ABSTRACT
The aim of present study was to extract the guava seed oil for its quantitative determination and to evaluate chemical characteristics including fatty acid composition and to compare with reported literature. Average oil content of guava seeds was found to be 11.12 g oil /100g seed. The iodine value, acid value, free fatty acid, peroxide value and saponification value were found to be 120.55 g of I2/100 g oil, 3.74 g/100g of oil, 1.86 g/100 g oil, 4.13 mEq/kg of oil and 190.74 mg/100 g of oil, respectively. Fatty acid composition was evaluated by gas chromatography-mass spectrometry and Fourier transform infrared spectroscopy. By GC-MS, a total of eighteen fatty acids were identified from which twelve were saturated fatty acids (SFAs) and six were unsaturated fatty acids (USFAs). Among SFAs, palmitic acid, stearic acid and arachidic acid were dominant, while linoleic and oleic acids were main USFAs found in guava seed oil. GC-MS results indicated that guava seed oil is a good source of essential fatty acids.
Abbreviations: FA: Fatty acid; FAMEs: Fatty acid methyl ester; PUFA: Polyunsaturated fatty acids; SFA: Saturated fatty acids; MUFA: Monounsaturated fatty acids;
Introduction
Psidium guajava L. (genus: Psidium; family: Myrtaceae) is a perennial tree that grows in tropical and subtropical areas of the world.[Citation1,Citation2] Psidium guajava is growing in India, Pakistan, Brazil, Mexico, Bangladesh, USA, Thailand and number of other countries. The main world producers of guava are India and Mexico. Pakistan is the fourth major country in production of guava fruit (0.55 million tonnes per year).[Citation3] In Pakistan, guava (locally called Amrood) is grown widely in the province of Sindh and Punjab. After citrus and mango fruits, the guava has third position with respect to cultivation and area. In Sindh province, Larkana is the largest guava producing district followed by Mirpurkhas, Hyderabad, Nawabshah and Nausheroferoz. Guava fruit is extremely nutritious, delicious in taste, low price and fruit produced round the year.[Citation4]
Psidium guajava is a well-known medicinal plant which contains number of phytochemicals. Its different parts are used in various indigenous systems of medicine and health such as cholera, bowels, ulcers and wounds.[Citation5] Leaves extract of guava tree are used to cure number of disease such as anti-microbial, anti-inflammatory, anti-oxidant and anti-cough effects.[Citation6] A decoction of leaves is used for the treatment of cholera, vomiting and diarrhoea.[Citation7] Leaves contain tannin, resin, eugenol, essential oil, caryophyllene, terpenoids and flavonoids.[Citation8] The plant bark is used as an astringent as well as antidiarrhoeatic in children.[Citation9]
Guava fruit is high in vitamins and minerals such as vitamin E, vitamin C (Ascorbic acid), vitamin A, niacin (vitamin B3), thiamine (vitamin B3) and Fe, Ca, K, P, Mg, etc. Guava fruit also has important medicinal properties such as anti-cancer, sedative, digestive, antispasmodic, carminative, antihyperglycemic, antipyretic, anti-bacterial, anti-inflammatory and analgesic activities.[Citation10,Citation11] Various chemical constituents of guava fruit have been identified such as phenolic compounds (tannins and phenols), carotenoids, triterpenes, fatty acids, flavonoids, saponins, vitamins, essential oils and lectins.[Citation12]
Guava seed extract has been reported as corrosion inhibitor (decreases corrosion rate of a material) for carbon steel in HCl (acidic) medium.[Citation13] It has been reported that guava seed posses significant amount of tryptophan, methionine, linoleic acid and fiber content confines its use as wheat flour alternate in the preparation of cookies.[Citation14] The plants contain a range of fatty acids with different structure commonly found in edible oils, and some of them are highly beneficial for health.[Citation15]
The skin and seeds of guava fruit contain carotenoids, glycosides, flavonoid and phenolic compounds. They are also rich in other nutrients, essential fatty acids, and contain good amount of iodine. The fruits are very rich in seeds, which contain 14 % oil content (on dry weight), with 13% starch and 15% proteins.[Citation15] Guava oil has moisturizing retention properties due to the presence of lycopene (powerful antioxidant).[Citation16]
Guava oil contains fatty acid such as palmitic acid, stearic acid, oleic acid, linoleic acid and linoleic acid .[Citation15–Citation18] Omega-6 (linoleic acid) is main fatty acid identified in guava seed oil. Linoleic acid provides oleochemical uses in oil paints, surfactants, lubricants, varnishes, cosmetics and pharmaceuticals.[Citation19] Saturated fatty acids such as palmitic acid and stearic acid are used in manufacturing of soaps, cosmetics, lubricants and softeners.[Citation20] Therefore, oil present in guava seed oil further requires improvements in fatty acid compositions. The objective of present study was to assess the chemical characteristics including fatty acid composition of guava seed oil collected from Mirpurkhas, Sindh, Pakistan by chromatographic and spectroscopic techniques and to compare results with published literature.
Material and methods
Collection of plant material
The mature fruit of Psidium guajava was collected from Mirpurkhas, Sindh, Pakistan (longitude: 25.4725° N and latitude: 68.7376° E) in March 2015. The plant was deposited (voucher specimen, 53141) in the herbarium of Institute of Plant Sciences, University of Sindh, Jamshoro, Pakistan. The plant was identified by taxonomist of same institution. The seeds were separated from fruit, cleaned and dried in shadow. The seeds were ground (using grinder), and oil was extracted using organic solvent.
Extraction of guava seed oil
Extraction of oil from guava seeds was carried out by Soxhlet extraction method. Around 30 g powdered guava seeds were taken in a cellulose thimble and placed in a Soxhlet apparatus using n-hexane as an oil extracting solvent. The n-hexane extract was concentrated by a rotary evaporator under reduced pressure to obtain crude oil. The sample was kept under fume hood to evaporate remaining n-hexane from oil. The percentage of oil content was reported on dry basis of guava seeds.
Moisture content and chemical analysis
Moisture content of guava seed was determined by oven drying at 105°C for 3 h according to AOAC method.[Citation21] The results of moisture content of seeds expressed in percentage. The iodine value (IV), acid value (AV), free fatty acid value (FFA), peroxide value (PV) and saponification value (SV) were determined by Official Method (AOCS, 1992).[Citation22]
Preparation of fatty acid methyl ester (fames)
FAMEs of guava seed oil were prepared by adding 20 mL of methanol two pellets of potassium hydroxide along with 20 mg of guava seed oil in round bottom flask. The mixture was refluxed about half an hour on heating mental and then cooled reaction mixture at room temperature. The reaction mixture was transferred to the separatory funnel and added 10 mL of hexane and 20 mL of water and shaken vigorously to separate aqueous and organic layer. The organic layer (hexane) was separated and dried over anhydrous sodium sulphate then analysed by GC-MS according to reported procedure.
Fourier transform infrared (FT-IR) spectroscopic analysis
FT-IR analysis of FAMEs of guava seed oil was performed using Thermo Nicolet 5700 spectrometer (Thermo Nicolet Analytical Instruments, Madison, WI) equipped with removable ZnSeattenuated total reflectance (ATR) accessory and deuterated triglycine sulfate (DTGS) detector. The FT-IR spectrum of guava seed oil FAMEs was recorded (at room temperature, 25°C) in the mid infrared range 4000–650 cm−Citation1 at 32 scans with resolution of 4 cm−Citation1. Spectrum of sample was obtained against background of air spectrum. After recording spectrum, ATR crystal (sample compartment) was carefully cleaned with organic solvents (hexane and methanol).
Analysis of fatty acids by GC-MS
Finally, FAMEs of guava seed oil were analysed on GC-MS instrument (Agilent 6890 N GC) attached with mass selective detector (MS-5975 inert XL), injector (auto sampler 7683-B) and column (HP-5MS; 30 m × 0.25 mm i.d. film thickness 0.25 µm). Helium was used as a carrier gas with flow rate of 1.5 mL/min. About 1.0 µL of sample was injected, using split mode (split ratio, 1:50). The MS was operated in the electron impact mode (EI) at 70 eV using 50–550 m/z scan range. Initial oven temperature was set at 90°C for 5 min, raised to 220°C at 5°C/min (2 min hold) and then increased to 270°C at 5°C/min (5 min hold). The injector and MS transfer line temperature were set at 220–290°C, respectively.
Identification of fatty acids and statistical analysis
Individual fatty acid of guava seed oil was identified by matching their mass spectra of known compounds (> 90%) in database incorporated in GC-MS software provided by NIST (National Institute of Standards and Technology) and Wiley. The percent area (relative %) of fatty acids was calculated from the peak areas. FAMEs of guava seed oil were analysed in triplicate and results expressed as mean ± standard deviation (SD).
Results and discussion
The moisture content of guava seed in present study was found to be 8.33g/100 g. Moisture content is important parameter in determining the shelf life and storage condition of seeds. Guava oil was extracted from seeds by Soxhlet extraction method using n-hexane as extraction solvent. Total oil content of Psidium Guajava seeds was found to be 11.12 g of oil /100 g of seed on dry bases. The oil content found in present study is higher than Habib[Citation23] 9.1 g of oil /100 g of seed and Opute[Citation24] 9.4 g of oil/100 g of seed, whereas slightly lower than Uchôa-thomaz et al.[Citation12] 13.93 g of oil/100 g of seed[Citation12]. To check the quality of guava seed oil, iodine value (IV), acid value (AV), free fatty acids value (FFA), peroxide values (PV) and saponification value (SV) were calculated as shown in . The IV is a measurement of degree of unsaturation and reflects the susceptibility of oil to oxidation. In present study, oil contains high IV (120.55 g I2/100g oil), indicating that oil is composed of mainly unsaturated fatty acids. Acid value was found to be (3.74 g/100 g oil) indicating the content of FFA in oil. It is an important variable when considering the quality of oil. FFA value was found to be (1.86 g/100 g oil). Generally, it is considered that lower the value of FFA better the oil quality. PV is the measure of rancidity index of oil, and it was observed that guava seed oil contains (4.13mEq/kg of oil) PV. Higher level of PV is the indication of a rancid oil/fat, but in guava seed oil, PV is relatively low which indicates that there is less chance of rancidity. The SV of guava seed oil was found to be (190.74 mg/100 g oil) which indicated that oil contains high-molecular weight fatty acids. The obtained SV of guava seed oil is almost comparable with most of the vegetable oils. Fatty acid composition and characterization of guava seed oil were evaluated by chromatographic and spectroscopic methods. To analyse fatty acid composition of guava seed oil, the oil was subjected to preparation of FAMEs. The esters were analysed by different techniques such as FT-IR and GC-MS.
Table 1. Chemical parameters of guava seed oil.
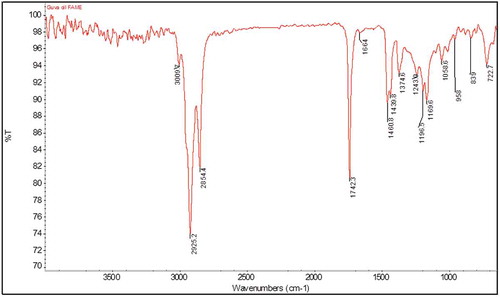
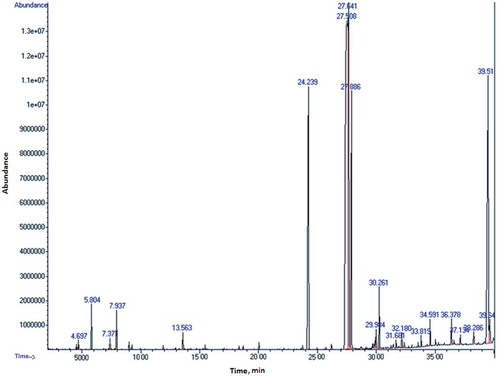
FT-IR spectral analysis of FAMEs of guava seed oil confirmed the presence of C = O and C-O characteristic bands at 1742 cm−Citation1 and 1169 cm−Citation1. Bands due to unsaturated fatty acids at 3009 cm−Citation1 and 1664 cm−Citation1were also observed as shown in . FT-IR spectral data supported the results FAMEs obtained by GC-MS. The GC-MS results of guava seed oil provided number of fatty acids that turned out to be different from previously published data.[Citation12] The results revealed that a total of 18 fatty acids methyl esters were separated and quantified based on their elution order as shown in .
Among 18 fatty acids, twelve saturated (26.45%) and six unsaturated (73.55%) fatty acids were identified as shown in . Among unsaturated fatty acids, the predominant fatty acids were linoleic acid (60.03%) and oleic acid (12.57%), whereas palmitic acid (14.81%), stearic acid (9.08%) and arachidic acid (1.31%) found as a main saturated fatty acids.
Table 2. Oil content (%) and fatty acid composition (%) of P. guajava seed oil by GC-MS.
Previous study, on guava seed oil, revealed the identification of lauric acid, myristic acid, palmitic acid, heptadecanoic acid, stearic acid, oleic acid (n-9), linoleic acid (n-6), arachidic acid, gondoic acid, linoleic acid and behenic acid.[Citation12] Habib[Citation23] reported that high levels of unsaturated fatty acids (86.9%) mainly linoleic acid (79.1%) and oleic acid (7.8%) were identified by Opute.[Citation24] In present study, on guava seed oil showed some new fatty acids which has been identified first time in guava seed oil such as palmitoleic acid, nonadecanoic acid, methyl ricinoleate, 10,13-eicosadienoic acid, 11-eicosenoic acid, heneicosanoic acid, tricosanoic acid, lignoceric acid and cerotic acid in minor concentration. The major unsaturated fatty acids such as linoleic acid and oleic acid were found to be common, but in different concentration from previously reported studies.[Citation11,Citation12,Citation23,Citation24] The possible reason between obtained results and reported data may be due to either geographical location or variety or the extraction technique employed.[Citation24]
Comparative results of fatty acids classes in indicated that highest concentration of SFAs was reported by Habib[Citation23] (26.49%). In comparison with other reported works, our finding of SFA (26.45%) is higher than Opute[Citation24](13.1%) and Uchôa-thomaz et al.,[Citation12](13.01%). On the other hand, higher concentrations of USFAs were observed by Uchôa-thomaz et al.,[Citation12] (86.77%) and Opute[Citation24] (86.9%) but in present study (73.55%) that is higher than Habib[Citation23] (66.1%). While these results are comparatively lower than the findings of Opute[Citation24] (86.9%) and Uchôa-thomaz et al .,[Citation12] comparative results of the major fatty acids are shown in which indicates that highest concentration of linoleic acid was reported by Opute[Citation24] (79.1 %), followed by Uchôa-thomaz et al[Citation12](77.35%), present study (60.03%) and Habib[Citation23] (52.10%). Whereas other major fatty acid was oleic acid, maximum concentration of oleic acid reported by Habib[Citation23] (14.0%), followed by present study (12.57%), Uchôa-thomaz et al[Citation12] (9.42%) and Opute[Citation24](7.80%). The highest concentration of SFA includes stearic acid, which was reported by Habib[Citation23](11.10%), followed by present study (9.08%), Uchôa-thomaz et al[Citation12](4.48%) and Opute[Citation24](3.40%). On the other hand, palmitic acid was observed as a major saturated fatty acid by present study (14.81%), followed by Habib[Citation23] (13.30%), Opute[Citation24](9.70%) and Uchôa-thomaz et al[Citation12] (8.00%).
General classification of guava seed oil with regard to fatty acids discussed in has been found in the sequence of MUFA (13.40%) < SFA (26.45%) < PUFA (60.15%). The minimum ratio of PUFA/SFA set by the department of Health UK is 0.45. In this study, the ratio of PUFA/SFA found 2.27, which was much higher than the recommended value. In comparison with the published data on guava seed oil, this ratio is much lower than the reported value of 8.11.[Citation12] This variation is due to the presence of higher concentrations of PUFA.
From the identified components of guava seed, linoleic acid and oleic acid are particular importance because of their many properties and uses. The presence of these fatty acids in high quantity makes guava seed oil beneficial to human health, especially for heart patients. Therefore, guava seed oil can be considered as a good source of linoleic acids and has a wide range of applications in the nutrition, cosmetics and medicine. On the other hand, the results of this study are also useful for phytopharmaceutical industries to establish their quality control profile. This study helps to understand that after extracting the juice seeds could be utilized in above mentioned applications.
Conclusion
Based on our findings, it is recommended that extraction of oil from guava seeds should be carried out on industrial scale for food, cosmetic and pharmaceutical industries. The fatty acid composition of guava seed oil varies from different geographic locations as reported in the literature, but in all studies, linoleic acids has been reported as dominant fatty acid, and it has number of application. More harvesting of Psidium guajava may increase production of health beneficial fruit and better environment as well as greater production of valuable oil from the seeds.
Declaration of interest
The authors have declared no conflict of interest.
Funding
The authors are grateful for the financial support provided by National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro.
Additional information
Funding
References
- Flores, G.; Wu, S.B.; Negrin, A.; Kennelly, E.J. Chemical Composition and Antioxidant Activity of Seven Cultivars of Guava (Psidiumguajava) Fruits. Food Chemistry 2015, 170, 327–335.
- Moussa, T.A.; Almaghrabi, O.A. Fatty Acid Constituents of Peganumharmala Plant Using Gas Chromatography–Mass Spectroscopy. Saudi Journal of Biological Sciences 2015. doi:10.1016/j.sjbs.04.013
- Usman, M.; Samad, W.A.; Fatima, B.; Shah, M.H. Pollen Parent Enhances Fruit Size and Quality in Intervarietal Crosses in Guava (Psidiumguajava). International Journal of Agriculture and Biology 2013, 15, 125–129.
- Mehmood, A.; Jaskani, M.J.; Khan, I.A.; Ahmad, S.; Ahmad, R.; Luo, S.; Ahmad, N.M. Genetic Diversity of Pakistani Guava (Psidiumguajava L.) Germplasm and Its Implications for Conservation and Breeding. Scientia Horticulturae 2014, 172, 221–232.
- Choudhury, S.; Sharan, L.; Sinha, M.P. Phytochemical and Antimicrobial Screening of Psidiumguajava L. Leaf Extracts against Clinically Important Gastrointestinal Pathogens. Journal of Natural Product and Plant Resources 2012, 2, 524–529.
- Moura, P.M.; Prado, G.H.C.; Meireles, M.A.A.; Pereira, C.G. Supercritical Fluid Extraction from Guava (Psidiumguajava) Leaves: Global Yield, Composition and Kinetic Data. Journal of Supercritical Fluids 2012, 62, 116–122.
- Jaiarj, P.; Khoohaswan, P.; Wongkrajang, Y.; Peungvicha, P.; Suriyawong, P.; Saraya, M.S.; Ruangsomboon, O. Anticough and Antimicrobial Activities of Psidiumguajava Linn. Leaf Extract. Journal of Ethnopharmacology 1999, 67, 203–212.
- Kumar, A.; Importance for Life Psidium guava. International Journal of Research in Pharmaceutical and Biomedical Sciences 2012, 3, 2229–3701.
- Gayathri, V.; Kiruba, D. Preliminary Phytochemical Analysis of Leaf Powder Extracts of Psidiumguajava L. International Journal of Pharmacognosy and Phytochemical Research 2014, 6, 332–334.
- Chen, K.C.; Peng, C.C.; Chiu, W.T.; Cheng, Y.T.; Huang, G.T.; Hsieh, C.L.; Peng, R.Y. Action Mechanism and Signal Pathways of Psidiumguajava L. Aqueous Extract in Killing Prostate Cancer Lncap Cells. Nutrition and Cancer 2010, 62, 260–270.
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Guava (Psidiumguajava L.) Seed Oil Obtained with a Homemade Supercritical Fluid Extraction System Using Supercritical CO2 and Co-Solvent. Journal of Supercritical Fluids 2011, 56, 238–242.
- Uchôa-Thomaz, A.M.A.; Sousa, E.C.; Carioca, J.O.B.; Morais, S.; Lima, A.D.; Martins, C.G. Chemical Composition, Fatty Acid Profile and Bioactive Compounds of Guava Seed (Psidiumguajava L.). Food Science and Technology (Campinas) 2014, 34, 485–492.
- Kumar, K.V.; Pillai, M.S.N.; Thusnavis, G.R. Seed Extract of Psidiumguajava as Ecofriendly Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Medium. Journal of Materials Science & Technology 2011, 27, 1143–1149.
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidiumguajava: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Journal of Ethnopharmacology 2008, 117, 1–27.
- Aitzetmüller, K.; Matthäus, B.; Friedrich, H. A New Database for Seed Oil Fatty Acids the Database SOFA. European Journal of Lipid Science and Technology 2003, 105, 92–103.
- Pelegrini, P.B.; Murad, A.M.; Silva, L.P.; Dos Santos, R.C.; Costa, F.T.; Tagliari, P.D.; Bloch, C., Jr.; Noronha, E.F.; Miller, R.N.; Franco, O.L. Identification of a Novel Storage Glycine-Rich Peptide from Guava (Psidiumguajava) Seed with Activity against Gram-Negative Bacteria. Peptides 2008, 29, 1271–1279.
- Norshazila, S.; Syed Zahir, I.; Mustapha Suleiman, K.; Aisyah, M.R.; Kamarul Rahim, K. Antioxidant Levels and Activities of Selected Seed of Malaysian Tropical Fruits. Malaysian Journal of Nutrition 2010, 16, 149–159.
- Bontempo, P.; Doto, A.; Miceli, M.; Mita, L.; Benedetti, R.; Nebbioso, A.; Altucci, L.; Molinari, A.M. Psidiumguajava L. Anti‐Neoplastic Effects: Induction of Apoptosis and Cell Differentiation. Cell Proliferation 2012, 45, 22–31.
- Chandrika, U.G.; Fernando, K.S.S.P.; Ranaweera, K.K.D.S. Carotenoid Content and in Vitro Bioaccessibility of Lycopene from Guava (Psidiumguajava) and Watermelon (Citrulluslanatus) by High-Performance Liquid Chromatography Diode Array Detection. International Journal of Food Sciences and Nutrition 2009, 60, 558–566.
- Atolani, O.; Adeniyi, O.; Kayode, O.O.; Adeosun, C.B. Direct Preparation of Fatty Acid Methyl Esters and Determination of in Vitro Antioxidant Potential of Lipid from Fresh Sebalcausarium Seed. Journal of Applied Pharmaceutical Science 2015, 5, 24–28.
- Helrich, K.C.; Official Methods of Analysis of the AOAC; Vol. 2. No. Ed. 15. Association of Official Analytical Chemists: Arlington, VA, 1990.
- AOCS Official Methods and Recommended Practices of the American Oil Chemists Society; 4th ed.; American Oil Chemists’ Society: Champaign, USA, 1992.
- Habib, M.A. Studies on the Lipid and Protein Composition of Guava Seeds (Psidiumguajava). Food Chemistry 1986, 22, 7–16.
- Opute, F.I. The Component Fatty Acids of Psidiumguajava Seed Fats. Journal of the Science of Food and Agriculture 1978, 29, 737–738.