ABSTRACT
The aim of this work was to determine the influence of the addition (5, 10, or 20 g/100 g) of different sugars (sucrose, maltose, or trehalose) prior to freeze-drying on the key volatile compounds of the sour cherry puree. The key volatile compounds of sour cherries were benzaldehyde, benzyl alcohol, and 2-hexenal. The results obtained for the samples with the addition of sugars were compared to the control sample, the sample without sugar addition. The control sample contained 3.92 mg/kg of benzyl alcohol, 856.61 µg/kg of benzaldehyde, and 15.58 µg/kg of 2-hexenal. Benzaldehyde, the compound described as the typical cherry volatile compound, was determined in the highest amount in the sample with the addition of 10 g/100 g of trehalose (937.51 µg/kg). The highest amount of 2-hexenal was determined in samples with the addition of 10 or 20 g/100 g of trehalose (17.45 and 18.96 µg/kg, respectively). All samples with the sugar addition had lower content of the benzyl alcohol than the control sample. Generally, samples with the addition of sucrose had the lowest amount of examined volatile compounds.
Introduction
Fruits are very perishable raw material, so in order to consume it out of their season, it is often transformed into the products (such as jams, jellies, juices) or semi products (such as puree) that can be later used for the consumption or the preparation of the final product. The volatile and non-volatile compounds are responsible for the sensorial quality characteristics of fruits. It is highly desirable that the obtained fruit product retains as much as possible typical properties of fruits, especially the flavor and color. The volatile compounds are mainly associated with the product flavor, thus the stability of those compounds in the products during preparation as well as during storage is of increasing interest to the food industry. The retention and the stability of the products’ flavor are in the strong relationship with the consumer’s acceptability of the products, and it is necessary to control it. In order to retain as much as possible of volatile compounds especially the most typical ones for the selected fruit, huge effort has been made during the fruit product formulation. Sugars are very often used in the foods’ formulation for the preservation, but also in order to achieve the desired properties. Selected sugars for this study were the sucrose, as the sugar that is commonly used in the fruit product formulation, and the maltose and trehalose, the sugars that are less sweet than the sucrose. Nowadays, especially the trehalose is getting more attention in the food industry due to its positive effect on the products’ quality. The positive effect of trehalose addition on volatile compounds in the products that were prepared by freeze-drying was already observed, like in strawberry purees,[Citation1,Citation2] apricot purees,[Citation3] pear products,[Citation4] and strawberry cream fillings.[Citation5,Citation6]
The equilibrium between the different volatile compounds and their concentrations is responsible for the overall flavor profile of fruits and their products.[Citation7] Sour cherries are well known for their antioxidant properties and rich sours of phenolics and anthocyanins,[Citation8–Citation10] as well as by the specific flavor. The flavor of sour cherry (Prunus cerasus) is composed of a great number of organic components, including carbonyls, alcohols, esters, acids, and terpenes. Next to benzaldehyde which is described as a character-impact compound, the key flavor compounds of sour cherry are benzyl alcohol, 2-phenyl ethyl alcohol, eugenol, 2-hexenal, α-ionon, and β-ionon.[Citation11–Citation13] In this study, the sour cherry puree was freeze-dried with the addition of the disaccharides (sucrose, maltose, or trehalose) in different amounts (5, 10, or 20 g/100 g) in order to investigate the influence of the different sugars as well as their amounts on the key volatile compounds of sour cherry (benzaldehyde, benzyl alcohol, and 2-hexenal).
Materials and methods
Materials
Sour cherry var. Maraska ware obtained from a local market. Standards, namely benzaldehyde, benzaldehyde-d6, benzyl alcohol, and 2-hexenal, were purchased from Sigma-Aldrich (Germany). Sucrose was obtained from Kemika (Croatia), and trehalose and maltose were obtained from Hayashibara, Nagase group (Japan). Model cherry juice was prepared in MilliQ water with the addition of 150 g/L glucose and 3 g/L malic acid. The pH of model juice solution was adjusted to 3.3 with 6 M NaOH. Stock solutions of each standard were prepared in methanol, and further dilutions were made with the model cherry juice solution.
Preparation of freeze-dried puree
Prior to disintegration (Braun Multiquick Professional 600 Watt Turbo), sour cherries were washed and the pits were removed. After disintegration of fruits, sugars (maltose, sucrose or trehalose) in different amounts (5, 10, or 20 g/100 g) were added and the mixtures were well homogenized. Before freeze-drying, sour cherry puree mixtures were frozen at −18°C for 24 h and then freeze-dried in a laboratory freeze-dryer (Christ Freeze Dryer, Gamma 2–20, Germany). Conditions for the drying process were as follows: freezing temperature −55°C; the temperature of sublimation −35°C to 0°C; and the vacuum level 0.220 mbar. The temperature of isothermal desorption varied from 0°C to 22°C under the vacuum of 0.060 mbar. Freeze-drying lasted about 48 h until the total solid content was 95–97%. The total solids were determined by drying the samples in the vacuum dryer at 70°C.
SPME extraction
Prior to extraction, samples were rehydrated with the water in ratio 1:5 (sample:water). The samples were then vortex mixed and centrifuged for 3 min at 8000 rpm (Beckman, Avanti JXN-26). 2.5 mL of supernatant, 10 μL benzaldehyde-d6 (internal standard; 0.35 g/L standard solution in water) and 2.5 mL of saturated solution of CaCl2 were introduced into a 10 mL vial. The extraction of volatiles was carried out using a solid-phase microextraction (SPME) fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) sorbent (1 cm long, 50/30 µm thickness, StableFlex™, Supelco, USA).[Citation14] The sample vials were conditioned in a temperature-controlled heating module at 40°C for 45 min and agitated at 350 rpm. After extraction, the fiber was removed from the sample and the volatiles were thermally desorbed in the injector port of the GC.
Gas chromatography/mass spectrometry (GC/MS) analysis
Analysis was conducted on a GC 7890A gas chromatograph (Agilent Technologies, CA, USA) equipped with a MPS2 Multipurpose autosampler (Gerstel GmbH, Mülheim van der Ruhr, Germany) and 5975C mass spectrometer (Agilent Technologies). Volatile compounds were desorbed into a GC injector port at 250°C in a splitless mode for 2 min. The gas chromatograph was fitted with a ZB-WAX capillary column, 60 m × 0.32 mm i.d. with 1 μm film thickness. Helium was the carrier gas at a flow rate of 1.2 mL/min at 40°C. Oven temperature was programmed as follows: initial temperature 40°C held for 5 min, then 4°C/min to 230°C. The volatile compounds were identified with a mass selective detector (5975C, Agilent Technologies, CA, USA). The detector operated in the m/z range between 30 and 250, ion source and quadrupole temperature were maintained at 250 and 150°C, respectively. Identification of compounds was performed by comparison of their mass spectra with those of available commercial standards. All compounds were also confirmed by matching of their mass spectra with NIST 2.0 mass spectral database (National Institute of Standards and Technology, USA).
Quantification of the volatile compounds was carried out by the preparation of the calibration curves for the each volatile compound. Concentration of volatiles in samples was calculated from the peak areas of selected ions and the corresponding standards of the known concentration. Determination was conducted in the triplicates.
Statistical analysis
The results were expressed as the mean values of the triplicates ± standard deviation. Data of the volatile compounds amount were analyzed by analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) with the significance defined at p < 0.05. All statistical analyses were carried out using software program STATISTICA 7 (StatSoft, Inc, USA).
Results and discussion
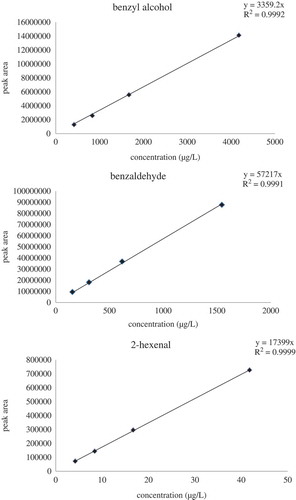
Quantification of volatiles was carried out from calibration curves. For quantification, the ions used were m/z 83 (2-hexenal), m/z 106 (benzaldehyde), m/z 112 (benzaldehyde d6—internal standard) and m/z 108 (benzyl alcohol). The calibration curves were linear in the range from 0.0042 to 0.0418 mg/L for 2-hexenal, 0.154–1.546 mg/L for benzaldehyde and 0.4178 to 4.178 mg/L for benzyl alcohol. In all calibration curves regression coefficient were higher than 0.999 ().
In this study, an influence of disaccharides, sucrose, maltose, or trehalose, on volatile compounds of the freeze-dried sour cherry puree was investigated. The selected key volatile compounds of the sour cherry were benzaldehyde, benzyl alcohol, and 2-hexenal. Benzaldehyde is described with floral, rose, phenolic flavor note, benzyl alcohol with sweet, almond, fruity, green flavor note and 2-hexenal with strong, sharp, bitter, almond flavor note. The results of the evaluation of the amount of the volatile compounds in samples are presented in . The control sample had 3.92 mg/kg of benzyl alcohol, 856.61 µg/kg of benzaldehyde and 15.58 µg/kg of 2-hexenal. All the samples with the sugar addition had lower amount of the benzyl alcohol (2.65–3.19 mg/kg) than the control sample. Comparing the results of the amount of the sugar addition, it can be seen that in the samples with sucrose or maltose addition with the increase of the sugar amount decrease of the benzyl alcohol occurred. In the samples with trehalose addition, the amount of trehalose didn’t have influence on the amount of benzyl alcohol (~3 mg/kg). Comparison of the addition of the same amount of the different sugars showed that addition of 5 and 10 g/100 g of the sugars (sucrose, maltose or trehalose) affected the benzyl alcohol amount. When 20 g/100 g of sugar was added the highest amount of the benzyl alcohol was in sample with trehalose addition (2.94 mg/kg), and the lowest with sucrose addition (2.65 mg/kg). In the case of the benzaldehyde different tendency was observed. All the samples with the sucrose addition had lower amount of the benzaldehyde (~750 µg/kg) than control sample. In the samples with addition of the maltose with the increase of added amount, the increase of benzaldehyde amount was determined (697.78, 779.67, and 871.34 µg/kg). In the sample with 20 g/100 g of maltose addition, the same amount was determined as in the control sample, while other two samples (with 5 or 10 g/100 g) had lower amount of this volatile compound. In comparison to the control sample, the sample with 5 g/100 g of trehalose addition had lower (731.62 µg/kg), with 10 g/100 g higher (937.51 µg/kg) and with 20 g/100 g the same (815.87 µg/kg) amount of the benzaldehyde. Comparing the effect of the same amount of different sugars, the samples with addition of 5 g/100 g of sucrose and trehalose had the same amount, while the sample with maltose had lower amount of the benzaldehyde. The samples with addition of 10% sucrose and maltose had the same amount, while sample with trehalose had higher amount of the benzaldehyde. When 20 g/100 g of sugar was added to the puree, the highest amount of the benzaldehyde was determined in sample with maltose addition (871.34 µg/kg), while sample with sucrose had the lowest amount (734.01 µg/kg). The samples with addition of the sucrose (all amounts) had lower (~13 µg/kg) amount of 2-hexenal than control sample (15.58 µg/kg). Samples with 5% of maltose addition had lower (12.7 µg/kg), while other two samples had the same (~16.5 µg/kg) amount of 2-hexenal as the control sample. Samples with 5% of trehalose addition had lower, while other two samples (with 10 or 20 g/100 g) higher amount (17.45 and 18.96 µg/kg, respectively) of 2-hexenal than the control sample. Comparing the influence of the sugars amount, the samples with 5 g/100 g of sugars had the same amount of 2-hexenal, while in the case of 10 and 20 g/100 g addition, samples with maltose and trehalose had higher amount than samples with sucrose. The positive effect of the trehalose addition to freeze-dried puree samples was observed in other studies, too. The addition of trehalose had a positive influence on the benzaldehyde retention in the freeze-dried strawberry puree, nevertheless the difference between samples without sugar and with sugar addition was not so high as in the case of 2-hexenal. In the case of the 2-hexenal addition of both sugars, sucrose, or trehalose had positive influence on its retention, with trehalose having a more pronounced effect.[Citation2] In addition, Komes at al. reported that there was a positive effect of the sugar addition to the freeze-dried apricot puree on 2-hexenal and benzaldehyde, but a higher positive effect was observed for the benzaldehyde.[Citation3] Nevertheless, a higher effect on retention of both compounds had trehalose. Galmarini et al. reported that in freeze-dried strawberry puree majority of volatile compounds were determined in higher amount when trehalose was added (30%), but there were some exceptions like nerodilol, octyl butyrate, and γ-dodecalactone that were determined in higher amount in the samples with the sucrose addition.[Citation1] In freeze-dried strawberry cream fillings, Kopjar et al. reported that the samples with addition of the trehalose had higher amount of fruity esters but on the other hand trehalose positive influence was not reported for γ-decalactone and furaneol.[Citation5,Citation6] Also, the same group reported that with increase of the trehalose addition proportional increase of the amount of fruity esters wasn’t observed, as it was the case with the results reported in this study.
Table 1. Amount of volatile compounds in freeze-dried sour cherry puree without and with addition of sugars.
Generally, aroma release depends on a number of the volatile parameters and the matrix composition. Among the volatile parameters, the most important ones are hydrophobicity, water solubility and volatility that are usually connected to the volatile’s structural properties, like chain length, branching and the presence and position of a functional group.[Citation15–Citation18] It is difficult to explain the behavior of the sugars in the complex food matrix due to different interactions that can occur between compounds. Trehalose, maltose, and sucrose are isomers with same chemical formula but different structures resulting in different behavior of each sugar which could be reason for retention of some volatile compounds during dehydration when trehalose or maltose were present in the system.[Citation19,Citation20] Trehalose has higher effectiveness as a hydrogen bond donor than other disaccharides resulting in stabilization of biomolecules during dehydration.[Citation19] Compounds that have unsaturated bonds similar in the shape to the cis-type olefinic double bond like benzene and p-cresol can bind to trehalose in the aqueous solutions but not with other disaccharides due to unique nature of the trehalose. The aromatic ring approaches to the dehydrated, hydrophobic pocket of trehalose leading to formation of the complex.[Citation21] Correlation between the heats of solution (trehalose 19.1, maltose 15.6, and sucrose 5.95 kJ/mol)[Citation22] of this disaccharides with the effectiveness in cryogenic preservation was done.[Citation20] Superior cryoprotecting characteristics of trehalose can be attributed to trehalose ability to disrupt the water structure.[Citation20] Glass transition theory could also be one of mechanism of sugar action in food matrix. Comparing the glass transition temperature of used sugars, trehalose has the highest glass transition temperature (115°C), than maltose (84°C) and sucrose (60°C).[Citation20,Citation23] The stability of low-moisture foods can be explained through the concept of the glassy state, emphasizing that thermodynamically unstable state can achieve apparent structural and chemical stability by virtue of high viscosity.[Citation24] The formation of a glassy state results in a significant arrest of translational molecular motion thus translational mobility and diffusion are considered to be virtually non-existent.[Citation25] Consequently, in that state, chemical reactions that are dependent upon the diffusion of molecules would slow down or would not occur at all.[Citation26–Citation28] Also, it is very important that disaccharides are behaving differently in water solutions during freezing and formation of ice due to different interactions between disaccharides and water molecules that were proven by the study of the structural properties of disaccharide (trehalose, maltose and sucrose) water systems. In the presence of trehalose, water molecules are arranged in a particular configuration, which avoids ice formation, so preserving biomolecules from damage due to freezing and cooling. Addition of the trehalose, in comparison to the maltose and especially sucrose, completely destroys the tetrahedral intermolecular network of water, which by lowering the temperature would give rise to ice.[Citation29–Citation35] The diffusion of water in trehalose water mixtures was strongly affected by trehalose, and trehalose and water form a unique entity, creating a rigid environment where biomolecules can be protected.[Citation35] Also, trehalose molecules have a greater inhibitory effect on the growth of ice crystals in comparison to sucrose[Citation34] and aqueous trehalose has a larger amount of unfrozen water content in comparison with the other disaccharide systems.[Citation36] Possibility to avoid ice formation is associated with disaccharides molecular structure like combination of constitutive monosaccharides and the position and type of the glycosidic bond between the monosaccharides. More unfrozen water is induced in the presence of disaccharides having a poorer compatibility with the three-dimensional hydrogen-bond network of the water.[Citation35] It is very difficult to point out only one factor that could influence retention of volatiles in freeze-dried sour cherry purees, matrix that is quite complex. More likely, the combination of different factors, like properties of sugars and volatile compounds, influence of sugars on water dynamics, but also applied in the processing method, were responsible for the behavior of the volatile compounds in investigated fruit matrix. Also, it is evident that there is no regularity on retention of volatile compounds with increase of sugar amount. As it was stated sugars do change water dynamics, but also interactions (hydrogen bonding or week hydrophobic interactions) between sugars and volatiles can occur causing this irregularity.
Conclusion
Formulation of the fruit products is very important since the ingredients in the matrix can interact with each other resulting in the quality loss or retention. In this study, change of the flavor of sour cherry purees was monitored depending on the addition of different disaccharides (sucrose, trehalose, and maltose) and their amounts. Overall looking, the trehalose addition prior freeze-drying of sour cherry puree had the most positive effect on the key volatile compounds of the mentioned fruit product. Even if all investigated sugars are isomers having the same chemical formula, their structure and behavior, as well as properties of the volatile compounds were probably the factors that caused different tendency of the retention of the selected volatile compounds of the freeze-dried sour cherry puree.
Acknowledgments
We are grateful to Hayashibara, Nagase Group, Japan, for generous donation of trehalose and maltose.
Funding
This work has been fully supported by the Croatian Science Foundation under the project Trehalose: fruit product quality improvement project (6949).
Additional information
Funding
References
- Galmarini, M.V.; Van Baren, C.; Zamora, M.C.; Chirife, J.; Di Leo Lira, P.; Bandoni, A. Impact of Trehalose, Sucrose And/Or Maltodextrin Addition on Aroma Retention in Freeze Dried Strawberry Puree. International Journal of Food Science and Technology 2011, 46, 1337–1345.
- Komes, D.; Lovrić, T.; Ganić, K.K.; Gracin, L. Study of Trehalose Addition on Aroma Retention in Dehydrated Strawberry Puree. Food Technology and Biotechnology 2003, 41, 111–119.
- Komes, D.; Lovrić, T.; Ganić, K.K.; Kljusurić, J.G.; Banović, M. Trehalose Improves Flavour Retention in Dehydrated Apricot Puree. International Journal of Food Science and Technology 2005, 40, 425–435.
- Komes, D.; Lovrić, T.; Kovačević Ganić, K. Aroma of Dehydrated Pear Products. LWT – Food Science and Technology 2007, 40, 1578–1586.
- Kopjar, M.; Piližota, V.; Hribar, J.; Simčič, M.; Zlatič, E.; Tiban, N.N. Influence of Trehalose Addition and Storage Conditions on the Quality of Strawberry Cream Filling. Journal of Food Engineering 2008, 87, 341–350.
- Kopjar, M.; Hribar, J.; Simčič, M.; Zlatić, E.; Tomaž, P.; Piližota, V. Effect of Trehalose Addition on Volatiles Responsible for Strawberry Aroma. Natural Product Communicastions 2013, 8, 1767–1770.
- Seuvre, A.M.; Philippe, E.; Rochard, S.; Voilley, A. Retention of Aroma Compounds in Food Matrices of Similar Rheological Behaviour and Different Compositions. Food Chemistry 2006, 96, 104–114.
- Siddiq, M.; Iezzoni, A.; Khan, A.; Breen, P.; Sebolt, A.M.; Dolan, K.D.; Ravi, R. Characterization of New Tart Cherry (Prunus cerasus L.): Selections Based on Fruit Quality, Total Anthocyanins, and Antioxidant Capacity. International Journal of Food Properties 2011, 14, 471–480.
- Taghadomi-Saberi, S.; Omid, M.; Emam-Djomeh, Z.; Ahmadi, H. Development of an Intelligent System to Determine Sour Cherry’s Antioxidant Activity and Anthocyanin Content during Ripening. International Journal of Food Properties 2014, 14, 1169–1181.
- Kopjar, M.; Oršolić, M.; Piližota, V. Anthocyanins, Phenols and Antioxidant Activity of Sour Cherry Puree Extracts and Their Stability during Storage. International Journal of Food Properties 2014, 17, 1393–1405.
- Schmid, W.; Grosch, W. Quantitative Analyse Flüchtiger Aromastoffe Mit Hohen Aromawerten in Sauerkirschen (Prunus cerasus L.), Süßkirschen (Prunus avium L.) Und Kirschkonfitüren. Zeitschrift Für Lebensmittel-Untershuncung Und Forschung 1986, 183, 39–44.
- Poll, L.; Barixtofte, M. Influence of Harvest Year and Harvest Time on Soluble Solids, Titrateable Acid, Anthocyanin Content and Aroma Components in Sour Cherry (Prunus cerasus L. Cv. “Stevnsbær”). European Food Research and Technology 2003, 216, 212–216.
- Xiao, Z.-B.; Zhang, N.; Niu, Y.-W.; Fena, T.; Tian, H.-X.; Zhu, J.-C.; Yu, H.-Y. Multivariate Classification of Cherry Wines Based on Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry of Volatile Compounds. International Journal of Food Properties 2015, 18, 1272–1287.
- Howerd, K.L.; Mike, J.H.; Riesen, R. Validation of a Solid-Phase Microextraction Method for Headspace Analysis of Wine Aroma Components. American Journal of Enology and Viticulture 2005, 56, 37–45.
- Hansson, A.; Leufven, A.; Van Ruth, S. Partition and Release of 21 Aroma Compounds during Storage of a Pectin Gel System. Journal of Agricultural and Food Chemistry 2003, 51, 2000–2005.
- Kopjar, M.; Andriot, I.; Saint-Eve, A.; Souchon, I.; Guichard, E. Retention of Aroma Compounds: An Interlaboratory Study on the Effect of the Composition of Food Matrices on Thermodynamic Parameters in Comparison with Water. Journal of the Science of Food and Agriculture 2010, 90, 1285–1292.
- Van Ruth, S.M.; King, C. Effect of Starch and Amylopectin Concentrations on Volatile Flavour Release from Aqueous Model Food Systems. Flavour and Fragrance Journal 2003, 18, 407–416.
- Zafeiropoulou, T.; Evageliou, V.; Gardeli, C.; Yanniotis, S.; Komaitis, M. Retention of Selected Aroma Compounds by Gelatine Matrices. Food Hydrocolloids 2012, 28, 105–109.
- Branca, C.; Faraone, A.; Magazu, S.; Maisano, G.; Migliardo, F.; Migliardo, P., Villari, V. Structural and Dynamical Properties of Threalose-Water Solutions: Anomalous Behaviour and Molecular Models. Recent Research Developments in Physical Chemistry 1999, 3, 361–386.
- Patist, A.; Zoerb, H. Preservation Mechanisms of Trehalose in Food and Biosystems. Colloids Surfaces B Biointerfaces 2005, 40, 107–113.
- Sakakura, K.; Okabe, A.; Oku, K.; Sakurai, M. Experimental and Theoretical Study on the Intermolecular Complex Formation between Trehalose and Benzene Compounds in Aqueous Solution. Journal of Physical Chemistry B 2011, 115, 9823–9830.
- Miller, D.P.; De Pablo, J.J. Calorimetric Solution Properties of Simple Saccharides and Their Significance for the Stabilization of Biological Structure and Function. Journal of Physical Chemistry B 2000, 104, 8876–8883.
- Crowe, L.M.; Reid, D.S.; Crowe, J.H. Is Trehalose Special for Preserving Dry Biomaterials?. Biophysical Journal 1996, 71, 2087–2093.
- Schebor, C.; Burin, L.; Buera, M.; Chirife, J. Stability to Hydrolysis and Browning of Trehalose, Sucrose and Raffinose in Low-Moisture Systems in Relation to Their Use as Protectants of Dry Biomaterials. Food Science and Technology 1999, 32, 481–485.
- Schiraldi, C.; Di Lernia, I.; De Rosa, M. Trehalose Production: Exploiting Novel Approaches. Trends in Biotechnology 2002, 20, 420–425.
- Koster, K.L. Glass Formation and Desiccation Tolerance in Seeds. Plant Physiology 1991, 96, 302–304.
- Schebor, C.; Buera, M.D.P.; Karel, M.; Chirife, J. Color Formation Due to Non-Enzymatic Browning in Amorphous, Glassy, Anhydrous, Model Systems. Food Chemistry 1999, 65, 427–432.
- Sun, W.; Leopold, C. Glassy Stated and Seed Storage Stability: A Viability Equation Analysis. Annals of Botany 1994, 74, 601–604.
- Wang, G.M.; Haymet, D.J. Trehalose and Other Sugar Solutions at Low Temperature: Modulated Differential Scanning Calorimetry (MDSC). Journal of Physical Chemistry B 1998, 102, 5341–5347.
- Magazù, S.; Migliardo, F.; Ramirez-Cuesta, J. Inelastic Neutron Scattering Study on Bioprotectant Systems. Journal of the Royal Society Interface 2005, 2, 527–532.
- Magazù, S.; Migliardo, F.; Telling, M.T.F. Structural and Dynamical Properties of Water in Sugar Mixtures. Food Chemistry 2008, 106, 1460–1466.
- Cesaro, A.; Magazù, V.; Migliardo, F.; Sussich, F.; Vadalà, M. Comparative Study of Structural Properties of Trehalose Water Solutions by Neutron Diffraction, Synchrotron Radiation and Simulation. Physica B: Physics of Condensed Matter 2004, 350, 367–370.
- Magazù, S.; Migliardo, F.; Ramirez-Cuesta, J. Changes in Vibrational Modes of Water and Bioprotectants in Solution. Biophysical Chemistry 2007, 125, 138–142.
- Uchida, T.; Nagayama, M.; Shibayama, T.; Gohara, K. Morphological Investigations of Disaccharide Molecules for Growth Inhibition of Ice Crystals. Journal of Crystal Growth 2007, 299, 125–135.
- Magazù, S.; Migliardo, F.; Gonzalez, M.; Mondelli, C.; Parker, S.; Vertessy, B. Molecular Mechanisms of Survival Strategies in Extreme Conditions. Life 2012, 2, 364–376.
- Furuki, T. Effect of Molecular Structure on Thermodynamic Properties of Carbohydrates. A Calorimetric Study of Aqueous Di- and Oligosaccharides at Subzero Temperatures. Carbohydrate Research 2002, 337, 441–450.