ABSTRACT
In the present study, the effect of different parameters on the correct geographical differentiation of Greek fir honey was investigated. Forty-three honey samples were collected from four regions and subjected to physicochemical and melissopalynological analyses, using official and instrumental methods. Results showed that fir honeys met the European regulatory quality standards, whereas significant differences (p < 0.05) were recorded for all the determined parameters according to geographical origin. The highest differentiation rate (81.4%) was recorded using the combination of six physicochemical parameters and five phenolic compounds, as assessed by multivariate analyses.
Introduction
Fir honey is produced by honey bees from excretions of plant sucking insects (Hemiptera) in the living parts of plants or secretions of living parts of plants (honeydew honeys). Typical examples of honeydew honeys are pine and fir tree honeys. Species of plant sucking insects in Greek fir trees include Physokermes hemicryphus Dalman (Homoptera genus, Coccidae spp.) (major) and Eulecanium sericeum (Homoptera genus, Coccidae spp.), and the aphides Mindarus abierinus Koch (Homoptera genus, Aphididae spp.), Cinara confinis Koch, (Homoptera genus, Aphididae spp.), and Cinara pectrinatae Nordl (Homoptera genus, Aphididae spp.) producing excretions exploited by honey bees.[Citation1]
Honey is considered as part of traditional medicine. Apitherapy has recently become the focus of attention as a form of folk and preventive medicine for treating certain conditions and diseases as well as promoting overall health and wellbeing.[Citation2] The main constituents of honey are sugars (mainly fructose and glucose), moisture, and other valuable micro-nutrients such as vitamins, minerals, enzymes, free amino acids, and numerous volatile compounds;[Citation3,Citation4] as well as different amounts of phenolic compounds depending on its botanical/geographical origin, bee species, harvesting season, climatic conditions, and honey extraction techniques.[Citation5]
Honey quality control regarding its floral or geographical origin, as well as potential adulteration, is usually carried out at any stage of production, processing, and marketing. In that sense, the EU honey regulation 110/2001[Citation6] aims to guarantee product quality, authenticity, protect consumers from product adulteration and fraud, by setting specific qualitative standards.
Fir honey is produced in Greece, Turkey, Germany, Italy, Slovenia, Croatia. In Greece, this type of honey is a less common type, representing the 10–15% of the annual production (13,000–15,000 tons). The standard approach in determining the botanical origin of honey is based on microscopic examination of its pollen (melissopalynology); however, this method has certain limitations.[Citation7] Contradicting opinions have been reported, regarding the differentiation of honey’s geographical origin, based on pollen data. For example, in the case of honeydew honeys from Greece, geographical discrimination based on pollen data in combination with chemometrics was not accomplished.[Citation8] This was not the case for Moroccan Eucalyptus honeys, in which the presence of Quercus, Plantago, and Thymelaea pollen allowed the differentiation of these honeys from those of a different geographical origin.[Citation9]
For this reason, in recent studies it has been suggested that chemical approaches may be more accurate and easily undertaken in the characterization of the geographical/and or botanical origins of honey. Methods used for this purpose include volatile compounds,[Citation4,Citation10] volatile compounds and physicochemical parameters,[Citation11–Citation13] volatile compounds and phenolic compounds,[Citation14–Citation17] mineral content and isotopic data,[Citation18,Citation19] free amino acids, mineral content and physicochemical parameters[Citation20,Citation21] in combination with chemometrics.
Based on the above, the aim of the present study was to investigate the impact of certain physicochemical parameters including phenolic compounds and specific pollen grains on the correct geographical differentiation of Greek fir honeys, using multivariate analysis of variance (MANOVA) and linear discriminant analysis (LDA). In an effort to maximize the correct prediction rate of differentiation, samples from multiple harvesting periods were used.
Materials and methods
Honey samples
A total of 43 commercial fir honey samples were collected from local beekeepers and ATTIKI Honey SA, during the harvesting periods 2010–2013 from four different regions in Greece: Messinia (7 samples: A. cephalonica and fir honeydew), Lakonia (11 samples: A. cephalonica and fir honeydew), Arkadia (9 samples: A. cephalonica and fir honeydew), and Evritania (16 samples: A. cephalonica), where fir honey is traditionally produced (see Figure S1 and Table S1 in the online supplementary information (SI)). Samples were stored in glass containers, shipped to the laboratory, and maintained at 4 ± 1°C until analysis.
Chemicals and instrumentation
Sodium hydroxide and hydrochloric acid (37%) used for the determination of free and lactonic acidity were purchased from Sigma Aldrich (Riedel-de Haën, GmbH, Germany). Potassium chloride (0.1 M, 1413 μS/cm), used for the calibration of conductivity meter (Delta OHM, model HD 3456.2, Padova, Italy), was obtained from Hanna (HI 7031, Hanna Instruments, Inc., Woonsocket, USA). pH of honey samples was measured using model HD 3456.2 pH meter (Delta OHM) with a precision of 0.002 pH units. The instrument was calibrated with buffer solution (pH = 7.0 ± 0.002, Catalogue 22835–49) prior to measurements, which was obtained from HACH (UK). Standard phenolic compounds [syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid), quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one), myricetin (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone), kaempferol (3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one), and chrysin (5,7-dihydroxy-2-phenyl-4H-chromen-4-one)] were obtained from Sigma-Aldrich (Steinheim, Germany). Ethyl acetate and acetonitrile were of HPLC grade and purchased from Merck (Darmstadt, Germany). Finally, hydrochloric acid and sodium chloride used in the isolation of phenolic compounds were purchased from Merck (Darmstadt, Germany).
Physicochemical and colour parameters L*, a*, b*
The following physicochemical parameters: pH, moisture content, electrical conductivity (EC), free acidity, lactonic acidity, total acidity, ash content, lactonic/free acidity ratio were determined according to the international honey commission methods of analysis.[Citation22] CIELAB colour parameters (L*, a*, b*) were determined according to CIE (Commission Internationale de l’ Eclairage) recommendations. This system uses three parameters to evaluate colour in foodstuffs: colour parameter L* corresponds to degree of brightness; parameter a* (positive values) corresponds to degree of redness, a* (negative values) to degree of greenness; parameter b* corresponds to yellowness of colour (when positive) and to blueness of colour (when negative). The preparation and analysis conditions of honey samples prior to colour measurements are given in a previous study.[Citation12]
Melissopalynolgical analysis
In order to ensure that the honeys were unifloral the melissopalynological methodology was applied to all samples.[Citation7] In particular, 10 g of each honey sample were diluted in 20 mL of distilled water and centrifuged at 3000 rpm for 10 min. The sediment of the solution was dried at 40°C and mounted on Entellan Rapid (Merck, 1.07961.0500). The honeydew elements and pollen grains were counted and identified in 20 optical areas at 200× magnification using an OLYMPUS BX 40 light microscope (Table S2 in the online SI).
Analysis of phenolic compounds
Extraction
Extraction of phenolic compounds from honey samples was carried out using ethyl acetate, as described previously.[Citation23]
HPLC instrumentation and conditions
The chromatographic analyses of phenolic compounds were performed using a HPLC system (Agilent 1100 series, USA). Syringic acid was detected at λ = 280 nm, whereas kaempferol, chrysin, myricetin, and quercetin were detected at λ = 254 nm. Gradient elution was used at a flow rate of 1 mL/min using a solution of 2.0% (w/v) acetic acid and acetonitrile (Merck) as the mobile phase. The solvent gradient system used was initially 10% of acetonitrile, increased to 30% for 20 min, further increased to 40% for 30 min, increased to 50% for 35 min, and finally increased to 50% for 40 min. Furthermore, the column was eluted isocratically for 10 min before next injection. Separations of the phenolic compounds were carried out using the reversed phase column Eclipse XDB C18 (Merck; 150 mm × 4.5 mm × 5 μm) at room temperature. Identification of phenolic compounds was carried out by comparing the retention times of individual chromatographic peaks to the retention times of standards. Quantification was achieved by comparing the chromatographic peak areas of each phenolic compound with that of the standards, using calibration curves. The calibration curves were constructed in triplicate for each individual standard at five different concentrations (0–200 mg/L). The determination coefficients for the calibration curves were RCitation2 = 0.984 for syringic acid, Citation2 = R 0.986 for kaempferol, and RCitation2 = 0.999 for quercetin, myricetin, chrysin, respectively. Limit of detection (LOD) and limit of quantification (LOQ) were determined using regression parameters from the calibration curves (3 Sy/x/a and 10 Sy/x/a, respectively); where Sy/x is the standard deviation of the residues, and a is the slope. The analysis of particular phenolic compounds in honey samples was carried out in triplicate (n = 3). A typical HPLC-DAD chromatogram of the five phenolic compounds is given in Figure S2 in the online SI.
Statistical analysis
Data processing was performed using the SPSS 20.0 statistics software (SPSS, Inc., 2012).[Citation12] Comparison of the means was achieved by MANOVA in order to determine those physicochemical parameter values or pollen grains that are significant in differentiating fir honeys collected from different production areas. Geographical origin was taken as the independent variable, while physicochemical parameter values or pollen grains were taken as the dependent variable. The Pillai’s trace and Wilk’s lambda indices were computed to determine a possible significant effect of physicochemical parameter values/pollen grains on geographical origin of fir honeys. LDA was then applied using the selected dependent variables to explore the possibility of classifying fir honey samples according to geographical origin.
In the MANOVA analysis, the independent variables were analysed simultaneously and the significant variables chosen by MANOVA have been already specified. Then, LDA analysis was used as a MANOVA follow-up test.[Citation24] Both original and leave-one-out cross-validation methods were used to test the classification ability.[Citation24,Citation25] It should be stressed that LDA is a supervised statistical method that should preferably be used in classification analysis.[Citation19,Citation26]
Results and discussion
Physicochemical parameters of Greek fir honeys
The physicochemical parameter values (average ± SD) for the 43 fir honey samples analysed are given in . Present values for each physicochemical parameter determined are within the range reported previously for dark-coloured honeys, including fir honeydew, produced in Morocco, Greece, Portugal, Turkey, and Slovakia.[Citation9,Citation12,Citation13,Citation27–Citation30] More specifically, moisture content (g/100 g) varied between 15.40 (fir honey samples from Lakonia) and 18.59 (fir honey samples from Arkadia). Present moisture content values are within the limits (≤20 g/100 g) set by the EU for commercial honey samples,[Citation6] but slightly higher than the limit of ≤18.50 g/100 g set by the Greek directive 127/2004[Citation31] regarding fir honeys.
Table 1. Physicochemical parameters of Greek fir honeys according to geographical origin and comparison with similar studies.
Honey moisture content depends on environmental conditions and specific beekeepers’ practices during the harvesting period, and may vary from year to year.[Citation13] High moisture content may accelerate crystallization in certain types of honey and increase its water activity to values where certain yeasts could grow. Moisture content of honey may also depend on the degree of honey maturity reached in the hive and moisture content of original plant.[Citation27] Sahinler et al.[Citation28] reported moisture content (%) values for pine honeys, sunflower, and citrus honeys of 17.15–17.27, 17.80–18.20, and 17.98–18.88, respectively.
Manzanares et al.[Citation29] reported moisture content (%) values 16.21–17.75 for honeydew honeys and 16.13–17.33 for blossom honeys. Finally, Pavelková et al.[Citation30] in a study dealing with the physicochemical and microbiological quality of 24 honey samples (8 rape, 8 lime, and 8 honeydew), from Liptov region in Slovakia, reported moisture content values ranging 16.47–17.20 for honeydew honeys tested. pH ranged from 4.80 (fir honey samples from Arkadia) to 4.97 (fir honey samples from Evritania). Manzanares et al.[Citation29] reported pH values of 3.89–5.27 for honeydew honeys and 3.68–4.24 for blossom honeys. The acid content in honey is characterized by the free acidity. Furthermore, the measurement of free acidity is useful for the evaluation of honey’s fermentation. Furthermore, it is helpful for the authentication of unifloral honeys and especially for the differentiation between nectar and honeydew honeys.[Citation32]
Free acidity ranged between 26.10 meq/kg (i.e. fir honey samples from Lakonia) and 31.28 meq/kg (fir honey samples from Karditsa). According to the EU,[Citation6] the upper limit for free acidity is 50.00 meq/kg. No tested honey sample exceeded this limit value. Lactonic acidity ranged between 4.16 meq/kg (fir honey samples from Evritania) and 5.78 meq/kg (fir honey samples from Lakonia). Finally, total acidity ranged between 31.89 meq/kg (fir honey samples from Lakonia) and 35.44 meq/kg (fir honey samples from Evritania). Manzanares et al.[Citation29] reported acidity values (meq/kg) of 23.98–45.32 for honeydew honeys, while in the case of blossom honeys respective values were 19.21–29.67.
Lactonic/free acidity ratio (L/FA) ranged from 0.15 (fir honey samples from Evritania) to 0.23 (fir honey samples from Lakonia). EC reflects the mineral content of honey. EC is used to distinguish between floral and honeydew honeys. It is also the most important physicochemical parameter measured for the authentication of unifloral honeys.[Citation33] EC values ranged between 1.24 mS/cm (fir honey samples from Evritania) and 1.83 mS/cm (fir honey samples from Arkadia). According to the European standards,[Citation6] honeydew honeys have specific range/limits regarding EC. EC in honeydew honeys must be no less than 0.8 mS/cm.[Citation6] Furthermore, in Greek directive 127/2004[Citation31] it is stated that fir honeys must have an EC of no less than 1.0 mS/cm. The high EC in fir honey samples from Arkadia could be an indication of lack of authenticity of fir tree honeys.
Manzanares et al.[Citation29] reported EC values of 0.88–1.54 for honeydew honeys and 0.30–0.64 for blossom honeys. These authors also stated that honeydew honeys usually have higher mean values of EC in comparison to blossom honeys. This statement is in excellent agreement with results of the present study. Ash content has been used to distinguish between honeydew and blossom honeys in combination with their different microscopic and sensory characteristics. Ash content (g/100 g) ranged from 0.75 (fir honey samples from Messinia and Evritania) to 1.15 (fir honey samples from Arkadia). Pavelková et al.[Citation30] reported lower ash values for 8 Slovakian honeydew honeys tested, ranging 0.06–0.08 (w/w), while Karabagias et al.[Citation12] reported ash values for 39 pine honeys tested ranging 0.39–0.92 g/100 g. This parameter proved significant (p < 0.05) for the differentiation of the geographical origin of pine honeys.
Honey colour is another quality criterion for its acceptance by consumers. L* colour parameter ranged from 69.91 (fir honey samples from Karditsa) to 72.68 (fir honey samples from Lakonia). Colour parameter a* values ranged from −4.73 (fir honey samples from Arkadia) to −3.46 (fir honey samples from Evritania). Finally, colour parameter b* ranged from 17.95 (fir honey samples from Lakonia) to 23.55 (fir honey samples from Evritania). Researchers have satisfactorily classified different honey types such as citrus, rosemary, lavender, eucalyptus, thyme, honeydew, heather, chestnut, avocado, and pine using among other CIE parameters L*a*b* values.[Citation12,Citation34]
Pollen content of Greek fir honeys
Fir honey is a less common type of honey with limited production or even no production at all depending on harvesting year in Greece. In this type of honey, the presence of different pollen percentages from other plants is fairly common. These include Cistaceae, Boraginaceae, Ephedra spp., Helianthemum spp., Cistus spp., Olea europaea, Labiatea, Caprifoliaceae, Verbascum spp., Polygonum aviculare, Pyrus/Prunus, etc.
Beekeepers often transfer their beehives in groups to lower the cost of apiculture. Thus, in the case of fir honey production, geographical regions of close proximity may be preferred. In that sense, the presence of pollen in fir tree honey is probably the result of the presence of pollen from both honeydew and blossom honeys. To estimate the pollen content of commercial fir honey samples, predominant pollen (>45%), secondary pollen (16–45%), important minor pollen (3–15%), minor pollen (<3%), and isolated pollen (ca. <1%) were considered. The most abundant pollen grains determined, serving as predominant pollen >45%, secondary pollen 16–45% and important minor pollen 3–15%, were Castanea sativa, Quercus ilex, and Erica spp., exhibiting variations according to the production areas of fir honeys analysed ().
Table 2. Most dominant mean content (% pollen grains) isolated from Greek fir honeys according to geographical origin.
On the other hand, since the production of this type of honey depends mainly on harvesting period, dark-coloured honeys produced in some regions, which cover the criteria set by the Greek directive,[Citation31] may be characterized as “fir honeys” or “fir honeydew” or “commercial fir” for marketing purposes. These criteria are EC ≥ 1.0 mS/cm, moisture content (g/100 g) ≤ 18.5, and variations in the ratio of honeydew elements/pollen grains with the presence of characteristic honeydew elements such as fungal spores. This was the case for some samples produced in Messinia, Lakonia, and Arkadia, which are regions of close proximity (average distance value < 85 km) and characterized as fir honeydew based on melissopalynological analysis. On the other hand, fir honey samples from Evritania were A. cephalonica Loudon, Pinaceae.
Furthermore, fir honeys had few honeydew elements/pollen grains (HDE/PG), with this ratio being < 3, the important value required for a honey to be characterized as predominately honeydew. However, because of the numerous over- or under-represented pollen types, the pollen percentages and the HDE/PG ratios may vary greatly among different unifloral honeys.[Citation7]
Phenolic content of Greek fir honeys
In the present study, significant variations in each phenolic compound were observed, among the 43 honey samples of different geographical origin (). Furthermore, amounts of phenolic compounds determined are within the range reported in similar studies involving honeys produced in different countries.[Citation14–Citation16] Differences in the phenolic profile among the four regions studied (including and regions of close proximity) may be attributed to several factors such as altitude, temperature, rainfall, sunlight, or even different contribution of nectar/pollen percentage (genotype) owed to different flowers present in the greater area (Tables S1 and S2 in the online SI). It has been previously reported in the literature that in case of natural products this is very likely to occur.[Citation35]
Table 3. Phenolic content in Greek fir honeys according to geographical origin, method’s analytical characteristics, and comparison with similar studies.
On the other hand, it should be stressed that the presence of pollen in fir tree honey is probably the result of the presence of pollen from both honeydew and blossom honeys such is the case of Q. ilex which is the predominant pollen in fir tree honey from Messinia and Lakonia (Table S2 in the online SI).
Geographical differentiation of Greek fir honeys based on physicochemical parameter values
Pillai’s trace = 1.735 (F = 3.867, p = 0.001 < 0.05) and Wilk’s lambda = 0.024 (F = 6.633, p = 0.001 < 0.05) indicate the existence of a significant effect of geographical origin on physicochemical parameter values of honeys. Seven physicochemical parameters () were found to be significant (p < 0.05) for the geographical discrimination of fir honeys. Thus, these were subjected to LDA. Results showed that two statistically significant discriminant functions were formed (Wilk’s lambda = 0.089, XCitation2 = 89.313, df = 18, p = 0.001 < 0.05) for the first function, and Wilk’s lambda = 0.492, XCitation2 = 26.271, df = 10, p = 0.002 < 0.05) for the second. Testing of the uniformity of variability (Box’s M index = 169.701, F = 2.539, p = 0.06 > 0.05) was insignificant at the 95% confidence level showing the existence of uniformity of sample variability for each geographical origin.
Table 4. Physicochemical parameters, pollen grains, and phenolic compounds used for the differentiation of Greek fir honeys according to geographical origin.
The first discriminant function accounted for 82.90% of total variance, while the second accounted for 14.5%. Both accounted for 97.43% of total variance, a truly satisfactory rate. In it is shown that only honey samples from Lakonia are well separated followed by those of Evritania. The overall correct differentiation rate was 86% for the original and 74.4% for the cross-validation method; the latter considered, in general, satisfactory for this method.
Figure 1. Differentiation of Greek fir honeys according to geographical origin based on seven physicochemical parameters.
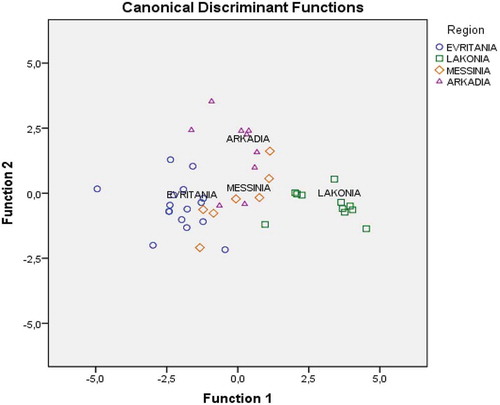
The correct geographical prediction rate was 87.5%, 90.9%, 28.6%, and 66.7% for Evritania, Lakonia, Messinia, and Arkadia fir honey samples, respectively. Especially, for fir honey samples produced in Messinia and Arkadia, an overlapping among samples was observed leading to a low differentiation rate. This may be attributed to the close proximity of these two regions (approximately 60 km) and to beekeepers’ practices. This was not the case for the Lakonia honey samples which gave a satisfactory differentiation rate.
Present results are in good agreement with those of Karabagias et al.,[Citation12] who indicated that physicochemical parameters may be used for the geographical differentiation of 39 pine honeys (honeydew honeys) from 4 different regions in Greece, providing a satisfactory prediction rate of 79.5% using the cross-validation method, and showing the general impact of geographical origin on honey physicochemical properties.
Habib et al.[Citation21] characterized 11 honey samples from arid regions (8 monofloral and 3 multifloral) and 5 from non-arid regions (3 monofloral and 2 multifloral) by determining selected physicochemical (pH, moisture, free, lactonic, and total acidity, EC, water activity, total sugar, Brix sugar, HMF, and colour intensity) and biochemical (minerals, total free amino acids, total carotenoids) parameters. Despite the limited number of honey samples analyzed, these authors concluded that there were considerable differences among the 16 honey samples, regarding physicochemical and biochemical parameters determined.
More recently, the use of selected heavy metals (As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn) determined in Romanian honeys from three different geographical regions (Suceava, Botosani, and Vaslui) in combination with chemometrics resulted in a correct classification rate of 80.8% according to botanical origin (acacia, sunflower, tilia, and polyfloral).[Citation36] This was not the case for the geographical origin differentiation efforts carried out by the same authors, where a poor differentiation rate of 21.2% was achieved. This may be attributed to the close proximity of two of the three investigated regions (i.e. distance from Suceava to Botosani being equal to 39.5 km).
Geographical differentiation of Greek fir honeys based on pollen content (pollen grains %)
Along the same line of reasoning, Pillai’s trace (=0.638, F = 3.515, p < 0.001 < 0.05) and Wilks’ lambda (=0.445, F = 3.954, p < 0.001 < 0.05) indicate the existence of a significant multivariable effect of geographical origin on the content of specific pollen grains investigated (Q. ilex and Erica spp.). This was not the case for C. sativa pollen grains (F = 0.560, p = 0.645 > 0.05). Thus, these two variables were subjected to LDA. Results showed that two statistically significant discriminant functions were formed (Wilk’s lambda = 0.472, XCitation2 = 29.258, df = 6, p = 0.000 < 0.05) for the first function and (Wilk’s Lambda = 0.857, XCitation2 = 6.012, df = 2, p = 0.049 < 0.05) for the second, respectively. The first discriminant function accounted for 83% of total variance, while the second accounted for 16%. Both accounted for 99% of total variance, a very satisfactory rate. The overall correct differentiation rate was 58.8% for the original and 48.8% for the cross-validation method; however, the values were considered unsatisfactory for both methods.
Correct prediction rates, based on Q. ilex and Erica spp. pollen grains were 28.6%, 63.6%, 0%, and 75% for honey samples from Messinia, Lakonia, Arkadia, and Evritania, respectively. The prediction rates obtained for Lakonia and Evritania honeys may be considered satisfactory. However, the overall classification rate obtained showed that pollen grains content do not provide a meaningful tool for the differentiation of fir honey according to geographical origin (). Present results are in very good agreement with those of Dimou et al.,[Citation8] who reported that the geographical discrimination of Greek honeydew honeys was not accomplished based on specific pollinic data. In contrast, in a more recent study, Dimou et al.[Citation37] reported that the determination of geographical origin of bee pollen was possible (correct classification rate of 75%) among northern, central, and southern Greece.
Figure 2. Differentiation of Greek fir honeys according to geographical origin based on two dominant pollen grains.
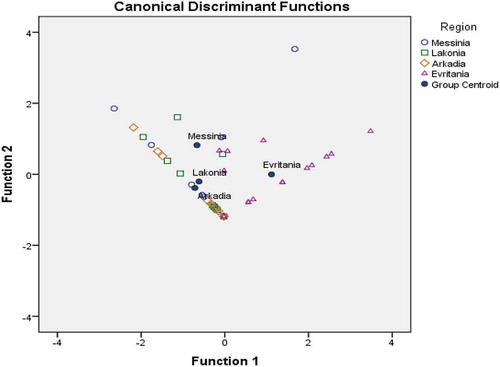
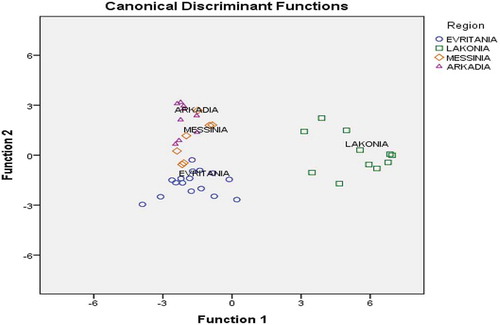
Figure 3. Differentiation of Greek fir honeys according to geographical origin based on the combination of six physicochemical and five phenolic compounds.
In contrast to present results, Terrab et al.[Citation9] reported that the presence of Quercus, Plantago, and Thymelaea pollen allowed the differentiation of Moroccan Eucalyptus honeys, according to geographical origin. We conclude that further research is needed on the validity of the use of specific pollen grains as markers of geographical origin of Greek fir honey.
Geographical differentiation of Greek fir honeys based on the combination of phenolic compounds and physicochemical parameter values
Since the differentiation rate of fir honey according to geographical origin was very low based on the use of specific pollen grains, an attempt was made to increase the overall differentiation rate by using the combination of physicochemical parameter values and phenolic compounds content. Pillai’s trace (=2.355, F = 5.930, p = 0.001 < 0.05) and Wilks’ lambda (=0.004, F = 8.547, p = 0.001 < 0.05) index values showed that there was a significant multivariable effect of geographical origin on the identity of phenolic compounds/physicochemical parameter values. Five phenolic compounds and six physicochemical parameter values () were found to be significant (p < 0.05) for the differentiation of honeys. Results showed that two statistically significant discriminant functions were formed (Wilks’ lambda = 0.012, ΧCitation2 = 154.833, df = 30, p = 0.001 < 0.05 for the first function, and Wilks’ lambda = 0.141, ΧCitation2 = 68.655, df = 18, p = 0.001 < 0.05 for the second). Testing of the uniformity of variability (Box’s M index = 205.821, F = 4.822, p = 0.058) was insignificant at the 95% confidence level showing the existence of uniformity of sample variability for each geographical origin. The first discriminant function accounted for 75.7% of total variance, while the second accounted for 16.0%. Both accounted for 91.7% of total variance, a very satisfactory rate. shows that honey samples from Lakonia and Evritania are well separated. The first discriminant function differentiates fully honey samples from Lakonia, while the second discriminant function differentiates honey samples from Evritania. The overall correct differentiation rate was 93.0% for the original and 81.4% for the cross-validation method, satisfactory for both methods. Correct prediction rate (100%) was achieved for honey samples from Lakonia and Evritania, followed by those of Messinia (85.7%) and Arkadia (77.8%). The standardized canonical discriminant function coefficients obtained from each model are also given in . Thus, these 11 variables were subjected to LDA.
Present results are in general agreement with those reported by other researchers, who showed that the use of specific volatiles, physicochemical parameters, minerals, isotopic data, selected flavonoids, and phenolic acids in combination with multivariate techniques could be used for the determination of both: botanical and geographical origin of honeys in lieu of melissopalynological analysis,[Citation13,Citation15,Citation18,Citation19] providing satisfactory differentiation rates higher than 75%. What is clearly shown in the present study is that the combined use of physicochemical parameter values and phenolic compound content increased the overall differentiation rate by 32.6% as compared with that of pollen grains.
Conclusion
Results of the present study showed that: (a) fir honeys met the standard quality criteria set by EU and (b) specific physicochemical parameters (p < 0.05) in combination with chemometrics could differentiate the geographical origin of Greek fir honeys. In this case, a correct prediction rate of 74.4% was achieved, despite the close proximity (average distance value less than 85 km) between Messinia, Arkadia and Lakonia regions. When the above physicochemical parameters were combined with specific phenolic compounds, the overall correct geographical differentiation rate of fir honey was increased to 81.4%. Differences in correct classification rates of Greek fir honey may be attributed also to the presence of pollen from different flowers (botanical origin) in proximity of fir trees as well as other environmental factors such as altitude, rainfall, sunlight, etc. Furthermore, when the most abundant pollen grains percentages determined (Q. ilex and Erica spp.) were subjected to chemometric analyses, in total, a poor discrimination rate was obtained (< 50%). On the other hand, these pollen grains resulted in a satisfactory discrimination rate for fir honeys from Lakonia/Evritania, and could serve as a fingerprint of their geographical origin. In general, further research is needed, especially in the case of pollen grains, if they are to be used as potential markers of geographical origin of Greek fir honey. Finally, physicochemical parameter analysis in combination with multivariate analyses proved to be a more effective tool for the geographical differentiation of honeydew honey such as fir.
LJFP_A_1300811_Supplementary_files.docx
Download MS Word (163.6 KB)Acknowledgements
The authors are grateful to Attiki Honey S.A., Athens, Greece, for the donation of fir honey samples from Evritania (Karditsa and Karpenisi) and to professional beekeepers from Messinia (G. Sentementes, E. Ntokos, P. Lagios), Lakonia (P. Sotiralis, D. Heliotis), and Karditsa (K. Mallios) for the donation of fir honey samples.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Santas, L.A.;. Insects Producing Honeydew Exploited by Bees in Greece. Apidologie 1983, 14(2), 93–103.
- Kassim, M.; Achoui, M.; Mohd-Rais, M.; Ali-Mohd, M.; Kamaruddin, M.Y. Ellagic Acid, Phenolic Acids, and Flavonoids in Malaysian Honey Extracts Demonstrate in Vitro Anti-Inflammatory Activity. Nutrition Research 2010, 30, 650–659.
- Baroni, M.V.; Nores, M.L.; Díaz, M.D.P.; Chiabrando, G.A.; Fassano, J.P.; Costa, C.; Wunderlin, D.A. Determination of Volatile Organic Compound Patterns Characteristics of Five Unifloral Honeys by Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Coupled to Chemometrics. Journal of Agricultural and Food Chemistry 2006, 54, 7235–7241.
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Perez-Coello, M.S. Differentiation of Monofloral Citrus, Rosemary, Eucalyptus, Lavender, Thyme, and Heather Honeys Based on Volatile Composition and Sensory Descriptive Analysis. Food Chemistry 2009, 112, 1022–1030.
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. Journal of Agricultural and Food Chemistry 2002, 50, 5870–5877.
- Council Directive 2001/110/EC relating to honey. Official Journal of the European Communities 2002, L 10, 47–52.
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25.
- Dimou, M.; Katsaros, J.; Tzavella-Klonari, K.; Thrasyvoulou, A. Discriminating Pine and Fir Honeydew Honeys by Microscopic Characteristics. Journal of Apicultural Research 2006, 45(2), 16–21.
- Terrab, A.; Díez, M.J.; Heredia, F.C. Palynological, Physico-Chemical and Colour Characterization of Moroccan Honeys: I. River Red Gum (Eucalyptus camaldulensis Dehnh) Honey. International Journal of Food Science and Technology 2003, 38, 379–386.
- Soria, A.C.; González, M.; De Lorenzo, C.; Martínez-Castro, I.; Sanz, J. Characterization of Artisanal Honeys from Madrid (Central Spain) on the Basis of Their Melissopalynological, Physicochemical and Volatile Composition Data. Food Chemistry 2004, 85, 121–130.
- Juan-Borrás, M.; Domenech, E.; Hellebrandova, M.; Escriche, I. Effect of Country Origin on Physicochemical, Sugar and Volatile Composition of Acacia, Sunflower and Tilia Honeys.. Food Research International 2014, 60, 86–94.
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and Classification of Greek Pine Honeys according to Geographical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Chemistry 2014, 146, 548–557.
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical Discrimination of Greek Unifloral Honeys with Physico-Chemical and Chemometric Analyses. Food Chemistry 2014, 165, 181–190.
- Michalkiewicz, A.; Biesaga, M.; Pyrzynska, K. Solid-Phase Extraction Procedure for Determination of Phenolic Acids and Some Flavonols in Honey. Journal of Chromatography A 2008, 1187, 18–24.
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using Flavonoids, Phenolics Compounds and Headspace Volatile Profile for Botanical Authentication of Lemon and Orange Honeys. Food Research International 2011, 44, 1504–1513.
- Jhou, J.; Yao, L.; Chen, L.; Zhao, J. Floral Classification of Honey Using Liquid Chromatography Authentication Detection Classification of Honey Using Liquid Chromatography. Food Chemistry 2014, 145, 941–949.
- Sousa, M.J.; Leite De Souza, E.; Marques, G.; Meireles, B.;, et al. Polyphenolic Profile and Antioxidant and Antibacterial Activities of Monofloral Honeys Produced by Meliponini in the Brazilian Semiarid Region. Food Research International 2016, 84, 61–68.
- Karabagias, I.K.; Casiello, G.; Kontakos, S.; Louppis, P.A.; Longobardi, F.; Kontominas, M.G. Investigating the Impact of Botanical Origin and Harvesting Period on Carbon Stable Isotope Ratio Values (13C/12C) and Different Parameter Analysis of Greek Unifloral Honeys: A Chemometric Approach for Correct Botanical Discrimination. International Journal of Food Science and Technology 2016, 51, 2460–2467.
- Karabagias, I.K.; Louppis, P.A.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and Geographical Discrimination of Commercial Citrus Spp. Honeys Produced in Different Mediterranean Countries Based on Minerals, Volatile Compounds and Physicochemical Parameters, Using Chemometrics. Food Chemistry 2017, 217, 445–455.
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of Ph and Electrical Conductivity upon Honey Dilution as a Complementary Tool for Discriminating Geographical Origin of Honeys. Food Chemistry 2007, 101, 695–703.
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and Biochemical Properties of Honeys from Arid Regions. Food Chemistry 2014, 153, 35–43.
- IHC (Harmonized methods of the International Honey Commision). IHC Responsible for the Methods: Stefan Bogdanov; Swiss Bee Research Centre FAM: Liebefeld, CH-3003 Bern, Switzerland, 1997.
- Wahdan, H.A.L.;. Causes of the Antimicrobial Activity Of. Honey. Infection 1998, 26, 30–35.
- Field, A.;. Discovering Statistics Using SPSS; 3rd, Sage Publications Ltd: London, UK, 2009; 384.
- Sharma, S. Applied Multivariate Techniques, John Wiley and Sons: NJ, USA, 1996.
- Granato, D.; De Oliveira, C.C.; Fernandes Caruso, M.S.; Farah Nagato, L.A.; Alaburda, J. Feasibility of Different Chemometric Techniques to Differentiate Commercial Brazilian Sugarcane Spirits Based on Chemical Markers. Food Research International 2014, 60, 212–217.
- Finola, M.S.; Lasagno, M.C.; Marioli, J.M. Microbiological and Chemical Characterization of Honeys from Central Argentina. Food Chemistry 2007, 100, 1649–1653.
- Sahinler, S.; Sahinler, N.; Gul, A. Determination of Honey Botanical Origin by Using Discriminant Analysis. Journal of Animal and Veterinary Advances 2009, 8(3), 488–491.
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, R.; Romero, C.D. Differentiation of Blossom and Honeydew Honeys Using Multivariate Analysis on the Physicochemical Parameters and Sugar Composition. Food Chemistry 2011, 126, 664–672.
- Pavelková, A.; Kačániová, M.; Čuboň, J.; Švecová, Z.; Kňazovická, V.; Felsöciová, S. Physicochemical and Microbiological Quality of Honey from Liptov Region. Journal of Microbiology, Biotechnology, and Food Sciences 2013, 2, 1185–1193.
- Directive 127/2004. Classification of Monofloral Honeys; Greek Ministry of Agricultural and Food Development, 67a Codex Alimentarius, 2004.
- Sanz, M.L.; Gonzalez, M.; De Lorenzo, C.; Sanz, J.; Martínez-Castro, I. A Contribution to the Differentiation between Nectar Honey and Honeydew Honey. Food Chemistry 2005, 91, 313–317.
- Mateo, R..; Bosch-Reig, F. Classification of Spanish Unifloral Honeys by Discriminant Analysis of Electrical Conductivity, Color, Water Content, Sugars, and Ph. Journal of Agricultural and Food Chemistry 1998, 46(2), 393–400.
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.A.; Heredia, F.J. Multivariate Correlation between Color and Mineral Composition of Honeys and by Their Botanical Origin. Journal of Agricultural Food Chemistry 2005, 53, 2574–2580.
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. ASEV Phenolics Symposium 2005, American Society for Ecology and Viticulture.
- Oroian, M.; Prisacaru, A.; Hretcanu, E.C.; Stroe, S.G.; Leahu, A.; Buculei, A. Heavy Metals Profile in Honey as a Potential Indicator of Botanical and Geographical Origin. International Journal of Food Properties 2016, 19, 1825–1836.
- Dimou, M.; Tananaki, C.; Liolios, V.; Thrasyvoulou, A. Pollen Foraging by Honey Bees (Apis mellifera L.) in Greece: Botanical and Geographical Origin. Journal of Apicultural Science 2014, 58(2), 11–23.
