ABSTRACT
The purpose of this study was to determine antioxidant activity and the most abundant phenolics of nine commercial dark beers. In order to estimate the content of both free and bound forms of beer phenolics, the beer samples were subjected to alkaline hydrolysis. The hydrolyzed beer samples exhibited the significantly higher antioxidant activity measured using 2,2'-azino-bis (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays as compared with nonhydrolyzed ones which was associated with the release of free forms of beer phenolics after hydrolysis. The predominant phenolic compounds in analyzed dark beers were hydroxycinnamic acids, especially ferulic and synapic acids, as well as gallic acid belonging to hydroxybenzoic acids. A principal component analysis (PCA) was applied in order to differentiate the investigated dark beers in terms of phenolic compounds and antioxidant activity for both hydrolyzed and nonhydrolyzed beer samples.
Introduction
Beer is one of the oldest alcoholic beverages in the world. In fact, it is a fermented beverage brewed from malt and usually flavored with hops. Of all the plant additives that have been used in beer production, only hop has gained a widespread acceptance and is regarded as an essential raw material in beer production process. Many epidemiological studies suggest a strong association between the consumption of food rich in phenolics and the prevention of many human diseases associated with oxidative stress.[Citation1–Citation4] Furthermore, several epidemiological studies have indicated the relationship between the mild or moderate ethanol consumption (especially in the form of beer or wine) and the beneficial healthy effects on the cardiovascular system.[Citation5–Citation8] In the recent years, the renewed interest has been focused on beer being a beverage with a moderate antioxidant activity (AA) and a low ethanol content.
Phenolic compounds present in beer originated from cereal grains used for malt production (e.g., barley, maize, rice, wheat, sorghum, millet, oat, rye, and triticale), hop as well as from other nonmalted raw materials.[Citation9] Among the phenolic compounds that occur in beer, the most important are as follows: xanthohumol and related prenylflavonoids (originated from hop),[Citation10] malt phenolics (e.g., phenolic acids, flavonols, and flavanols), as well as proanthocyanidins, tannins, and amino phenolic compounds.[Citation11] As a matter of fact, the beer phenolics are of particular interest to producers, due to their ability to delay or prevent oxidation processes occurring during brewing by the mechanisms involving both free radical scavenging activity and metal chelation.[Citation12] Besides the bioactive and prohealth properties (e.g., AA), the beer phenolics have also technological significance, since they play a crucial role in foam maintenance, physicochemical stability, and shelf-life of beer.[Citation9]
Phenolic compounds are very rarely present in plants and raw materials as free forms. More often, they occur in forms of esters, glycosides, and bound complexes (e.g., with polysaccharides). The methods used to qualitative and quantitative analysis of both free and total (free and bound) contents of phenolics are based mainly on alkaline procedure.[Citation13] These are used in order to release free forms of phenolics. Despite the presence of data dealing with phenolic profile and antioxidant properties of commercial beers,[Citation14–Citation16] there is, however, lack of studies concerning the dark beers, especially in the terms of content of free and bound phenolics evaluated chromatographically as well as their AA. This information also seems to be very important to consumers drawing attention to the health-promoting effect and antioxidant properties of dark beers. Furthermore, the analysis of phenolic profile in beers seems to be crucial for their quality control, and also may be used for evaluation of beer authenticity.
Thus, the first purpose of this study was to determine AA and the most abundant phenolics in nine commercial dark beers originated from Germany, Czech, and Poland. In order to quantify the amounts of free and bound forms of phenolics, an alkaline hydrolysis of dark beer samples was conducted. The AA of both forms of beer extracts (i.e., hydrolyzed and nonhydrolyzed) was evaluated using 2,2'-azino-bis (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays. The phenolic profile in both types of beer extracts was investigated using HPLC (High Performance Liquid Chromatography) method. The second purpose of this study was to differentiate the hydrolyzed and nonhydrolyzed beer samples on the basis of analytical variables (i.e., phenolic acids and flavonoids contents, as well as AA of beers measured in the reaction with ABTS and DPPH free radicals) using principal component analysis (PCA).
Materials and methods
Beer samples
The experimental materials were nine various brands of commercial dark beers, originated from various producers in Czech, Germany, and Poland, which are the traditional European brewing regions. The beers were obtained from the grocery stores. The beers analyzed are summarized and described in . The products were stored in the dark at 4ºC until further analysis.
Table 1. Characteristics of nine commercial dark beers.
Chemicals
Acetonitrile (HPLC gradient grade), ethyl acetate, methanol, sodium carbonate, sodium chloride, and potassium persulphate were purchased from POCh (Gliwice, Poland). Acetic and hydrochloric acid were obtained from Chempur (Piekary Śląskie, Poland). The Folin–Ciocalteu reagent, DPPH, and ABTS (3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). All the standards of phenolic acids (i.e., caffeic acid (CA), gallic acid (GA), p-coumaric acid (p-COA), ferulic acid (FA), and synapic acid (SA) and flavonoids (i.e., quercetin and kaempferol) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany) or Fluka Chemie AG (Buchs, Switzerland).
Extraction of phenolics from the nonhydrolyzed beer samples
In order to estimate the content of free forms of phenolics in the dark beers tested, the extraction process was performed according to the reported procedure.[Citation17] In summary, the beer sample (in the volume of 75 cmCitation3) was adjusted to pH = 2 with 4 N HCl solution and then saturated with NaCl in the amount of 300 mg per 1 cmCitation3 of sample. The resulting solution was extracted three times with ethyl acetate, using 25 cmCitation3 of the solvent. The obtained fractions of ethyl acetate were collected and evaporated to dryness in a vacuum evaporator at a temperature of 35°C. The dry residue was dissolved in a final volume of 5.0 cmCitation3 of methanol. For each beer sample, the extraction process was conducted in two replications.
Extraction of phenolics from the hydrolyzed beer samples
In order to estimate the total contents (free and bound) of phenolics in the dark beers tested, the hydrolytic process was performed according to Nardini and Ghiselli.[Citation13] In summary, the beer sample (in the volume of 10 cmCitation3) was subjected to alkaline hydrolysis with 90 cmCitation3 2 N NaOH containing 10 mM EDTA (Ethylenediaminetetraacetic acid) and 1% ascorbic acid at 30ºC for 30 min. At the end of incubation, the solution was cooled, adjusted to pH = 2 with 4 N HCl solution and then saturated with NaCl in the amount of 300 mg per 1 cmCitation3. The resulting solution was extracted three times with ethyl acetate, using 25 cmCitation3 of the solvent. The obtained fractions of ethyl acetate were collected and evaporated to dryness in a vacuum evaporator at a temperature of 35°C. The dry residue was dissolved in a final volume of 5.0 cmCitation3 of methanol. For each beer sample, the alkaline hydrolysis followed by extraction process was conducted in two replications.
Determination of antioxidant activity using a DPPH assay
The AA of both hydrolyzed and nonhydrolyzed beer samples was analyzed using a DPPH assay following the reported procedure.[Citation18] In summary, 0.1 cmCitation3 of an appropriately diluted methanolic extract of beer sample was mixed with 3.9 cmCitation3 of DPPH methanolic solution (25 mg per dmCitation3) and left for 15 min. The blank test was run in the same conditions, replacing beer extract with methanol. Then an absorbance of the obtained mixture was measured in an ultraviolet/visible (UV/Vis) V-530 spectrophotometer (Jasco, Japan) at λ = 515 nm against a methanol. The measurements were performed in triplicate. The AA of beer samples was expressed as trolox equivalent antioxidant capacity (TEAC) in mM of trolox per 1 dmCitation3 of beer, using a calibration curve plotted for trolox methanolic solutions in concentration range of 0–1.0 mM.
Determination of antioxidant activity using an ABTS assay
The AA of both hydrolyzed and nonhydrolyzed beer samples was analyzed using an ABTS assay following the reported procedure.[Citation17] In summary, 0.1 cmCitation3 of an appropriately diluted methanolic extract of beer sample was mixed with 6 cmCitation3 of ABTS cation radical solution and after 30 min an absorbance of the obtained mixture was measured in a UV/Vis V-530 spectrophotometer (Jasco, Japan) at a wavelength of 734 nm against phosphate buffer. The measurements were performed in triplicate. The AA of beer samples was expresses as TEAC in mM of trolox per 1 dmCitation3 of beer, using a calibration curve plotted for trolox methanolic solution in concentration range of 0–1.0 mM.
Chromatographic analysis of phenolic compounds
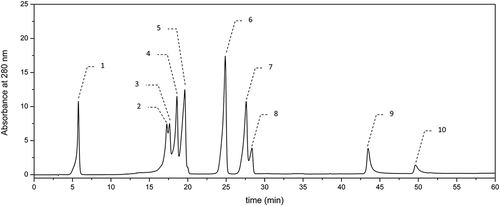
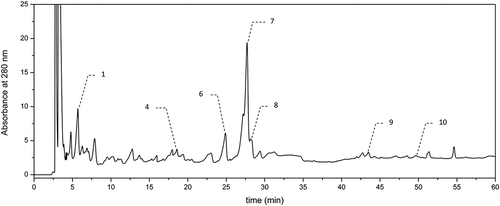
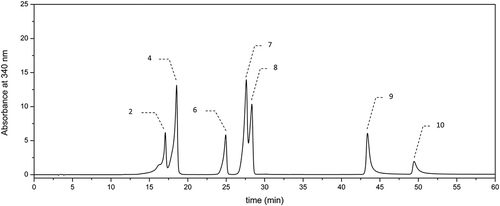
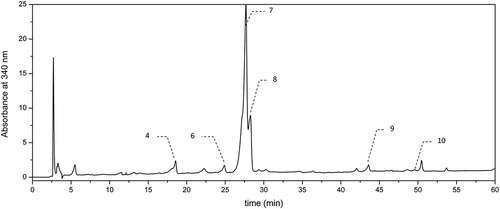
The methanolic extracts of both hydrolyzed and nonhydrolyzed beer samples were subjected to the chromatographic analysis using an HPLC system (La Chrom, Merck-Hitachi, Japan). Prior to the analysis, the extracts were filtered through a Millex-LCR (Millipore) filter with a pore diameter of 0.45 µm. The qualitative and quantitative assessment of phenolic compounds in the beer extracts examined was performed according to the reported procedure.[Citation17] GA was detected at λ = 280 nm (–), whereas the hydroxycinnamic acids (i.e., CA, p-COA, FA, and SA) and flavonols (i.e., quercetin and kaempferol) were detected at λ = 340 nm (–). The chromatographic analysis was performed with a reversed-phase ODS (Octadecylsilane) column (Thermo Scientific, USA: 250 × 0.46 mm, particle size 5 µm) at a temperature of 30ºC in a gradient mode with two solvents: solvent A—(2.5% acetic acid in water) and solvent B—acetonitrile. The eluent flow rate was of 1 cmCitation3 per min and the injection volume was 20 µl. The elution was carried out using a gradient procedure as follows: for the first 10 min—linear gradient with solvent B contribution increasing in the mobile phase from 3 to 8%, followed by an increase in phase B contribution to 15, 20, 30, and 40% at 20, 30, 40, and 50 min, respectively. Finally, the column was eluted isocratically for 10 min with acetonitrile before the next injection. Phenolic compounds were identified by comparison of their retention times with those of standards. In turn, the quantitative analysis was carried out using calibration curves plotted for particular standards at four different concentrations (0.02–0.2 mg cm−Citation3). The analyzes of particular phenolic compounds in beer samples were carried out in duplicate.
Statistical analysis
In order to determine the statistically significant differences between the means, the obtained data were treated by an one-way analysis of variance, and the least significant differences (LSD) using a Fischer test at a significance level α = 0.05 was calculated. The Pearson’s linear correlation coefficients between selected parameters were also estimated. Additionally, a PCA was applied in order to differentiate the commercial dark beers under study (including both hydrolyzed and nonhydrolyzed samples) in terms of phenolic acids content and AA measured using ABTS and DPPH assay. Calculations were performed with statistical software package Statistica 9.0 (StatSoft Inc. Tulsa, USA).
Results and discussion
Antioxidant activity
The values of AA for the tested dark beers, including both hydrolyzed and nonhydrolyzed beer samples, expressed as mM of trolox per 1 dmCitation3 are shown in . It was found that the values of AA for the hydrolyzed beer samples, measured using both ABTS and DPPH assays, several times exceeded the values obtained for nonhydrolyzed ones. This fact may indicate that during alkaline hydrolysis the bound forms of phenolics (i.e., esters and glycosides) being the majority of phenolic compounds in beer undergo decomposition to the free forms (i.e., phenolic acids and aglycons of flavonoids). Noteworthy is fact that the higher AA of the released forms of phenolics (occurring in the beer samples after alkaline hydrolysis), as compared to that of their bound forms, is associated with the higher number of free hydroxyl groups (OH) attached to the phenolic molecules. The values of AA of both hydrolyzed and nonhydrolyzed beer samples () were strongly diversified in terms of the brand of investigated dark beers. With respect to the beers under study, the most important compounds that are responsible for AA of beer may be divided into two groups. The first one includes the hop flavonoids (e.g., xanthohumol and related prenylflavonoids), whereas the second one includes the phenolics originated mainly from a malt (e.g., phenolic acids and flavonoids).[Citation10] On this regard, the observed variability in the values of AA of different brands of dark beers tested are likely related to the differences in raw materials (i.e., barley and hop), as well as malting and brewing processes.
Table 2. Antioxidant activity of the dark beer samples measured using ABTS and DPPH assays.
The values of AA of nonhydrolyzed beer samples () varied from 0.25 for Krušovice beer B5 to 0.72 mMT/dmCitation3 for Porter Żywiec beer B9, and from 0.23 for Krušovice beer B5 to 0.67 mMT/dmCitation3 for Porter beer B9, in the test with ABTS and DPPH radicals, respectively. The obtained results were in agreement with the data reported by Zhao[Citation11] who investigated Chinese nonhydrolyzed light beers of lager type in terms of AA measured using ABTS and DPPH assays. The latter authors observed a high and statistically significant (α = 0.01) linear correlation (r = 0.973) between these two assays. In our study, also the high and significant (α = 0.05) linear correlations between ABTS and DPPH assays, measured for the hydrolyzed and nonhydrolyzed beer samples, r = 0.826 and 0.793, respectively, were observed. In the case of nonhydrolyzed samples, the value of correlation was very similar to that observed for Indian beers[Citation19] (r = 0.73). The AA of hydrolyzed dark beer samples () varied from 2.73 for Krušovice beer B5 to 4.71 mMT/dmCitation3 for Samson beer B7, and from 2.60 for Krušovice beer B5 to 3.76 mMT/dmCitation3 for Samson beer B7, in the test with ABTS and DPPH radicals, respectively. It is interesting to note that Krušovice beer B5 which exhibited the lowest AA measured against free radicals before alkaline hydrolysis () was also characterized by the low content of original extract () amounting to 10% when compared with the remaining samples under study. In contrary, Żywiec Porter beer B9 which exhibited the highest AA against both ABTS and DPPH radicals before hydrolysis was also characterized by the highest content of extract amounting to 21.4% (). However, we did not find any significant linear correlation between the content of extract of the studied dark beers and the AA values of the samples with the exception of DPPH assay for the nonhydrolyzed samples, the value of which amounting to r = 0.74 (α = 0.05). Thus, it can be concluded that the content of original extract may not be treated as a satisfactory source of information about the level of antioxidants in dark beers, as well as their antioxidant properties. It is worth emphasizing that the information concerning both most abundant phenolic compounds and AA of beverages (e.g., beer) after alkaline hydrolysis may be interesting for consumers due to the fact that the phenolics that are absorbed in human gastrointestinal track are previously decomposed from bound to their free forms, and only in the last forms they are bioavailable for human.[Citation13]
Regarding the dark beers under study, their AA may also be determined by the Maillard reaction products. This group of compounds, also referred to melanoidins, exhibited a strong AA. Another type of malt that may be used in a dark beer production is a black malt that is responsible for a dark color of beer, as well as for coffee, chocolate, and nutty aroma of this alcoholic beverage. It is obtained by intensely roasting a barley malt using very high heat.
Phenolic compounds profile
The levels of most abundant phenolic acids and flavonoids determined chromatographically in dark beers tested are specified in . Out of phenolic acids evaluated chromatographically, GA (–) and the hydroxycinnamic acids (i.e., CA, p-COA, FA, and SA) (–) were identified and quantified. Furthermore, two flavonoids, namely quercetin and kaempferol (belonging to flavonols), were detected. Among the nonhydrolyzed beer samples (), Porter Żywiec beer B9 was characterized by the highest level of phenolic compounds determined chromatographically, whereas Krušovice beer B5 was the poorest in this respect. These results are in accordance with the previous data concerning AA of nonhydrolyzed samples measured using ABTS and DPPH assays (), where Porter Żywiec beer B9 exhibited the highest AA, while Krušovice beer B5 was the poorest in this respect. As regards, the dark beer samples after hydrolysis (), again Krušovice beer B5 was characterized by the lowest level of phenolic compounds, whereas German dark beer Andechs Doppelbock B1 was the richest one in this respect.
Table 3. Phenolic compounds determined by HPLC in the dark beers samples expressed in mg per 1 dmCitation3.
The occurrence of GA was observed in all the investigated brands, including both hydrolyzed and nonhydrolyzed beer samples (). In the beer samples before and after hydrolysis, the level of GA was in the range of 0.92–6.34 mg/dmCitation3 and of 1.21–8.08 mg/dmCitation3, respectively. Similarly, as in the case of AA (), all the hydrolyzed dark beer samples were characterized by a significantly higher content of this phenolic as compared to the nonhydrolyzed ones. As reported by the other authors investigating the light beers, GA was by far the predominant compounds among hydroxybenzoic acids.[Citation10,Citation11] The amounts of GA determined in dark beers before hydrolysis () are in accordance with the data reported by Gorinstein[Citation20] and by Zhao[Citation11] who investigated the light beers. According to the former authors, the level of this phenolic varied to 2.9 mg/dmCitation3, whereas according to the latter authors who investigated commercial light Chinese beers of lager type, GA level varied in the range from 1.81 to 10.43 mg/dmCitation3. Despite a high antiradical activity of this phenolic, the previous authors[Citation11] did not find any significant linear correlation between its level and AA of investigated light beer measured using antiradical assays (i.e., ABTS and DPPH). However, in our study, we observed the significant (α = 0.05) linear correlation between GA level and scavenging activity against ABTS radicals (r = 0.7848), in the case of beers after alkaline hydrolysis. Thus, it was proved that the total content of GA (sum of free and bound form) in the tested dark beers had a significant influence on their AA. The high AA of GA is likely related to the presence of three OH attached to an aromatic ring.
With respect to hydroxycinnamic acids, this group of phenolics seems to be a characteristic for dark beers under study (). Regarding the nonhydrolyzed beers examined, similar results were reported by Marova[Citation10] who investigated Czech light beers of lager type. The last authors reported that level of hydroxycinnamates (i.e., chlorogenic and FA) was significantly higher than that of GA belonging to hydroxybenzoic acids. In contrast, Zhao[Citation11] who also investigated commercial light beers originating mainly from China reported GA as the predominant phenolic, unlike the hydroxycinnamates (i.e., FA, p-COA, and CA) being the minor constituents. It is worth emphasizing that proportion between hydroxybenzoic and hydroxycinnamic acid contents in beer may serve as a source of valuable information concerning both authenticity of beer and the influence of production processes (e.g., malting and brewing) on changes in phenolic content. Furthermore, the content of hydroxycinnamates, in particular FA, is strongly associated with the type of malt used during beer production, as well as with degree of degradation process of malt phenolic during brewing, since it has been known that this group of phenolics originated mainly from malt. On the other hand, the content of GA may also give some information about degree of oxidation processes during beer production due to a very high reactivity and susceptibility this phenolic to oxidation and degradation. Regarding the amounts of p-COA and CA in the hydrolyzed and nonhydrolyzed dark beer samples (), similar values to our results were reported by Piazzon[Citation14] who investigated commercial beers of different types, However, in the case of FA and SA, their level before and after hydrolysis in the dark beers tested () was significantly higher than that observed by the latter authors.
Out of the phenolic compounds determined chromatographically (), FA was by far the predominant in the both hydrolyzed and nonhydrolyzed beer samples followed by SA. FA is very characteristic for a barley malt used in beer production. Thus, the observed variability in level of this phenolic appeared to be related mainly to the type of barley malt used. Regarding the phenolic profile of most investigated light beers, FA was also the predominant phenolic. In the case of nonhydrolyzed samples, a slight higher level of this phenolic was observed in Czech light beers,[Citation10] and also in the light beers tested by Gorinstein[Citation20] as compared to our results. In contrast, a nearly twofold lower content of this phenolic (averaging at 1.07 mg/dmCitation3) was reported in the dark specialty beers investigated by Vanbeneden.[Citation21] With respect to our study, we observed a significant increase of FA level, as well as the level of other hydroxycinnamates after alkaline hydrolysis (). Furthermore, in the case of samples B1, B3, and B8, total content of FA was much higher (exceeded 20 mg/dmCitation3) as compared to the level reported by the other authors for the light beers.[Citation13] Dark beer Andechs Doppelbock B1 characterized by the highest total content of both GA and FA () also exhibited the highest AA after hydrolysis measured using ABTS and DPPH assays (). It is worth emphasizing that also a significant increase of FA level after alkaline hydrolysis was reported in Italian, Austrian, and German light beers,[Citation13] from 2.31, 1.89, and 1.36‒13.88, 12.64, and 16.75 mg/dmCitation3, respectively. Similar tendency was observed by Piazzon[Citation14] who investigated commercial beers of different types for which FA level increased on average from 1.99 to 17.66 mg/dmCitation3. Regarding the dark speciality beers, as reported by Vanbeneden,[Citation21] also an increase of FA content was observed after hydrolysis from 1.07 to 12 mg/dmCitation3. According to the last authors, some differences in fermentation and brewing processes may occur between dark malts (e.g., caramel, colored, and roasted) used for dark beers production and light malts with respect to activity of cinnamoyl esterase and phenylacrylic acid decarboxylase. Cinnamoyl esterases that have been reported in a barley and a barley malt are responsible for releasing of free hydroxycinnamic acids (e.g., FA, p-CA, and SA) during mashing. However, phenylacrylic acid decarboxylase reported in fermenting yeasts strains is responsible for decarboxylation of the hydroxycinnamates to vinylphenols. Thus, inhibition of the decarboxylase in dark malts during fermentation or inhibition of the cinnamoyl esterase activity during brewing by compounds present in dark malts may occur.[Citation21] Moreover, native enzymes existed in malt may be partially degraded during kilning and roasting of malt at higher temperatures during production of dark malts. It is worth emphasizing that with respect to dark beers in our study, we observed a significantly higher total content of both FA and SA () as compared to data reported for most light beers. The significant (α = 0.01 and 0.05) linear correlation was observed between the level of FA and the ABTS scavenging activity in the case of both hydrolyzed and nonhydrolyzed beers (r = 0.807 and 0.673, respectively).
Among the hydroxycinnamic acids evaluated chromatographically, SA was the second most abundant phenolic in the investigated beers (). Similarly to the other hydroxycinnamates, this phenolic originated mainly from the malt using for beer production. The level of this phenolic varied between 0.21 and 0.99 mg/dmCitation3 in nonhydrolyzed beer samples (). Similarly to FA, the amount of SA significantly increased after alkaline hydrolysis, and then its level ranged from 3.25 to 13.94 mg/dmCitation3 () depending on the beer types. This tendency was also observed for Italian, Austrian, and German light beers,[Citation13] as well as for beers studied by Piazzon.[Citation14] The content of this phenolic in nonhydrolyzed samples was in accordance with that found in dark specialty beers studied by Vanbeneden,[Citation21] averaging to 0.375 mg/dmCitation3, and also in Italian, Austrian, and German light beers,[Citation13] amounting to 0.2, 0.84, and 0.54 mg/dmCitation3, respectively. However, in the mentioned above beers, as well as in nonhydrolyzed dark beers studied () this phenolic was a minor constituent. It is interesting to note that amount of SA in the dark beers tested after hydrolysis (), averaging 6.5 mg/dmCitation3, was higher than that reported for dark specialty beers[Citation21] (averaging 2.61 mg/dmCitation3), as well as for Italian light beers[Citation13](amounting 3.00 mg/dmCitation3). However, in the case of Austrian and German light beers,[Citation13] the level of this acid after hydrolysis (amounting to 6.16 and 5.05 mg/dmCitation3) was similar to our results (). Thus, the commercial dark beers under study may be supposed to be a more valuable source of hydroxycinnamic acids in human diet as compared to light beers type. Among the hydroxycinnamates, p-COA and CA turned out to be the minor constituents in the dark beers tested (). With respect to the nonhydrolyzed samples, the average level of CA and p-COA (0.08 and 0.27 mg/dmCitation3, respectively) was a slight lower than that reported for the light commercial Chinese[Citation11] and Czech[Citation10] beers of lager type. However, CA as well as p-COA acids were also reported to be the minor components in the light beers investigated by the previous authors.[Citation10,Citation11] With respect to our study, upon alkaline hydrolysis, a significant amount of these phenolic acids was released in all the dark beers tested (). The amount of these phenolics including both hydrolyzed and nonhydrolyzed dark beer samples was in accordance with data reported by Piazzon[Citation14] for commercial beers of different types.
There is a little information regarding the content of flavonoids in beer.[Citation10,Citation11] Marova[Citation10] identified and quantified the content of following flavonoids: quercetin, kaempferol, morin, and rutin by using PDA (Photodiode Array) and MS (Mass Spectrometry) detection in the case of Czech light beer of lager type. Gorinstein[Citation20] tested the level of quercetin and epicatechin in order to compare the phenolic profile among the white wine, red wine, and light beer. There is, however, lack the data in the available literature concerning the attempts to investigate the level of flavonoids in dark beers. The amounts of flavonoids determined chromatographically (i.e., quercetin and kaempferol) (–) in dark beers tested are specified in . The mentioned above flavonoids seemed to be the minor constituents in the dark beers tested, and their average level for the nonhydrolyzed samples was below 0.1 mg/dmCitation3. Among the nonhydrolyzed beer samples (), Porter Żywiec beer B9 was characterized by the highest level of flavonoids.
Upon an alkaline hydrolysis, the amounts of flavonoids content were much higher than those before hydrolysis (), and Erdinger Wiessbier beer B2 was characterized by the lowest amount of flavonoids, whereas German dark beer Andechs Doppelbock B1 was the richest one in this respect. However, the amounts of flavonoids evaluated by HPLC method were insignificant as compared to those observed for phenolic acids after hydrolysis (). On the other hand, we found a significant (α = 0.05) linear correlation between total quercetin content and AA measured using ABTS assay (r = 0.791) after hydrolysis. A significant higher level of flavonols (i.e., quercetin and kaempferol) was reported by Marova[Citation10] for the nonhydrolyzed Czech light beers. According to the last author, the level ranged from 0.41 to 0.46 mg/dmCitation3 for quercetin, whereas from 0.92 to 1.27 mg/dmCitation3 for kaempferol. Again, Gorinstein[Citation20] reported a much higher level of quercetin (averaging 0.95 mg/dmCitation3) for the light beer tested when compared with our results (). Similarly, the significant higher content of this phenolic was observed for Indian beers.[Citation19] Thus, on the basis of obtained results it should be stated that the studied dark beers have not been supposed to be the valuable source of flavonoids. However, the further investigation has to be done in order to estimate if some other groups of phenolics which were not investigated (e.g., flavanols, proanthocyannidins, tannins, etc.) may play a role in shaping the phenolic content and AA of the commercial dark beers under study.
Principal component analysis
The analytical data (i.e., individual phenolic acids and flavonoids contents determined chromatographically, and AA measured using ABTS and DPPH assays) of the both nonhydrolyzed and hydrolyzed beer samples were processed statistically using a PCA in order to show grouping of the dark beers tested. The results of PCA of the nonhydrolyzed beer samples based on chosen dimensions are shown in and . The PCA explained 79.29% of the analytical variables. With respect to the hydroxycinnamic acids and flavonoids, as well as AA measured against DPPH radical, the variables were “split” into one group loaded on I factor. In contrast, GA content and radical scavenging activity against ABTS were “split” into a separate group loaded positively on factor II ().
Figure 5. Principal component analysis with distribution of analyzed parameters for the nonhydrolyzed beer samples: CAF: caffeic acid; COU: p-coumaric acid; FA: ferulic acid; GAL: gallic acid; KAE: kaempferol; QUE: quercetin; and SYN: synapic acid.
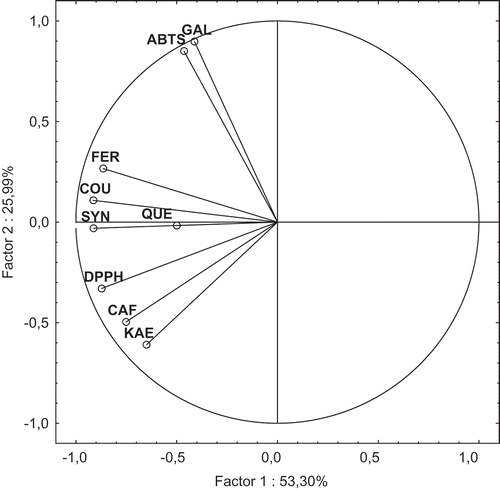
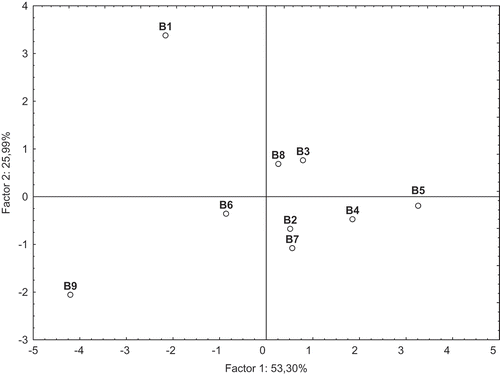
The projection of nonhydrolyzed beer samples tested on the surface of factors I and II is shown in , and strongly differentiated Porter Żywiec beer B9, located in the lower left quadrant from the others in terms of the first factor which explained 53.30% of the analytical variables. This sample was characterized by the highest AA measured against DPPH radicals, and beside the sample B1 also had the high content of phenolic compounds determined chromatographically (). The results of PCA analysis () also strongly differentiate German Andechs Doppelbock beer B1 from the others in terms of the second factor, which explained 25.99% of the analytical variables. This nonhydrolyzed beer sample (B1) could be best described by GA content that contributed for more than 50% of total phenolic acids content.
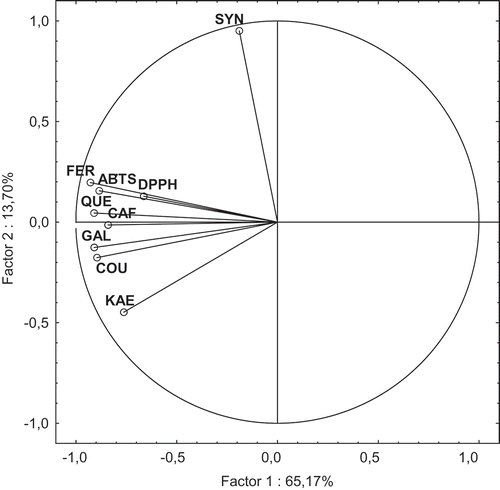
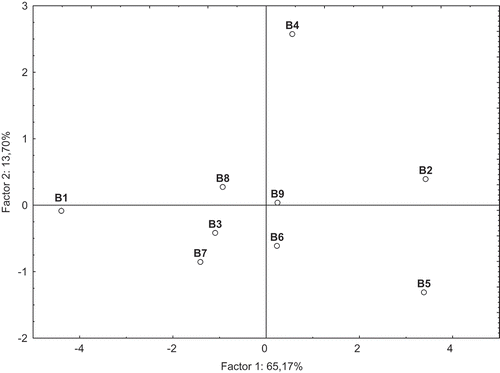
The results of PCA of the hydrolyzed beer samples, based on chosen dimensions, are shown in and . In this case, the PCA explained 78.87% of the analytical variables, with 65.17% and 13.70% explained by the first and second factors, respectively. With respect to the phenolic acids evaluated chromatographically, all the analytical variables (except of SA) were split into one group loaded on Factor I (). Unlike the nonhydrolyzed samples, in the case of hydrolyzed ones the radical scavenging activities against ABTS and DPPH radicals were loaded with factor I, and strongly correlated with each other (r = 0.826) (α = 0.05). The ABTS cation radical scavenging activity of dark beers after hydrolysis strongly correlated with FA and quercetin content, r = 0.807 and 0.791, respectively. Grouping of the hydrolyzed beer samples was performed visually using the PCA plot (). The PCA analysis strongly differentiates German beer Andechs Doppelbock B1 from the others. After alkaline hydrolysis, this beer was characterized by the highest level of total phenolic acid contents (45.60 mg/dmCitation3), including sum of free and bound forms of phenolics (). Among the phenolics (including their total content), this beer may be best described by CA, COA, GA, and FA content, as well as quercetin content (). Furthermore, the hydrolyzed beer sample B1 possessed a very high AA () measured using ABTS and DPPH assays (4.60 and 3.53 mMT/dmCitation3, respectively) as compared with the remaining samples. With respect to the first factor which explained 65,17% of the analytical variables, beside sample B1 also two other groups may be identified: the first one, including the samples B3, B4, B6, B7, B8, and B9 and the second one, including the samples B2 and B5. The former group includes the beer samples with the medium content of phenolic acids and flavonoids that were released after alkaline hydrolysis (), as well as the medium AA against ABTS and DPPH radicals (). It is interesting to note that in the case of this group of beers, the PCA analysis strongly differentiates the sample B4 from the others in terms of the second factor which explained 13.70% of the analytical variables. On this regard, sample B4 could be best described by SA content. With respect to the first factor, also the same similarities could be visible between samples B2 and B5. These samples seemed to be the poorest source of phenolic acids and flavonoids released after hydrolysis, and also exhibited the low AA, especially in the case of ABTS assay. It is interesting to add that Krušovice beer B5 was also the poorest in the case of analyzed parameters as compared to the nonhydrolyzed beer samples.
Conclusions
On the basis of the obtained results, it was proved that the studied dark beers varied considerably in both phenolic profiles evaluated chromatographically and antioxidant activities measured spectrophotometrically across different brands. Also, a considerable variation in phenolic profile and AA of commercial dark beers of different brands was found when compared the samples before and after hydrolysis. An alkaline hydrolysis of the investigated beer samples resulted in an increase of their AA measured using ABTS and DPPH assays due to releasing of free forms of phenolics which exhibited the higher antiradical activity than bound forms. This enabled to assess the investigated beers in terms of total content of most abundant phenolics, what seems to be of a great importance for consumers, since it has been known that only free forms of phenolics are supposed to be bioavailable in human gastrointestinal tract. The hydroxycinnamic acids, i.e., FA and SA, seemed to be a characteristic for the dark beers under study. Moreover, we observed that the total content of these phenolic acids, occurring in majority as bound forms, was higher than the most literature data concerning light beers. The investigation carried on hydrolyzed dark beer samples enabled to find the statistically significant correlations between the level of some phenolics, namely the total content of GA and FA, as well as quercetin, that occur mainly as the bounded forms, and AA of the beers measured using ABTS method. It should be emphasized that correlation between total content of individual phenolics and AA of beers has not been tested before. The PCA enabled to differentiate the investigated dark beers in terms of phenolic content and AA for both the hydrolyzed and nonhydrolyzed samples. With respect to the total most abundant phenolic acid content and AA, German beer Andechs Doppelbock B1 was the highest in this respect, being also the richest source of FA, GA, p-COA, and CA, as well as the quercetin content.
References
- Pulido, R.; Hernández-Garcia, M.; Saura-Calixto, F. Contribution of Beverages to the Intake of Lipophilic and Hydrophilic Antioxidants in the Spanish Diet. European Journal of Clinical Nutrition 2003, 57, 1275–1282.
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. Journal of Nutrition 2000, 130, 2037S–85S.
- Block, G.; Patterson, B.; Subar, A. Fruit, Vegetables, and Cancer Prevention; A Review of the Epidemiological Evidence. Nutritional Cancer 1992, 18, 1–29.
- Fuhrman, B.; Lavy, M.; Aviram, M. Consumption of Red Wine with Meals Reduces the Susceptibility of Human Plasma and Low-Density Lipoprotein to Lipid Peroxidation. American Journal of Clinical Nutrition 1995, 61, 549–554.
- Bourne, L.; Paganga, G.; Baxter, D.; Hughes, P.; Rice-Evans, C. Absorption of Ferulic Acid from Low-Alcohol Beer. Free Radical Research 2000, 32, 273–280.
- Shelnutt, S.R.; Cimino, C.O.; Wiggins, P.A.; Ronis, M.J.J.; Badger, T.M. Pharmacokinetics of the Glucuronide and Sulfate Conjugated of Genistein and Daidzein in Men and Women after Consumption of a Soy Beverage. American Journal of Clinical Nutrition 2002, 76, 588–594.
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van der Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The Metabolism of Dietary Polyphenols and the Relevance to Circulating Levels of Conjugated Metabolites. Free Radical Research 2002, 36, 1129–1141.
- Denke, M.A. Nutritional and Health Benefits of Beer. American Journal of Medical Science 2000, 320, 320–326.
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of Full-Scale Brewing Process on Polyphenols in Italian All-Malt and Maize Adjunct Lager Beers. Journal of Food Composition and Analysis 2011, 24, 568–573.
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of Phenolic Compounds in Lager Beers of Different Origin: A Contribution to Potential Determination of the Authenticity of Czech Beer. Chromatographia 2011, 73, S83–S95.
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic Profiles and Antioxidant Activities of Commercial Beers. Food Chemistry 2010, 119, 1150–1158.
- Guido, L.F.; Boivin, P.; Benismail, N.; Goncalves, C.R.; Barros, A.A. An Early Development of the Nonenal Potential in the Malting Process. European Food Research and Technology 2005, 220, 200–206.
- Nardini, M.; Ghiselli, A. Determination of Free and Bound Phenolic Acids in Beer. Food Chemistry 2004, 84, 137–143.
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolic Contents and Antioxidant Activity of Different Beer Types. Journal of Agriculture and Food Chemistry 2010, 58, 10677–10683.
- Granato, D.; Branco, G.F.; Faria, J.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. Journal of Science of Food and Agriculture 2011, 91, 563–571.
- Mitić, S.S.; Paunović, D.D.; Pavlović, A.N.; Stojković, M.B.; Tošić, S.B. Phenolic Profiles and Total Antioxidant Capacity of Marketed Beers in Serbia. International Journal of Food Properties 2014, 17, 908–922.
- Socha, R.; Gałkowska, D.; Robak, J.; Fortuna, T.; Buksa, K. Characterization of Polish Wines Produced from the Multispecies Hybrid and Vitis Vinifera L. Grapes. International Journal of Food Properties 2015, 18, 699–713.
- Sánchez-Moreno, C.; Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Free Radical Scavenging Capacity of Selected Red, Rosé, and White Wines. Journal of Scientific Food and Agriculture 1999, 79, 1301–1304.
- Pai, T.V.; Sawant, S.Y.; Ghatak, A.A.; Chaturvedi, P.A.; Gupte, A.M.; Desai, N.S. Characterization of Indian Beers: Chemical Composition and Antioxidant Potential. Journal of Food Science and Technology 2015, 52, 1414–1423.
- Gorinstein, S.; Caspi, A.; Zemser, M.; Trakhtenberg, S. Comparative Contents of Some Phenolics in Beer, Red and White Wines. Nutrition Research 2000, 20, 131–139.
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-Vinyl and 4-Ethyl Derivatives from Hydroxycinnamic Acids: Occurrence of Volatile Phenolic Flavor Compounds in Beer and Distribution of Pad1-Activity among Brewing Yeasts. Food Chemistry 2008, 107, 221–230.