ABSTRACT
The objective of this study was to investigate the effects of adding monoacylglycerols (MAGs) to triacylglycerols (TAGs) on the crystallisation properties in a fat system. Differential scanning calorimetry (DSC) and polarised light microscopy (PLM) methods were used for the analysis. Different MAGs (monoolein—O, monopalmitin—P, and monolaurin—L) were added at 1, 3, and 5% (w/w) to TAG samples, namely (triolein—OOO, tripalmitin—PPP, and tristearin—SSS). DSC results showed that the addition of MAGs changed the crystallisation of the TAGs (PPP, SSS, and OOO). The same MAG may exhibit different behaviours (induction or retardation of crystallisation) depending on the proportion added. The addition of 5% (w/w) of MAG to TAG (PPP and SSS) delayed the crystallisation process, while the best proportion of added MAGs to promote crystallisation was 3% (w/w).
Introduction
Lipids are present in living organisms and are hence present in most foods, differing in composition and properties. Owing to their diverse chemical composition, lipids perform a number of biological functions and contribute to the sensory characteristics of many foods as well as to their nutritional value and health effects.[Citation1] Triacylglycerols (TAGs) account for more than 95% of edible fats and oils, but these may also contain a variety of other minor constituents that influence their physical and chemical properties. These minor constituents include lipids with higher polarity and an amphiphilic structure, such as diacylglycerols (DAG), monoacylglycerols (MAG), free fatty acids (FFA), phospholipids (PLs), glycolipids, and sterols, besides water and minerals.[Citation2]
In most foods, the isolated crystallisation of TAGs is considered the most important event, although crystallisation of minor lipids (MLs) has a strong influence on the quality of a wide variety of products.[Citation3] MLs can be naturally present in fats and oils, as is the case of palm oil, which contains a high DAG content, ranging between 4% and 7.5%, or they can be added, for example, as food emulsifiers, of which MAGs and DAGs are considered the most important group.[Citation3] Additives can be employed, or minor lipids removed, to influence crystallisation, surface gloss, temperature stability, rheology, and polymorphic stability.[Citation4] Several studies have reported alterations in the crystallisation behaviour of TAGs following the addition of minor lipids, having the effect of either promoting or retarding crystallisation, depending on the type of additive used.[Citation5] Alfutimie et al.[Citation6] studied the influence of saturated and unsaturated MAGs on the crystallisation of TAGs present in miglyol oil, palm oil, and olive oil. Their results suggested that saturated MAGs affect the oil crystallisation behaviour. Conversely, TAGs composed mainly of unsaturated fatty acids underwent little influence from the presence of MAGs. Their results suggest that saturated and unsaturated MAGs are incorporated into the α-phase of palm oil.
The literature proposes some potential mechanisms to support the assumption that MLs can affect crystallisation. Possible interactions between MLs and TAG crystal growth have been considered, creating a competitive effect during the structural, permanent incorporation of MLs into the crystal structure, thereby preventing or enhancing growth. MLs may limit the transfer rate of TAGs to incorporation sites in the crystal lattice. By these means, MLs could affect crystallisation rates, polymorphic forms, and crystalline microstructure, by preferential inhibition or promotion of the development of certain crystalline phases. Some authors have also linked the effect of MLs with the induction of heterogeneous nucleation, according to the proposition that these compounds organise separately in micellar structures, acting as templates for the nucleation process. However, the distinction of these effects and their selectivity between nucleation and crystal growth steps are not yet fully understood, representing an issue of intense interest in lipid science.[Citation3,Citation7,Citation8] An understanding of the relevant potential mechanisms to support the assumption that MLs can affect crystallisation is essential. Smith et al.[Citation4] described several possible modes of action of additives during the processes of nucleation, crystal growth, and recrystallisation or polymorphic transition. It has been proposed that additives may coat heterogeneous nuclei, preventing their use as such, or may form nuclei by crystallising first. During early crystal growth, it has been suggested that minor components crystallise first to form seeds, evolving to accelerate growth by forming additional growth sites. Finally, these lipids might stabilise unstable polymorphs, as well as destabilise stable polymorphs, and also increase the energy barrier for transformation. Nevertheless, authors point out that such additives can also slow crystal growth or recrystallisation by blocking growth sites and also alter crystal morphology by blocking specific faces.
Our previous studies have focused on the influence of DAGs on TAG crystallisation as DAGs are generally one of the most abundant minor components naturally present in edible fats and oils.[Citation9] MAGs are present in lesser amounts than DAGs, explaining the fewer studies on the effect of these compounds on lipid crystallisation behaviour. The studies available to date indicate acceleration of fat crystallisation, modifications in crystal number and polymorphic form, and a decrease in yield value and solid fat content across a wide temperature range.[Citation7] The effect of MAG addition depends on the degree of supercooling, the concentration, and the type of MAG.[Citation10]
Sambuc et al.[Citation11] investigated the effect of MAGs on the crystallisation of different vegetable fats. The addition of 4% (w/w) of a monopalmitin plus monostearin blend decreased induction time in all samples. Smith, Cebula, and Povey[Citation12] showed that the incorporation of monolaurin accelerated trilaurin crystallisation, with further decrease in crystal size. Miura, Yamamoto, and Sato[Citation13] reported the effect of myristic, palmitic, stearic, lauric, and behenic MAGs (0.4% w/w) on palm oil crystallisation behaviour. Palm oil solid fat content decreased upon the addition of myristic, palmitic, and stearic MAGs, whereas lauric and behenic MAGs had no effect on this property. Vereecken et al.[Citation14] showed that appropriate selection of saturated and unsaturated MAGs led to the modification of the solid fat content of lipids. More recently, Vestringe et al.[Citation15] noted that the addition of saturated MAGs accelerated the crystallisation process of palm oil during non-isothermal crystallisation, a phenomenon that could reduce costs, provided the desired characteristics of PO, such as plasticity, are preserved.
Although blends of MLs are generally applied in industrial products, fundamental research on this topic is however missing. For this reason, studying the crystallisation behaviour of a pure lipid system is of great scientific importance as a means of gaining a deeper understanding of the phenomena involved, serving as basic knowledge to help guide the addition or removal of these compounds in different raw materials. The aim of this study was to evaluate the effects of adding different proportions (1, 3, and 5% (w/w)) of pure MAGs (monopalmitin, monoolein, and monolaurin) to pure triglycerides (tripalmitin, triolein, and tristearin) using DSC and polarised light microscopy analysis.
Material and methods
Material
Pure (>99%) triacylglycerol samples, namely glyceryl trioleate (OOO), glyceryl tripalmitate (PPP), and glyceryl tristearate (SSS), were acquired from Sigma-Aldrich (United Kingdom). Pure (>99%) monoacylglycerol samples were acquired from Nu-Chek Prep. Inc. (USA), namely monolaurin (L), monopalmitin (P), and monoolein (O).
Methods
To study effects of the incorporation of MAGs on the crystallisation properties of pure triacylglycerols, blends of TAGs (OOO, PPP, and SSS) plus MAGs (L, P, and O) at 1, 3, and 5% (w/w) were prepared. The components of the blends were melted (±80ºC) when in a solid state and then blended at the proportions 1, 3, and 5% to yield 2 grams for each blend. All the measurements were performed in triplicate.
Differential scanning calorimetry
The DSC curves were obtained on a DSC 4000 Perkin Elmer device (Perkin Elmer Corp., Norwalk, CT, USA) equipped with an Intracooler SP cooling system, under a dynamic atmosphere of He (20 mL/min). The temperature and heat of melting were calibrated with indium (initial temperature of 156.6°C). The cooling rate was −10°C/min, at temperatures ranging from 80°C to −60°C with an isothermal time of 10 min at 80°C, using sealed aluminum capsules containing a sample mass of 5–10 mg.[Citation16–Citation18] Curves were processed by Pyris software, and crystallisation curves were analysed for onset of crystallisation (Tonset°C), peak crystallisation temperatures (Tpeak°C), end of crystallisation (TendsetºC), and crystallisation enthalpies (∆Hc J/g).[Citation19] All samples were analysed in triplicate. The time of crystallisation was defined by the Pyris software.
Polarised light microscopy
A model BX51 polarised light microscope (Olympus, Japan) was used to analyse crystalline structure at a magnification of 100 X. The microscope was coupled to a Qimaging digital camera (Media Cybernetics, USA), which transmitted images to the computer in real time using Image Pro-Plus version 7.0 software (Media Cybernetics, USA).
The samples of pure triacylglycerols and triacylglycerols plus pure MAGs were heated to 80°C in a microwave oven. Using a capillary tube, a single drop of sample was mounted onto a glass slide. A cover slip was then placed over the fat droplet producing a thin film of fat. Slides, coverslips, and capillary tubes were previously heated to 80°C. Crystallisation was assessed using the following procedure: the samples were kept at 100°C for 15 min to erase the crystal memory[Citation20] and then cooled to 15°C (rate of 10°C/min) using a model PE-120 Linkam temperature controller (Surrey, England). The crystallisation images were captured every 2 s from 45°C (PPP) and 55°C (SSS) until complete crystallisation of the sample. The diameter of the crystals after complete crystallisation was determined for each sample using Image-Pro Plus version 7.0 software (Media Cybernetics, USA). The diameter of the crystals for each sample were determined after complete crystallisation using the software Image-Pro Plus version 7.0 (Media Cybernetics, USA). All pictures captured during the crystallization process were used to calculated crystallised area. And from these data, curves of crystallisation were plotted. For each curve were determined, onset temperature (first point with an increment in the area that correspond to the first crystal formed) end temperature (first point in the plateau of the curve).
Results and discussion
Crystallisation profiles by DSC
The addition of MAGs (L, P, and O) accelerated the crystallisation process of OOO at all proportions tested (). This effect can be explained by the high melting points of the MAGs L, P, and O of 61, 74, and 34ºC, respectively.[Citation21,Citation22] Acceleration was greatest at 3% (w/w) MAG addition, with this behaviour representing the optimum interaction between MAG and TAG among the different proportions analysed (1, 3, and 5% w/w). The effect on crystallisation of the addition of 5% (w/w) MAGs L and O was weaker than for both 1 and 3% (w/w).
Figure 1. Crystallisation curves obtained by DSC of pure triolein added with MAGs (L, P, and O) in different proportions (1, 3, and 5%).s
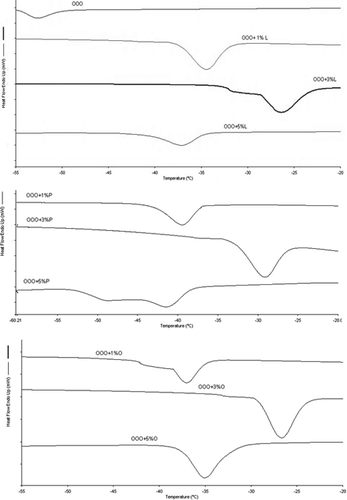
Although the addition of MAGs accelerated the crystallisation of OOO by raising onset, peak, and endset temperatures, the overall duration of the crystallisation event was longer with MAG inclusion (). The crystallisation time of OOO was 28 s. The greatest increase in crystallisation time was observed for the addition of MAG P, an effect that might be attributed to the higher melting point of P relative to the other MAGs assessed.[Citation21] The addition of 5% (w/w) P increased crystallisation time to 66 s.
Table 1 Crystallisation time and enthalpy of OOO, PPP, and SSS pure and added with monoacylglycerols (L, P, and O) in different proportions (1, 3, and 5%) by DSC.
Triolein plus MAG L promoted a larger crystallisation area, evidenced by higher crystallisation enthalpy for all proportions tested (). The greatest shift in crystallisation enthalpy of OOO occurred for the addition of 3% (w/w) O, most likely owing to the chemical similarity of the acyl group. This indicates that the MAG affects the energy released in the crystallisation of triolein and the crystallisation temperature.
The addition of L, P, and O to PPP produced a similar effect at both 1 and 3% (w/w), accelerating the onset of crystallisation, most likely due to co-crystallisation between TAG and the MAG (). The addition of MAGs L and O at 5% (w/w) delayed the onset of crystallisation, indicating that interaction may have occurred between the two components at this proportion, hampering nucleation. Some authors have attributed this effect to induction of heterogeneous nucleation, based on the proposition that these compounds group separately in micellar structures, acting as bases for the nucleation process.[Citation7,Citation8,Citation18]
Figure 2. Crystallisation curves obtained by DSC of pure tripalmitin added with MAGs (L, P, and O) in different proportions (1, 3, and 5% w/w).
The duration of the crystallisation process in PPP was also affected by the addition of MAGs. Although the addition of L, P, and O accelerated the onset of crystallisation, overall crystallisation time increased at all proportions of added MAGs (). This effect is due to the increase in crystallisation energy, evidenced by increased crystallisation enthalpy for all proportions tested ().
Based on these observations, it can be affirmed that nucleation of crystals of PPP was favoured by the addition of MAGs, particularly at 1 and 3% (w/w), and that growth of crystals was also affected given that the process of crystallisation took longer (). The addition of 1% (w/w) MAGs was insufficient to accelerate crystallisation of SSS (). This is due to the high melting point of pure SSS (72ºC). At this percentage, the presence of minor lipids hindered the process, delaying the attainment of crystallisation temperatures (onset, peak, and endset) ().
Figure 3. Crystallisation curves obtained by DSC of pure tristearin added with MAGs (L, P, and O) in different proportions (1, 3, and 5% w/w).
The addition of 3% (w/w) L, P, and O to SSS significantly increased crystallisation enthalpy (). This result indicated that MAGs exerted an effect on the energy released in the crystallisation of SSS. The onset of crystallisation was accelerated by the addition of all MAGs (L, P, and O) at 3% (w/w). This can be regarded as the optimal proportion of added MAGs for accelerating crystallisation of this triacylglycerol.
While the onset of crystallisation was accelerated by the addition of all MAGs at 3% (w/w), increases in overall crystallisation time were observed. The addition of these minor lipids can favour the onset of crystallisation and consequently its nucleation but have a negative effect on the development of crystals and thus on their growth.[Citation8,Citation23] The temperature of onset of crystallisation upon addition of 5% (w/w) MAGS was only slightly affected, but crystallisation enthalpies were increased. This effect is due to elevation in crystallisation energy.
Polarised light microscopy
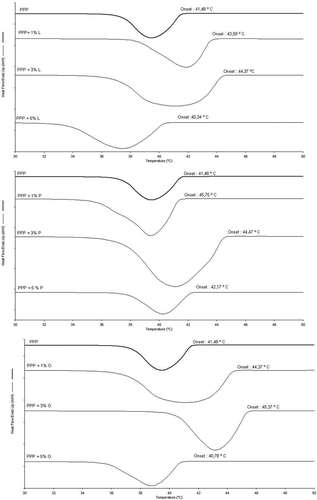
Crystallisation curves: The images obtained from the microscope were used to determine the initial and final times of crystallisation, induction time, and crystallisation time of SSS and PPP with MAG additives (). The onset of crystallisation of pure PPP () occurred 78 s (induction time) after starting to cool (rate of 10ºC/min) at a temperature of 42.6°C. After 36 s, PPP fully crystalised at a temperature of 39.1°C (). These results are consistent with those found by DSC for crystallisation of PPP ( and ).
Table 2 Initial and final crystallisation temperatures and related induction and crystallisation times of PPP and SSS pure and added with 1, 3, and 5% of monoacylglycerols, obtained by PLM.
Figure 4. Crystallisation curves obtained by image analysis under polarised light microscopy of the pure triacylglycerols and added 1, 3, and 5% (w/w) of monoacylglycerols.
The addition of 1% (w/w) L increased the induction time of PPP by only 4 s, where the first spherulite formed after 82 s. On the other hand, crystal growth time was accelerated by the addition of 1% (w/w) L, where PPP was 100% crystallised after 14 s. However, this result conflicts with those observed on DSC, which showed that the addition of 1% (w/w) L reduced the speed of crystal growth. The hypothesis explaining this disparity in crystallisation time is that full crystallisation on the glass slide in the α form did not correspond with the end of the energy release process during crystallisation observed on DSC.
The addition of 3% (w/w) L accelerated the onset of crystallisation of PPP by 4 s, while the process of crystal growth occurred 14 s faster than for pure PPP. Akin to that observed in crystallisation by DSC, the addition of 5% (w/w) L also changed the crystallisation curve obtained by microscopy, delaying the onset of crystallisation. The first spherulite formed after 84 s, and the time for full crystallisation of PPP occurred only after 82 s. The results observed for the incorporation of 5% (w/w) of this MAG were consistent with those obtained on the crystallisation curve by DSC. This is possibly due to the long crystallisation time, allowing the formation of more stable polymorphic forms.
The presence of 1% (w/w) P changed the crystallisation of PPP (). At this percentage, the time of onset of crystallisation was accelerated to 66 s. Crystal growth time was also accelerated, where the sample was fully crystallised after 24 s. The addition of 3% (w/w) P also accelerated the onset of crystallisation, which commenced after 72 s, but the most significant effect was the acceleration in crystal growth which was complete after 14 s.
Based on the microscopy results, it can be concluded that the addition of 5% (w/w) P delayed the crystallisation process, particularly in terms of spherulite growth. These data are consistent with the DSC results, showing that the addition of 5% (w/w) P also increased crystallisation time ( and ). For crystallisation of PPP with added O, induction and crystallisation times were accelerated at both 1 and 3% (w/w) addition (Figure 4C). The same behaviour was apparent from the crystallisation curve determined by DSC. The addition of 5% (w/w) accelerated induction time of PPP, whereas crystallisation time was increased (). This suggests that unlike saturated MAGs that co-crystalise with PPP, 5% (w/w) O had a greater influence on this process. The longer crystal growth time is attributed to the heterogeneity of the structures of TAG and MAG, which hampers the construction of the crystalline network of the different forms.[Citation12]
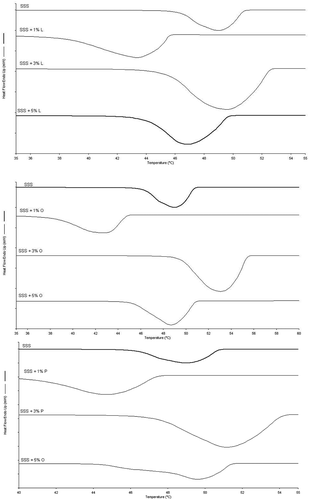
The onset of crystallisation of SSS occurred 12 s (induction time) after starting to cool (at rate of 10ºC/min). After 16 s, SSS was fully crystalised (Figure 4D) at a temperature of 51.3°C (). Regarding the crystallisation process of SSS, the first crystals were formed at around 53ºC, consistent with DSC results. The induction times of samples containing 1, 3, and 5% (w/w) L were increased by 8, 2, and 10 s, respectively. At 1% (w/w), longer cooling was necessary (50.7ºC) for nucleation to take place. The addition of 3% (w/w) L led to similar crystallisation temperatures and times to those of pure SSS. The main effect of the addition of 5% (w/w) L was an increase in crystallisation time, showing that the addition of the MAG at this proportion (5% (w/w)) had a greater impact on crystal growth compared to the other proportions (1 and 3% (w/w).
The addition of 1% (w/w) P to SSS () hindered the crystallisation process, leading to lower crystallisation temperatures, as well as longer induction and crystallisation times, compared to pure SSS. The addition of 3 and 5% (w/w) accelerated the crystallisation process, leading to higher crystallisation temperatures and shorter induction times, where the crystallisation time of SSS plus 5% (w/w) was increased considerably (26 s). These results are consistent with those for crystallisation determined by DSC, where the addition of 3 and 5% (w/w) of this MAG promoted nucleation and accelerated induction time relative to pure SSS.
The addition of 1, 3, and 5% (w/w) O led to a greater shift in the crystallisation of SSS regarding the onset of crystallisation (), that is, induction time was increased. In general, the addition of MAG O hampered the crystallisation process of SSS, given that TAG required lower temperatures to conclude the crystallisation process (). The growth of spherulites was also affected by the addition of this MAG at 5% (w/w).
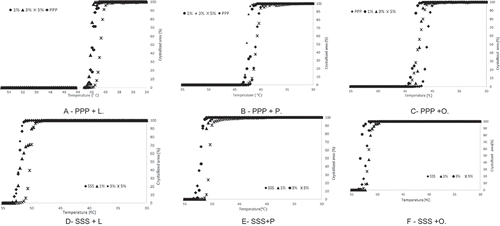
Crystalline microstructure: The crystallised area (%) obtained from the microscope images, of pure TAGs and of TAGs plus MAGs, was used to build the crystallisation curves shown in . The crystalline microstructures of pure PPP and PPP plus MAGs are given in , depicting images of PPP acquired in the temperature range of 15–55ºC for crystallisation.
Figure 5. Images of crystal structures obtained at 15ºC during the crystallisation of pure PPP and PPP added with 1, 3, and 5% monoolein (O), monopalmitin (P), and monolaurin (L). The bar represents 200 µm.
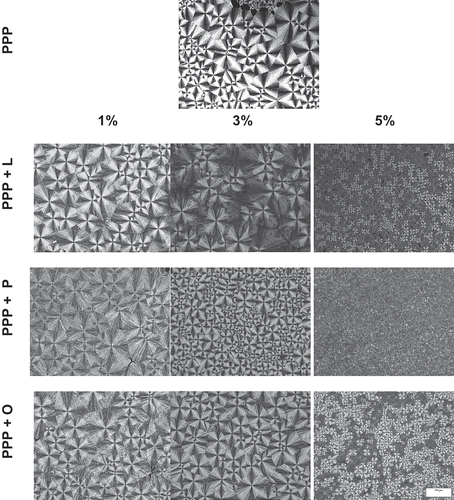
The structure seen for the α form of PPP closely resembles that described by Kellens, Meeusen, and Reynaers,[Citation24] in which the α form is characterised by a structure of tightly packed bright spherulites, resulting in well-defined, straight edges. The size of the spherulites of α form depends strongly on nucleation and can vary considerably between different points in the same sample. The α form of PPP exhibited crystals in the 11–74 µm size range, confirming the observation above.
The addition of the MAGS L, P, and O at 1% (w/w) resulted in a reduction of birefringence and change in the size of the spherulites, which crystalised in a more homogenous manner in terms of size. The addition of 3% (w/w) L promoted the formation of a completely different crystalline microstructure to those of pure PPP and of PPP plus 1% (w/w) L. The microstructure, in this case, had larger spherulites with variable birefringence, which may be indicative of changes in the polymorphic form of this sample. The anisotropy of the spherulites is much less evident than that observed for pure PPP (). The edges of spherulites are only partially visible. The size range of the crystals formed with the addition of 3% (w/w) L was wider (≈85 µm). The addition of 3% (w/w) P and O also led to a reduction in birefringence. The resultant spherulites were highly homogenous and well defined. Smaller size and increased nucleation were also observed, particularly upon the addition of 3% (w/w) P.
The greatest change in crystalline microstructure occurred for the addition of 5% (w/w) MAGs. The size of the spherulites was drastically reduced (≈15 µm) and nucleation increased, resulting in high-density spherulites. Moreover, the microstructures of PPP plus 5% (w/w) L and O exhibited two types of birefringence, a phenomenon previously attributed to the concomitant presence of two polymorphic forms.[Citation9,Citation25] Garti, Wellner, and Sarig[Citation26] reported that emulsifiers containing unsaturated fatty acids promoted polymorphic transitions, probably owing to the angulation of the chain of unsaturated acid, which results in greater mobility of PPP thereby reducing interaction between the emulsifier and PPP.
The morphology of the crystals of PPP was altered by the addition of MAGs while the stability of the meta-stable polymorphic forms was increased. These results are consistent with those reported by Smith, Cebula, and Povey.[Citation12] The samples containing 1 and 3% (w/w) MAGs crystalised faster, whereas the incorporation of 5% (w/w) MAGs favoured slower crystallisation. Thus, this allowed greater stability of the polymorphic form, in contrast to pure PPP, which exhibited a crystalline microstructure typical of the polymorphic α form.
According to the results reported in an earlier study by our research group, SSS begins crystallisation with the formation of the first spherulites at temperatures of around 53ºC.[Citation9] These results closely resemble those found on microscopy and by DSC in the present study. The crystalline microstructures of pure SSS and SSS plus MAGs are depicted in . During the crystallisation process, images were acquired in the temperature range of 15–55ºC. The addition of MAGs to SSS promoted changes in the crystallisation of the α form. The addition of P and L resulted in a reduction of birefringence and in the size of the spherulites, which crystalised in a more homogenous manner in terms of size.
Figure 6. Images of crystal structures obtained at 15ºC during the crystallisation of pure SSS and SSS added with 1, 3, and 5% monoolein (O), monopalmitin (P), and monolaurin (L). The bar represents 200 µm.
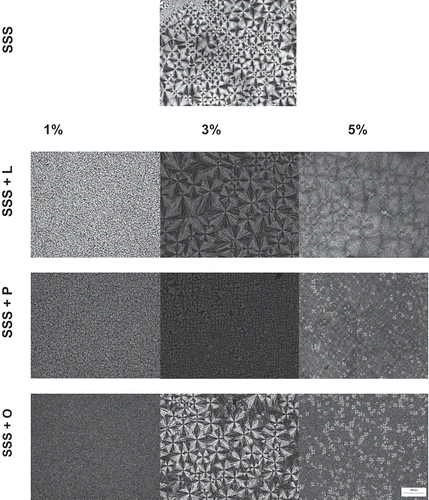
The size of the spherulites of the α form depends strongly on nucleation. The microstructure seen for the α form of SSS closely resembled the structure described by Oh et al.[Citation27] which exhibited the α form with small bright spherulites in the 9–38 μm range. In the present study, the α form of SSS had spherulites in the 8–31 µm range. The addition of 1% (w/w) MAGs (L, O and P) considerably changed the microstructure of SSS, which had a large number of very small homogenous spherulites with poorly defined edges. This behaviour is due to the increased induction time observed on microscopy analysis ().
The addition of 3% (w/w) L changed only the anisotropy of SSS. The size of spherulites was altered only slightly, whereas the addition of 5% (w/w) of this MAG changed the microstructure of these TAGs considerably, affording less-defined, larger spherulites. Akin to that observed for PPP, the addition of 5% (w/w) P and O to SSS produced concomitant crystallisation of two polymorphic forms, favoured by the slower crystallisation. Thus, this allowed greater stability of the polymorphic form, in contrast to pure SSS, which exhibited a crystalline microstructure typical of the polymorphic α form.
Conclusion
The addition of MAGs changed the crystallisation profile of the pure triacylglycerides. The same MAG may have different behaviours (induction or retardation of crystallisation) depending on the proportion added. The incorporation of MAGs to unsaturated TAG OOO favoured the crystallisation process at all proportions for all MAGs (L, P, and O), although crystallisation was most effectively promoted with the addition of 3% (w/w) of each MAG. Based on the results from DSC analyses and polarised light microscopy, it can be concluded that the addition of 5% (w/w) MAG to saturated TAGs (PPP and SSS) delayed the crystallisation process and that the optimum proportion of added MAGs for promoting crystallisation was 3%.
Funding
The authors gratefully acknowledge the generous support of the Brazilian research funding agencies, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Additional information
Funding
References
- Kolakowska, A.; Sikorski, Z.E. The Role of Lipids in Food Quality. Chemical and Functional Properties of Food Lipids; CRC Press: New York, USA, 2003; 3–4.
- Chen, B.; McClements, D.J.; Decker, E.A. Minor Components in Food Oils. A Critical Review of Their Roles on Lipid Oxidation Chemistry in Bulk Oils and Emulsions 51, 2011, 901–916.
- Metin, S.; Hartel, R.W. Crystallization of Fats and Oils. In Bailey’s Industrial Oil and Fat Products; Shahidi, F.; Ed.; Wiley Interscience: New York, USA, 2005; 45–76. Vols. 1.
- Smith, K.W.; Bhaggan, K.; Talbot, G.; Van Malssen, K.F. Crystallization of Fats: Influence of Minor Components and Additives. Journal of the American Oil Chemists’ Society 88, 2011, 1085–1101.
- Saitou, K.; Homma, R.; Kudo, N.; Katsuragi, Y.; Sato, K. Retardation of Crystallization of Diacylglycerol Oils Using Polyglycerol Fatty Acid Esters. Journal of the American Oil Chemists’ Society 91, 2014, 711–719.
- Alfutimie, A.; Al-Janabi, N.; Curtis, R.; Tiddy, G.J.T. The Effect of Monoglycerides on the Crystallisation of Triglyceride. Colloids and Surfaces A: Physicochemical and Engineering Aspects 494, 2016, 170–179.
- Foubert, I.; Vanhoutte, B.; Dewettinck, K. Temperature and Concentration Dependent Effect of Partial Glycerides on Milk Fat Crystallization. European Journal of Lipid Science and Technology 106, 2004, 531–539.
- Wright, A.J.; Hartel, R.W.; Narine, S.S.; Marangoni, A.G. The Effect of Minor Components on Milk Crystallization, Microstructures and Rheological Properties. In Physical Properties of Lipids; Marangoni, A.G.; Narine, S.S.; Eds.; Marcel Dekker: New York, USA, 2002; 125–161).
- Silva, R.C.; Soares, F.A.S.M.; Maruyama, J.M.; Dagostinho, N.R.; Silva, Y.A.; Calligaris, G.A.; Ribeiro, A.P.B.; Cardoso, L.P.; Gioielli, L.A. Effect of Diacylglycerol Addition on Crystallization Properties of Pure Triacylglycerols. Food Research International 55, 1, 2014, 436–444.
- Daels, E.; Rigolle, A.; Raes, K.; Block, J.; Foubert, I. Monoglycerides, Polyglycerol Esters, Lecithin, and Their Mixtures Influence the Onset of Non-Isothermal Fat Crystallization in a Concentration Dependent Manner. European Journal Lipid Sciences Technological 117, 2015, 1745–1753.
- Sambuc, E.; Dirik, Z.; Reymonf, G.; Naudet, M. Etude de la cristallisation des corps gras plastiques VI – Influence des glycerides partiels et des phosphatides en absence et presence d’eau A. Cas des monoglycerides stearopalmitiques. Revue Française des Corps Gras 1980, 27, 505–512.
- Smith, P.R.; Cebula, D.J.; Povey, M.J.W. The Effect of Lauric-Based Molecules on Trilaurin Crystallization. Journal of the American Oil Chemists’ Society 71, 1994, 1367–1372.
- Miura, S.; Yamamoto, A.; Sato, K. Effect of Monoacylglycerols on the Stability of Model Cream Using Palm Oil. European Journal of Lipid Science and Technology 104, 2002, 819–824.
- Vereecken, J.; Foubert, I.; Smith, K.W.; Dewettinck, K. Effect of Satsatsat and Satosat on Crystallization of Model Fat Blends. European Journal of Lipid Science and Technology 111, 2009, 243–258.
- Vestringe, S.; Danthine, S.; Blecler, C.; Depypere, C.; Dewettinck, K. Influence of Monopalmitin on the Isothermal Crystallization Mechanism of Palm Oil. Food Research International 2013, 51, 344–353.
- AOCS. American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society 5th ed ed.; Champaign, AOCS Press: Illinois, USA, 2004.
- GrimaIdi, R.; Gonçalves, L.A.G.; Gioielli, L.A.; Simões, I.S. Interactions in Interesterified Palm and Palm Kernel Oils Mixtures. II – Microscopy and Differential Scanning Calorimetry. Grasas Y Aceites 52, 6, 2001, 363–368.
- Metin, S.; Hartel, R.W. Thermal Analysis of Isothermal Crystallization Kinetics in Blends of Cocoa Butter with Milk Fat or Milk Fat Fractions. Journal of the American Oil Chemists’ Society 75, 1998, 1617–1624.
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; Gonçalves, L.A.G. Effect of Chemical Interesterification on Physicochemical Properties and Industrial Applications of Canola Oil and Fully Hydrogenated Cottonseed Oil Blends. Lipids 16, 2009, 362–384.
- Mazzanti, G.; Guthrie, S.E.; Sirota, E.B.; Marangoni, A.G.; Idziak, S.H.J. Effect of Minor Components and Temperature Profiles on Polymorphism in Milk Fat. Crystal Growth Design 4, 6, 2004, 1303–1309.
- Faergemand, M.; Krog, N. Using Emulsifier to Improve Food Texture. In Texture in Food – Semi Solid Foods; Mackena, B.M.; Ed.; Woodhead Publishing Limited, 2003.
- Fredrick, E.; Heyman, B.; Moens, K.; Fischer, S.; Verwijlen, T.; Moldenaers, P.; Van der Meeren, P.; Dewettinck, K. Monoacylglycerols in Dairy Recombined Cream: II. the Effect on Partial Coalescence and Whipping Properties. Food Research International 2013, 51, 936−945.
- Lawler, P.J.; Dimick, P.S. Crystallization and Polymorphism of Fats. In Food Lipids: Chemistry, Nutrition and Biotechnology 3nd ed.; Akoh, C.; Min, D.B.; Eds.; CRC Press: New York, USA, 2008.
- Elisabettini, P.; Desmedt, A.; Gibon, V.; Durant, F. Effect of Sorbitan Tristearate on the Thermal and Structural Properties of Monoacid Triglycerides: Influence of a Cis or Trans Double Bond. Fat Science Technology 97, 1995, 65–69.
- Kellens, M.; Meeusen, W.; Reynaers, H. Study of the Polymorphism and the Crystallization Kinetics of Tripalmitin: A Microscopic Approach. Journal of the American Oil Chemists’ Society 69, 1992, 906–911.
- Garti, N.; Wellner, E.; Sarig, E.S. Crystal Structure Modification of Tristearin by Food Emulsifiers. Journal American Oil Chemists’ Society 1982, 59, 181–185.
- Oh, J.H.; McCurdy, A.R.; Clark, S.; Swason, B.G. Characterization and Thermal Stability of Polymorphic Forms of Synthesized Tristearin. Journal of Food Science 67, 2002, 2911–2917.
