harvested at different developmental stages based on external color and size uniformity, including young fruit (9–12 days), cell expansion (14–19 days), turning (20–25 days), green maturation (25–30 days), and maturation (34–45 days). For every cultivar, each replicate included 200 fruits sampled from 10 trees. Among them, 50 fruits were used to determine the firmness, TA (titratable acid), and total soluble solid (TSS). The other 150 fruits were ground into a fine powder with liquid nitrogen in a freezer-mill (6750) apparatus (Glen Creston) and then passed through a 40 mesh sieve. Three replicates were carried out for each genotype. The resulting powders were stored at −80°C until further use.
Table 1. Ningxia goji cultivars used in the present study and the berry harvest time at each developmental stage.
TSS and TA determination
TSS and TA were measured according to the method described by Ramful et al.[Citation14] The berry juice was dropped on a digital refractometer (Atago PR-101 R, Tokyo, Japan) and the value was read. Each replicate contained 20 fruits and all determinations were performed in triplicate. The temperature of the sample at the time of measurement was also recorded. The degree (°) Brix of the juice was then calculated and the results were rectified by temperature. After determining the TSS, 20 berries were homogenized in a Waring blender and filtered through muslin cloth. Three mL of juice were diluted to 30 mL with distilled water and transferred to a 100 mL beaker, which was placed over a magnetic stirrer to provide continuous stirring of the sample solution. A pH meter probe was then immersed in the solution, and 0.1 N NaOH was added until the pH of the sample exceeded 8.1. TA was expressed as a percentage of citric acid (%) and three replicates were performed.
Determination of aromatic volatiles
The concentrations of volatiles were determined according to the previously reported method with some modifications.[Citation15] The volatile compounds were extracted from 1.5 g of lyophilized berry powder using 3 mL of saturated sodium chloride solution. Then 20 μL of authentic n-hexanol were added as the internal standards to quantify the volatile compounds. The homogenate was incubated at 40°C for 30 min. Volatiles were extracted by a solid-phase microextraction (SPME) needle with a 1-cm long fiber coated with 65 μm of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fibers (Supelco Co., Bellefonte PA, USA).
A GC–MS-QP2010 gas chromatograph–mass spectrometer system (Shimadzu Corporation, Kyoto, Japan) with an Rtx-5 MS (Restek)-fused silica capillary column (5% diphenyl, 95% dimethyl polysiloxane) (0.32 mm, 30 m, 0.5 μm, J&W Scientific, Folsom CA, USA) was used for compound confirmation. The injection port temperature was 240°C and the injection volume was 1 μL. Helium was used as the carrier gas at a rate of 1.0 mL/min. The GC oven temperature was held at 40°C for 3 min, increased by 4°C/min to 250°C, and finally held at 250°C for 5 min. Mass spectra were obtained by electron ionization (EI) at 70 eV with a scan range of 40–500 mass units. The detector, ion source and transfer line temperature were set to 150°C, 200°C and 250°C, respectively.
The chromatograms and mass spectra were evaluated with GC–MS Postrun Analysis software (Shimadzu, GC–MS-QP2010, Japan). The compounds were tentatively identified by comparing their mass spectra with those in the data system library (NIST08). RIs were calculated using a homologous series of n-alkanes (C7–C30), analyzed on the TR-5 and HP-Innowax columns. The concentration of these compounds was quantified by standard curves obtained in the selective ion monitoring mode and the results were expressed as μg/g FW. Three replicates were used for each sample. Odor activity values (OAVs) were determined by using the concentrations of the aroma active compounds and their respective odor threshold values in water obtained from http://www.leffingwell.com/.
GC–O and AEDA analyses
GC–O and AEDA were determined according to the method described by Liu et al.[Citation16] with some modifications. Simultaneous distillation extraction (SDE) was used to extract the volatiles. The sample flask contained 50 g of the berries with 100 mL of distilled water; 20 mL of dichloromethane was used to dissolve the sample. After distillation for 3 h, the extract was cooled to room temperature and dried over anhydrous Na2SO4. The extract was collected and concentrated in a rotary evaporator over a 30°C water bath to a final volume of 1 mL under a gentle stream of nitrogen.
Dichloromethane was used as a solvent to 3-fold dilute the berry extract for AEDA. Samples were analyzed by an expert in fragrance chemicals and flavor technology. The odor characters of the volatiles were evaluated by sniffing and flavor dilution (FD) factors were obtained. The above GC–MS-QP2010 gas chromatograph–mass spectrometer system was equipped with the olfactory detection port Gerstel ODP-2 (Gerstel AG Enterprise, Mülheim an der Ruhr, Germany) for the GC–O analysis.
Statistical analysis
All data are expressed as the means ± standard deviation of three replicates. Statistical analysis was performed using the SPSS v19.0 software (SPSS Inc., Chicago, IL, USA). Significant differences among the samples were calculated using a one-way ANOVA followed by Duncan’s multiple-range test at the 5% level (p ≤ 0.05).
Results and discussion
Berry weight, total soluble solids, and titratable acidity
The berry weight, TSS and TA of the Ningxia goji berries are shown in . The berry weight of the NJ1, BH and NXH berries increased from 0.05 to 0.54 g, 0.07 to 0.55 g, and 0.04 to 0.52 g, respectively. The TSS of the goji cultivars tested increased from 5.13 to 13.22°Brix, 4.38 to 11.26°Brix and 4.17 to 13.67°Brix throughout the developmental period and peaked at the ripening stage, NXH berries had the highest TSS value. For all goji cultivars, the TA increased at first and then decreased after the S3 stage. The TA of the NJ1, BH and NXH goji cultivars tested decreased to 0.75%, 0.89% and 0.58%, respectively, NXH presented the lowest level of TA at stage 5 (S5).
Table 2. Basic quality index of Ningxia goji during fruit development and ripening a, b, c, d.
Identification of key aroma compounds from the ningxia goji berry
In the present study, a total of 193 volatiles were detected in berries tested. The majority were trace compounds and their peak areas were below 1. Among them, only 56 volatile compounds have been identified by LRI, MS and RI, including 17 aldehydes, 5 alcohols, 5 terpenoids, 8 esters, 11 terpenes, 6 ketones, 2 furans, and 3 acids (). Kim et al.[Citation17] identified 130 volatile components from Lycium chinensis Miller, including 8 acids, 21 alcohols, 23 aldehydes, 10 alkanes, 5 aromatics, 15 esters, 7 furans, 21 ketones, 3 miscellaneous compounds, 2 naphthalenes, 3 phenols, 2 pyrazines, and 10 terpenes. The main components in the oil of L. barbarum were found to be hexadecanoic acid (47.5%), β-elemene (5.4%), myristic acid (4.2%), and ethyl hexadecanoate (4.0%). The essential oil of L. ruthenicum had heptacosane (14.3%), ethyl linoleate (10.0%), hexacosane (7.0%), nonacosane (6.2%), and ethyl hexadecanoate (5.8%) as the main compounds.[Citation18] Chen et al.[Citation13] identified 57 volatile trace compounds by HS-trap-GC–MS based on low-temperature enrichment and MHE from the fruit of Lycium barbarum L., primarily including aliphatic alcohol, aliphatic aldehydes, aliphatic ketones, furan, and aliphatic hydrocarbon. Among these compounds, acetic acid was the highest peak ingredient (48.33%), followed by 1-methoxy-2-propanone (8.84%), 3-hydroxy-2-butanone (9.99%), and ethyl lactate (12.97%).
Table 3. Volatiles content (μg/g FW) and odor activity values of Ningxia goji samples a, b, c, d, e, f, g.
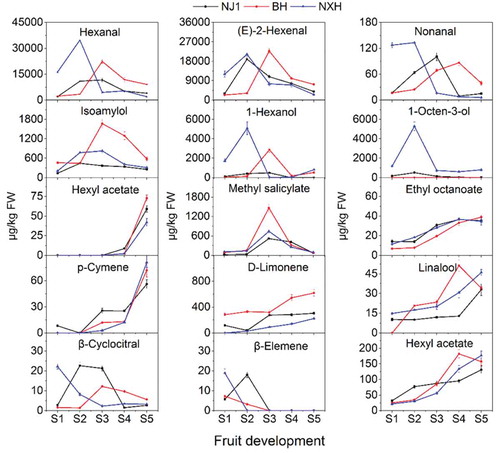
GC–O aimed to discover the active odor compounds by sensory evaluation of the eluate from the chromatographic column.[Citation19–Citation22] Properly educated personnel evaluated the samples using a specially constructed attachment called a sniffing port.[Citation23] AEDA is also suitable for screening potent odorants. In both procedures, an extract obtained from the food was diluted, usually as a series of 1:3 dilutions, and each dilution was analyzed by GC–O. In the case of AEDA, the results are expressed as a flavor dilution (FD)-factor, which is the ratio of the odorant concentration in the initial extract to its concentration in the most dilute extract in which the odor was detected by GC–O. Consequently, the FD-factor is a relative measure and is proportional to the odor activity value (OAV). Generally, the FD ≥27 means this compounds is suitable as indicator for the objective determination of flavor differences in foods.[Citation24] In the present study, GC–O coupled with AEDA was used to identify the characteristic aroma compounds in the Ningxia goji berry. The SDE extract was serially diluted in a 1:3 ratio and their FD factors are presented in . Fifty-eight were detected by GC–O and their aromas, RIs, and mass spectra. Seventeen compounds were detected by sniffing in the 1:27 dilution (FD = 27). These compounds were hexanal, (E)-2-hexenal, nonanal, isoamylol, 1-hexanol, 1-octen-3-ol, guaiacol, hexyl acetate, methyl salicylate, ethyl octanoate, p-cymene, d-limonene, linalool, β-cyclocitral, β-elemene, 3-octanone, and 2-pentylfuran, and their corresponding odor descriptors are given in . Hexanal and (E)-2-hexenal were the most abundant compounds in the Ningxia goji berry. Furthermore, isoamylol and 1-hexanol were the second-most rich compounds in the goji berries tested.
Table 4. Aroma active compounds of Ningxia goji samplesa, b.
To confirm the identities of the aroma compounds by GC–O, the OAVs of 58 compounds were calculated. Among these compounds, the OAVs of 17 compounds were more than 1. In particular, the OAV of hexanal was more than 300 and the OAV of hexyl acetate was beyond 20. It is important to note that (E)-2-hexenal was found at a very high level in goji berries tested, whereas its OAVs were more than 13. Combined with the GC–O identification, 15 aroma compounds were identified as the characteristic aroma compounds of the Ningxia goji berry.
Many studies have observed that goji berries not only contain functional components, such as phenolics and carotenoids, but are also rich in lycium barbarum polysaccharides (LBP). In the present study, 15 aroma compounds were identified as characteristic aroma components in the Ningxia goji berry and some of these components demonstrated bioactivity. P-cymene has been proven to have antimicrobial activity.[Citation25] As a major component in citrus essential oil, d-limonene has been reported to have important biological activities, such as antioxidant properties,[Citation26] anti-inflammatory activities,[Citation27] and chemopreventive or chemotherapeutic properties against several types of cancer.[Citation28] β-cyclocitral can dispel spider mites.[Citation29] β-elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells.[Citation30] Trace compounds, such as 6-di-tert-butyl-4-methylphenol identified from lycium barbarum, are one of the most commonly used food antioxidants in China. Safranal (2,6,6-trimethylcyclohexane-1,3-dien-1-carboxaldehyde) is also one of the major characteristic compounds of Crocussativus L. dry stigmas; modern pharmacological studies proved that this ingredient possessed anticancer, antioxidant and immunoregulatory properties.[Citation13] Therefore, these aromatic compounds not only provide necessary sensory clues to human beings, but also play an important role in goji berry nutritional value and enhancement of stress resistance in goji berries. The bioactivity of aroma compounds in the Ningxia goji berries should continue to be studied in the future.
Changes in characteristic aroma compounds during fruit development
To understand the dynamics of characteristic aroma compounds of Ningxia goji berries during fruit development, changes in 15 compounds are presented in . The content of hexanal, (E)-2-hexenal, and nonanal increased during the early development period. During the ripening period, the three aldehydes decreased rapidly. Similarly, the content of isoamylol, 1-hexanol, and 1-octen-3-ol increased to their peak values at the early stage and decreased to the lowest point at stage 4 (S4) or stage 5 (S5). The content of hexyl acetate and ethyl octanoate increased remarkably throughout the developmental period, whereas the content of methyl salicylate increased rapidly and then fell steeply at the ripening stage. The content of d-limonene increased slowly throughout the developmental period and it was the dominant terpene. The content of p-cymene and linalool increased throughout the developmental period of the goji berry, except for linalool, which showed a decrease at S5 in BH. For β-cyclocitral, different variations were observed in cultivars tested. The contents of β-cyclocitral in the NJ1 and BH berries increased during the early developmental period; however, β-cyclocitral content decreased during the late developmental period. β-cyclocitral content in the NXH berry declined throughout the developmental period. The level of β-elemene in all goji berries tested decreased during the developmental period. The content of 2-pentylfuran increased throughout the developmental period, with only a decrease at S5 in BH berries.
Aroma characterization of ningxia goji berries during development
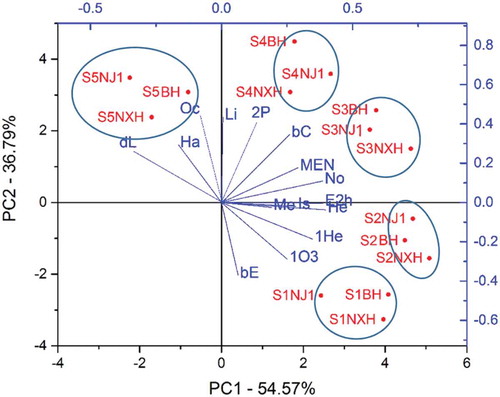
Principal component analysis (PCA) can provide an overview of relationships between samples and parameters. In the PCA model, PC1 and PC2 accounted for 54.57% and 36.79% of the total variable, respectively. And all samples were grouped into five different clusters (). Samples of stage 1 (S1) (S1NJ1, S1BH, and S1NXH) clustered with β-elemene (bE) and 1-0cten-3-ol (1O3), which was consistent with their high content at this stage. Samples of stage 2 (S2) (S2NJ1, S2BH, and S2NXH) clustered with 1-hexanol (1He), methyl salicylate (Me), isoamylol (Is), hexanal (He), and (E)-2-hexenal (E2 h), which were consistent with the content of these compounds at stage 2 (S2). Samples of stage 3 (S3) (S3NJ1, S3BH, and S3NXH) clustered with β-cyclocitral (bC), 2-pentylfuran (2P), ethyl octanoate (MEN), and nonanal (No). Samples of stage 4 (S4) (S4NJ1, S4BH, and S4NXH) clustered with linalool (Li). Finally, samples of stage 5 (S5) (S5NJ1, S5BH, and S5NXH) grouped with d-limonene (dL), o-cymene (Oc), and hexyl acetate (Ha), which is consistent with their high content in Ningxia goji berries at stage 5 (S5). Overall, the PCA result consistent with the GC–MS results.
Conclusion
A total of 56 aroma volatiles were identified from Ningxia goji berries. Hexanal and (E)-2-hexenal predominated in these fruits, accounting for 70–94% of the total volatiles. The GC–O and OAV results revealed that hexanal, (E)-2-hexenal, nonanal, isoamylol, 1-hexanol, 1-octen-3-ol, hexyl acetate, methyl salicylate, ethyl octanoate, o-cymene, d-limonene, linalool, β-cyclocitral, β-elemene, and 2-pentylfuran were the characteristic aroma volatiles in Ningxia goji berries. The content of hexyl acetate, linalool and 2-pentylfuran increased remarkably, while β-cyclocitral and β-elemene increased at the early stage and decreased at a later stage with other characteristic compounds. The results provide valuable information for Ningxia goji berry aroma quality regulation and breeding.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31060104 and 31360191).
Additional information
Funding
References
- Amagase, H.; Farnsworth, N.R. A Review of Botanical Characteristics, Phytochemistry, Clinical Relevance in Efficacy and Safety of Lycium Barbarum Fruit (Goji). Food Research International 2011, 44, 1702–1717. doi:10.1016/j.foodres.2011.03.027.
- Amagase, H.; Sun, B.; Borek, C. Lycium Barbarum (Goji) Juice Improves in Vivo Antioxidant Biomarkers in Serum of Healthy Adults. Nutrition Research 2009, 29, 19–25. doi:10.1016/j.nutres.2008.11.005.
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional Constituents and Antioxidant Activities of Eight Chinese Native Goji Genotypes. Food Chemistry 2016, 200, 230–236. doi:10.1016/j.foodchem.2016.01.046.
- Xin, T.; Yao, H.; Gao, H.; Zhou, X.; Ma, X.; Xu, C.; Chen, J.; Han, J.; Pang, X.; Xu, R.; Song, J.; Chen, S. Super Food Lycium Barbarum (Solanaceae) Traceability via an Internal Transcribed Spacer 2 Barcode. Food Research International 2013, 54(2), 1699–1704. doi:10.1016/j.foodres.2013.10.007.
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of Antioxidant and Antiproliferative Acidic Polysaccharides from Chinese Wolfberry Fruits. Food Chemistry 2012, 133, 978–989. doi:10.1016/j.foodchem.2012.02.018.
- Inbaraj, B.S.; Lu, H.; Kao, T.; Chen, B. Simultaneous Determination of Phenolic Acids and Flavonoids in Lycium Barbarum Linnaeus by HPLC–DAD–ESI-MS. Journal of Pharmaceutical and Biomedical Analysis 2010, 51, 549–556. doi:10.1016/j.jpba.2009.09.006.
- Wang, C.C.; Chang, S.C.; Chen, B.H. Chromatographic Determination of Polysaccharides in Lycium Barbarum Linnaeus. Food Chemistry 2009, 116, 595–603. doi:10.1016/j.foodchem.2009.03.015.
- Wang, J.; Hu, Y.; Wang, D.; Zhang, F.; Zhao, X.; Abula, S.; Fan, Y.; Guo, L. Lycium Barbarum Polysaccharide Inhibits the Infectivity of Newcastle Disease Virus to Chicken Embryo Fibroblast. International. Journal of Biological Macromolecules 2010, 46, 212–216. doi:10.1016/j.ijbiomac.2009.11.011.
- Li, X.; Li, X.; Zhou, A. Evaluation of Antioxidant Activity of the Polysaccharides Extracted from Lycium Barbarum Fruits in Vitro. European Polymer Journal 2007, 43, 488–497. doi:10.1016/j.eurpolymj.2006.10.025.
- Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium Barbarum Polysaccharides Administration on Blood Lipid Metabolism and Oxidative Stress of Mice Fed High-fat Diet in Vivo. Food Chemistry 2007, 113, 872–877. doi:10.1016/j.foodchem.2008.03.064.
- Wang, C.; Chang, S.; Inbaraj, B.S.; Chen, B. Isolation of Carotenoids, Flavonoids and Polysaccharides from Lycium Barbarum L. and Evaluation of Antioxidant Activity. Food Chemistry 2010, 120, 184–192. doi:10.1016/j.foodchem.2009.10.005.
- Chang, R.C.C.; So, K.F. Use of Anti-Aging Herbal Medicine, Lycium Barbarum, against Aging-associated Diseases. What Do We Know so Far? Cellular and Molecular Neurobiology 2008, 28, 643–652. doi:10.1007/s10571-007-9181-x.
- Chen, F.; Su, Y.; Zhang, F.; Guo, Y. Low‐temperature Headspace‐trap Gas Chromatography with Mass Spectrometry for the Determination of Trace Volatile Compounds from the Fruit of Lycium Barbarum L. Journal of Separation Science 2015, 38(4), 670–676. doi:10.1002/jssc.201400862.
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol Composition, Vitamin C Content and Antioxidant Capacity of Mauritian Citrus Fruit Pulps. Food Research International 2011, 44, 2088–2099. doi:10.1016/j.foodres.2011.03.056.
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of Key Odor Volatile Compounds in the Essential Oil of Nine Peach Accessions. Journal of the Science of Food and Agriculture 2010, 90, 1146–1154. doi:10.1002/jsfa.v90:7.
- Liu, C.; Cheng, Y.; Zhang, H.; Deng, X.; Chen, F.; Xu, J. Volatile Constituents of Wild Citrus Mangshanyegan (Citrus Nobilis Lauriro) Peel Oil. Journal of Agricultural and Food Chemistry 2012, 60, 2617–2628. doi:10.1021/jf2039197.
- Kim, J.S.; Chung, H.Y. GC-MS Analysis of the Volatile Components in Dried Boxthorn (Lycium Chinensis) Fruit. Journal of the Korean Society for Applied Biological Chemistry 2009, 52(5), 516–524. doi:10.3839/jksabc.2009.088.
- Altintas, A.; Kosar, M.; Kirimer, N.; Baser, K.H.C.; Demirci, B. Composition of the Essential Oils of Lycium Barbarum and L. Ruthenicum Fruits. Chemistry of Natural Compounds 2006, 42(1), 24–25. doi:10.1007/s10600-006-0028-3.
- Jiang, X.; Wu, S.; Zhou, Z.; Akoh, C.C. Physicochemical Properties and Volatile Profiles of Cold-Pressed Trichosanthes Kirilowii Maxim Seed Oils. International Journal of Food Properties 2016, 19(8), 1765–1775. doi:10.1080/10942912.2015.1107731.
- Wang, L.; Hu, G.; Lei, L.; Lin, L.; Wang, D.; Wu, J. Identification and Aroma Impact of Volatile Terpenes in Moutai Liquor. International Journal of Food Properties 2016, 19(6), 1335–1352. doi:10.1080/10942912.2015.1064442.
- Güler, Z.; Gül, E. Volatile Organic Compounds in the Aril Juices and Seeds from Selected Five Pomegranate (Punica Granatum L.) Cultivars. International Journal of Food Properties 2016, 19(1), 1–13.
- Bhouri, A.M.; Flamini, G.; Chraief, I.; Hammami, M. Aromatic Compounds and Soluble Carbohydrate Profiles of Different Varieties of Tunisian Raisin (Vitis Vinifera L.). International Journal of Food Properties 2016, 19(2), 339–350. doi:10.1080/10942912.2015.1027920.
- Plutowska, B.; Wardencki, W. Application of Gas Chromatography–olfactometry (GC–O) in Analysis and Quality Assessment of Alcoholic Beverages: A Review. Food Chemistry 2008, 107(1), 449–463. doi:10.1016/j.foodchem.2007.08.058.
- Grosch, W. Determination of Potent Odourants in Foods by Aroma Extract Dilution Analysis (AEDA) and Calculation of Odour Activity Values (Oavs). Flavour and Fragrance Journal 1994 9(4), 147–158. doi:10.1002/(ISSN)1099-1026.
- Dorman, H.; Deans, S. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. Journal of Applied Microbiology 2000, 88, 308–316. doi:10.1046/j.1365-2672.2000.00969.x.
- Yang, X.; Zhao, H.; Wang, J.; Meng, Q.; Zhang, H.; Yao, L.; Zhang, Y.; Dong, A.; Ma, Y.; Wang, Z. Chemical Composition and Antioxidant Activity of Essential Oil of Pine Cones of Pinus Armandii from the Southwest Region of China. Journal of Medicinal Plants Research 2010, 4, 1668–1672.
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.H.S.; Thomson, C.A. D-limonene: A Bioactive Food Component from Citrus and Evidence for a Potential Role in Breast Cancer Prevention and Treatment. Oncology Reviews 2010, 5, 31–42. doi:10.4081/oncol.2011.31.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils: A Review. Food and Chemical Toxicology 2008, 46, 446–475. doi:10.1016/j.fct.2007.09.106.
- Nyalala, S.O.; Petersen, M.A.; Grout, B.W.W. Volatile Compounds from Leaves of the African Spider Plant (Gynandropsis gynandra) with Bioactivity against Spider Mite (Tetranychus urticae). Annals of Applied Biology 2013, 162, 290–298. doi:10.1111/aab.2013.162.issue-3.
- Zou, B.; Li, Q.Q.; Zhao, J.; Li, J.M.; Cuff, C.F.; Reed, E. Β-Elemene and Taxanes Synergistically Induce Cytotoxicity and Inhibit Proliferation in Oavrian Cancer and Other Tumor Cells. Anticancer Research 2013, 33, 929–940.