ABSTRACT
The degradation kinetics of colour and individual carotenoids including lutein, α-carotene and β-carotene in pumpkin slices was investigated during microwave-vacuum drying (MVD) at selected microwave powers (4–8 W/g). The contents of lutein, α-carotene and β-carotene significantly decreased, while visual colour parameters such as the Hunter L* value decreased and the total colour difference (ΔE) value increased with the increase of processing time and microwave power. Degradation of lutein, α-carotene, β-carotene and Hunter L* value followed the first-order reaction kinetics; however, zero-order reaction kinetics was found adequate to describe changes in ΔE with R2 ≥ 0.9027. The relationship between individual carotenoid content and Hunter L* value was found to be more consistent through one-dimensional linear regression analysis with R2 ranging from 0.8697 to 0.9701. These results indicated that visual colour might be used to predict the lutein, α-carotene and β-carotene contents during drying pumpkin slices.
Introduction
The pumpkin (Cucurbita maxima L.), the member of Cucurbitaceae family, is a tropical and subtropical plant cultivated throughout the world.[Citation1] The reddish yellow colour of pumpkin solely depends on the varieties and contents of carotenoids, and the main carotenoids present in pumpkin include β-carotene (more than 80% of the total carotenoid content), α-carotene and a few xanthophylls.[Citation2–Citation4] Carotenoids in the diet are mostly associated with the beneficial effects on human health. Several studies have indicated that carotenoid intake may reduce the risk of certain types of cancer, degenerative and cardiovascular diseases, cataracts and macular degeneration, which plays an important role in disease prevention.[Citation5,Citation6] Carotenoid pigments are sensitive to light, heat, oxygen and acid.[Citation7] Pumpkins and pumpkin products are major sources of β-carotene and contribute significantly to carotenoid intake for humans. However, the thermal processing of pumpkin products usually causes carotenoid degradation and colour change in the final products, which is associated with various factors such as isomerization and oxidation reactions.[Citation7,Citation8]
Drying process assists in the extension of shelf life as well as a reduction in the volume of fruits and vegetables.[Citation9] Microwave-vacuum drying (MVD) is one of the advanced methods used for drying fruits and vegetables.[Citation10] However, various microwave drying conditions, such as the applied microwave power, vacuum levels and drying time, might have adverse effects on the colour and carotenoid contents in the processed products.[Citation11,Citation12] Thus, it is necessary to identify the optimal kinetic parameters to predict the change in colour and quality parameters during drying. Several researchers have reported the model of thermal degradation kinetics of carotenoid and colour during drying. Demiray and Tulek[Citation13] demonstrated that the degradation of β-carotene of carrot slices followed the first-order reaction kinetics model in convective drying, and the degradation rate is proportional to the drying temperature, which was consistent with the results for the degradation of β-carotene and the order of reaction found by Demiray et al.[Citation14] in hot-air drying of tomatoes. Similar results were described by Hadjal et al.,[Citation15] who reported that first-order reaction kinetics agreed well to describe the degradation of lutein and cryptoxanthin on microwave heating of orange Juice. However, Suvarnakuta et al.[Citation16] found that the degradation of β-carotene was in accordance with the third-order degradation kinetics in the vacuum microwave, hot air and low-pressure steam drying process. The difference in reaction order may be related to the fruit and vegetable matrix of carotenoids and the thermal processing. Carotenoid degradation is usually accompanied by changes in colour. Correlations between visual colour coordinates with carotenoids content have been widely discussed in the literature. Saxena et al.[Citation17] found that the degradation of colour parameters Hunter L*×b* value and the total carotenoids of jackfruit bulb slices agreed well with the first-order reaction kinetics during hot air drying. Moreover, there was a certain correlation between total carotenoids and the L*×b* value, and the combination of Hunter L*×b* value could replace carotenoid content for online quality control adequately. However, not all of the colour parameters in the system were correlated with carotenoids; Albanese et al.[Citation18] stated in their research that the degradation of colour parameters L* and C* of apricot are in line with the first-order reaction kinetics in the drying process, but with lack of correlation between β-carotene and colour. This could be explained by the assumption that the non-enzymatic browning reaction has more effect on the change of colour parameters of apricot than the degradation of carotenoid. Other reactions such as the Maillard reaction will affect the colour parameters, so it is necessary to examine whether there is a correlation between colour and carotenoid, which may be used as a substitute for the determination of carotenoid content.[Citation17,Citation19]
The objective of this study was to determine the kinetic parameters for the degradation of lutein, α-carotene, β-carotene and visual colour of pumpkin slices during MVD at different microwave powers as well as to evaluate the relationship between visual colour parameters and total carotenoids, which contributed to optimize the process through the control of processing parameters during drying and perform simulations using accurate kinetic parameters.
Materials and methods
Fresh material
The pumpkin used in this study is a Cucurbita maxima L. ‘Benmi’ variety, which was bought from a local market in Nanjing, China, washed in running tap water and then hand peeled. The washed pumpkins were cut into slices with a thickness of 6 mm using a chip cutter (FC-312, Zhaoqing Fengxiang Food Machinery Co., Ltd., Guangdong, China) and immersed in boiling water for 40s and then air-cooled to room temperature.
Chemicals and reagents
Carotenoid standards, namely lutein (95% pure, Sigma-Aldrich, catalogue no. C-9750) and β-carotene (≥95% pure, Sigma-Aldrich, catalogue no. C4582), were obtained from Sigma (St. Louis, MO). High performance liquid chromatography-grade (HPLC-grade) methyl tert-butyl ether (MTBE) and methanol were purchased from Tedia (Fairfield, USA). Analytical-grade hexane, anhydrous sodium sulphate, acetone, toluene, and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Purified water was obtained from a Milli-Q system (Millipore, Milford, MA, USA). All other chemicals and reagents were of analytical grade.
Microwave-vacuum drying
MVD was performed with a microwave-vacuum dryer (MVD-1, Nanjing Xiaoma Electrome-chanical Equipment Factory, Nanjing, China). Pumpkin slices were evenly placed on the turntable in the vacuum vessel, and subjected to a negative pressure of 90 KPa. MVD was intermittently conducted with 3 min heating-on followed by 1 min heating-off. The drying was continued until the final moisture content was approximately 5.0%. Pumpkin sampling under different microwave powers (4 W/g, 5 W/g, 6 W/g, 7 W/g and 8 W/g) at different dehydration times was carried out. Meanwhile, the core temperature of the pumpkin slices during MVD was detected and recorded by an optic fibre (Supplementary figure). All experiments were carried out in triplicates.
Colour measurement
Colour measurement was performed by our previous method.[Citation20] Visual colour of dried pumpkin slices was assessed using a colorimeter (WSC-S, Shanghai Precision & Scientific Instrument Co., Ltd., China) and reported as L*, a* and b* values. The L* value represents lightness, ranging from 0 (black) to 100 (white), and indicates how dark/light the sample is; the a* value exhibits redness (positive) and greenness (negative); and the b* value exhibits yellowness (positive) and blueness (negative). The total colour difference (ΔE) is used to characterize the overall change in colour during the drying process. The ΔE value is calculated by the following equation:
where L0, a0 and b0 are the control values for freeze-dried pumpkin.
Analysis of carotenoids
The method for the analysis of carotenoids in pumpkin slices is based on a previously published method.[Citation20–Citation22]
Carotenoid extraction
The slice samples were first ground into a fine powder using a herb grinder (FW100, Tianjin Taisite Instrument Co., Ltd, China), then a 0.5 g sample of pumpkin powder was mixed with 30 mL of acetone/petroleum ether (2:1, v/v) mixture in a 100-mL volumetric flask, followed by shaking for 4 h. Thereafter, 2 mL of 10% methanolic KOH was added for saponification at 25ºC in the dark under nitrogen gas for 12 h. After saponification, 30 mL of hexane was added for the partitioning of carotenoids, followed by shaking for 1 min, after which a 10% sodium sulphate solution was added, and the sample was diluted to volume. The mixture was allowed to stand until two phases separated clearly. The upper layer containing carotenoids was collected, evaporated to dryness, redissolved in 10 mL of methanol, and filtered through a 0.45-µm membrane filter for HPLC analysis.
HPLC-DAD-MS/MS analysis
The HPLC analysis was performed using an analytical-scale C30 reversed-phase column (250 mm×4.6 mm i. d.) with 5 µm particle size (YMC, Wilmington, MA, USA). Eluent A used consisted of methanol/MTBE/water (70: 25: 5, v/v/v), and eluent B consisted of MTBE/methanol/water (85: 10: 5, v/v/v). The separation was performed at a column temperature of 25ºC using gradient elution conditions within 30 min at a flow rate of 0.6 mL/min. Aliquots of 20 µL were used for HPLC analysis. The solvent gradient elution program employed was as follows: 0–4.5 min, linear gradient 95%A–80%A; 4.5–12.5 min, linear gradient 80%A–50%A; 12.5–18 min, linear gradient 50% A–25%A; 18–24 min, linear gradient 25%A–5%A; and finally, a return to the initial setting.
The MS procedure was carried out on an Agilent 1290 Infinity LC/Agilent Technologies 6530 Q-TOF MS (Agilent Technologies, Santa Clara, CA, USA). An atmospheric pressure chemical ionization (APCI) interface in positive ion mode was used to detect the carotenoids, with a total ion current (TIC) scanning range of 80–1000 m/z, a corona current of 4 μA, a capillary voltage of 2500 V, nitrogen of the highest purity (99.9%) as a nebulizer gas (flow rate 4 L/min) and a vaporizer temperature of 350ºC. Quantification was performed using the external standards method, after the preparation of a five-point external standard calibration curve for each available standard; standard calibration curve correlation coefficient (R2) values were in the range of 0.9991–0.9995.
Kinetic data analysis
To describe the changes in colour and carotenoid contents, zero-order (Eq. 2), first-order (Eq. 3) and second-order kinetic models (Eq. 4) were used. The R2 was one of the primary criteria to select the best equation to account for variation. In addition to R2, root mean square error (RMSE) was also used to determine the quality of the fit. Higher R2 value and lower RMSE value were chosen as the criteria for goodness of fit.[Citation23,Citation24] The above-mentioned parameters can be calculated as follows:
where Ct is the concentration (%) at time t; C0, the concentration (%) at time zero; k0, the zero-order rate constant (min−1); k1, the first-order rate constant (min−1); k2, the second-order rate constant (min−1); and t, the drying time (min). Moreover, the half time (t½), defined as the time required for carotenoids concentration in dried pumpkins to decay decreased to 50% of its initial concentration, was calculated by Eq. (5). The decimal reduction time (D values), defined as the time required for carotenoids concentration in dried pumpkins to decay decreased to 90% of its initial concentration, was calculated by Eq. (6).
where k is the reaction rate constant.
Statistical analysis
All experiments were performed in triplicate. Kinetic data were analysed by regression analysis using origin version 9.0 (Washington, USA). Regression results include the RMSE and coefficient of determination, R2.
Results and discussion
Predominant carotenoids of pumpkins
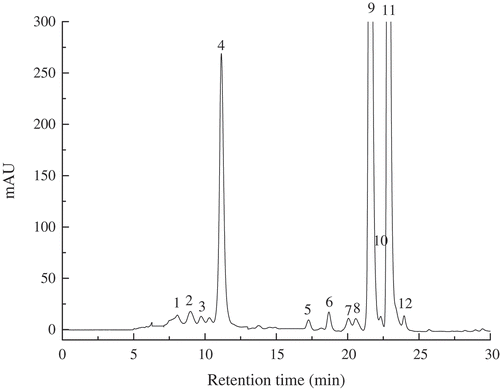
An HPLC chromatography profile of carotenoids from Cucurbita maxima L. ‘Benmi’ is shown in . The identification is discussed according to the combined information obtained from the retention times, UV-Visible absorption maxima, electron ionization mass spectroscopy (EIMS) and chemical ionization mass spectroscopy (CIMS) fragmentation patterns.[Citation25] Among the 12 different carotenoid compounds identified in this pumpkin sample, all-trans-α-carotene and all-trans-β-carotene, which was accompanied by four minor cis-isomers (15-cis-β-carotene, 13-cis-β-carotene, 9-cis-β-carotene and 9-cis-α-carotene), were the most abundant components (97%), followed by all-trans-lutein and traces of neoxanthin, neochrome, violaxanthin, α-cryptoxanthin and β-cryptoxanthin. In our study, zeaxanthin and antheraxanthin were not detected, which is not completely consistent with the literature.[Citation26] This might be due to the different composition of carotenoids in different varieties of pumpkin, but also related to the determination methods such as different extraction solvent and detection methods.[Citation4,Citation27] However, the major trans carotenoids, including all-trans-β-carotene, all-trans-α-carotene and all-trans-lutein, were similar to the results reported by others.[Citation26] The analysis of carotenoids in pumpkins during MVD focussed on the degradation of β-carotene, α-carotene and lutein in the following research.
Figure 1. HPLC chromatograms of carotenoids in fresh pumpkin pulp. The following peaks were identified: 1. neoxanthin, 2. neochrome, 3. violaxanthin, 4. all-trans-lutein, 5. α-cryptoxanthin, 6. β-cryptoxanthin, 7. 15-cis-β-carotene 8. 13-cis-β-carotene, 9. all-trans-α-carotene, 10. 9-cis-α-carotene, 11. all-trans-β-carotene, 12. 9-β-carotene.
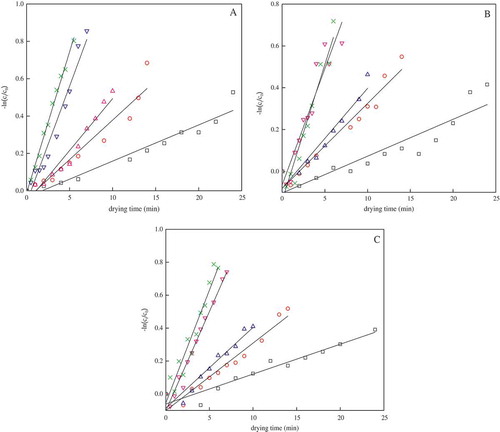
Fitting of kinetic modelling of carotenoids degradation
The reduction observed for lutein, α-carotene and β-carotene was, respectively, described by zero-, first- and second-order kinetic models in different microwave powers (4 W/g, 5 W/g, 6 W/g, 7 W/g, and 8 W/g). The R2 and RMSE used to determine the goodness of fit of the models are shown in and . At the fitting of the zero-order reaction rate model Eq. (2) (), the values of R2 showed 0.9461, 0.8802 and 0.9494 for lutein, α-carotene and β-carotene, respectively, but the values of RMSE for the zero-order kinetic models, which varied from 1.60391 to 22.82657, were the highest for all the models considered. To obtain higher R2 and lower RMSE, the first- (Eq. (3)) and second-order reaction rate models (Eq. (4)) were chosen in place of the zero-order reaction kinetic model. It was observed that the first-order kinetic model was found to better describe the carotenoids data. The R2 ranged from 0.9405 to 0.9527, while the RMSE values were less than 0.062 for all cases. Similar results have been reported by Fratianni et al.[Citation28] during apricots drying by hot air and microwave. By the time of second-order development, the R2 was decreased to 0.7867, 0.8191 and 0.9069 for lutein, α-carotene and β-carotene, respectively. Therefore, in the following discussion only the coefficients relating to the first-order kinetics will be considered ().
Table 1. Values of reaction rate constant and its coefficient of zero-, first- and second-order of the main trans carotenoids during microwave-vacuum drying.
Degradation kinetics of lutein
The lutein content of fresh pumpkin samples was found to be 47.51 μg/g DW. When the pumpkin slices were dried at the selected microwave power, its lutein content was decreased. The lutein content decreased to 28.03 μg/g DW after 24 min of drying at 4 W/g, accounting for approximately 41% loss. A similar trend was observed during drying of the pumpkin slices at higher power. The degradation of lutein increased with increase in microwave power and it lost more than 55% after only 6 min of drying at 8 W/g. Similar behaviours of lutein loss were observed by Aparicio-Ruiz et al.[Citation29] during heat treatment of virgin olive oils. Degradation occurs due to various reasons such as high temperature, long processing time, light and oxygen.[Citation7] Subagio et al.[Citation30] reported that the decline in lutein was due to destruction by isomerization and oxidation, resulting in cis isomers, epoxides, hydroxyl oxidation containing aldehyde or ketone group. The first-order model (Eq. (3)) for degradation of lutein during drying was fitted and the degradation parameters were obtained (). It was observed that as the microwave power increased, the degradation rate constant increased, with corresponding reduction in the half-life (t1/2) and the value of D. For example, the degradation rate constant of lutein was 0.0391 min−1 at 4 W/g, but it increased up to two times with increase in microwave power to 8 W/g. Results indicated that lutein was highly affected by microwave-induced tissue heating. The predicted values of lutein were obtained from the model and shown as a solid line in . The R2 values ranged from 0.9381 to 0.9942, whereas RMSE varied from 0.02117 to 0.06325, which adequately validated the first-order model for explaining the degradation behaviour. Similar studies on the thermal and oxidative degradation of lutein in safflower seed oil and virgin olive oils also concluded that the degradation of lutein fitted the first-order model with degradation rate and increased with increasing temperature.[Citation29,Citation31] The present findings concluded that the degradation of lutein followed the first-order model and the degradation rate constant increased with increase in microwave power and these results were similar to earlier reported observations.
Table 2. The kinetic parameters of three kinds of carotenoids, L* and ΔE at different microwave powers.
Degradation kinetics of α-carotene
Pumpkins are a good source of α-carotene and its content may vary. For example, Murkovic et al.[Citation4] reported that the α-carotene content of fresh pumpkins ranged from 0 to 7.5 mg/100 g, whereas de Carvalho et al.[Citation3] reported that the α-carotene content of landrace pumpkins (Cucurbita moschata Duch) ranged from 67.07 to 72.99 μg/g. In the present study, the most abundant carotenoid found in fresh pumpkin samples was α-carotene, the content of which was 386.22 μg/g DW, accounting for nearly 50% of the total carotenoid content. However, the thermal processing of pumpkins has been reported to have a very adverse effect on their α-carotene content. The α-carotene content of pumpkins dried at 4 W/g changed from 386.22 to 252.55μg/g DW at the end of drying. However, at 8 W/g, it dropped to 186.43μg/g DW. The results indicated that the degradation of carotenoids in tomatoes was highly influenced by the power and length of drying. Previous studies, such as Fratianni et al.,[Citation32] reported a significant loss of 56% in the α-carotene content of orange juice after 10 min microwave heating at 70°C. However, Dueik et al.[Citation33] found only 10.5% loss in α-carotene when carrots were dehydrated by vacuum frying at 60°C for 5 min, which may be due to the low temperatures employed and minimal exposure to oxygen under vacuum condition. The degradation reaction of α-carotene followed a first-order kinetic model, and this was in good agreement with the first-order degradation of α-carotene reported in previous studies.[Citation32] The α-carotene degradation at various powers, reported as the natural logarithm plot of the carotenoid content, as a function of drying time, is indicated in . The linear R2 was 0.8280–0.9527, confirming that the reaction of α-carotene degradation is of the first-order. The calculated rate constants (k) and other kinetic parameters are given in for various drying conditions. The reaction rate constants for α-carotene loss were in the range of 0.0353–0.0650 min−1 and were significantly influenced by drying power. The degradation rate of α-carotene increased gradually with microwave power. The half-life time (t1/2) of α-carotene in pumpkins was 19.66 min at 4 W/g microwave power, but it decreased to 10.66 min at 8 W/g.
Degradation kinetics of β-carotene
A large range of β-carotene content in pumpkins was reported in the literature, depending on the variety, maturation stage and extraction procedure as well as instruments used for analysis.[Citation27,Citation34] The initial β-carotene content was found to be 320.53 μg/g DW in fresh pumpkins. The β-carotene contents of pumpkin at 4, 5, 6, 7 and 8 W/g after completion of drying were experimentally determined to be 214.77, 190.39, 180.21, 153.22 and 149.52 μg/g, respectively. According to Fratianni et al.[Citation28] when apricot was hot dried at 60°C and 70°C, losses of carotenoids were approximately 20% and 40%, respectively. Several studies report the decrease of all-trans-carotenoid and the concomitant increase in cis-isomers in vegetables subjected to thermal processes.[Citation35–Citation37] Our results suggest that the β-carotene decreased, whereas three cis-isomers (15-cis-β-carotene, 13-cis-β-carotene and 9-cis-β-carotene) increased on prolonging the heating time, which is accelerated at higher microwave power. Therefore, suitable kinetics for carotenoids degradation was evaluated at different drying conditions to minimize its loss during drying.
As shown in , degradation of β-carotene was found to follow first-order reaction kinetics because the linear R2 was higher than 0.9299 and the mean square error was lower than 0.07155, which was in agreement with previous studies.[Citation8,Citation13,Citation16] First-order kinetic rate constants at different drying conditions are presented in . The range of reaction rate constants for β-carotene loses was 0.0363–0.0686 min−1. The reaction rate constant of β-carotene degradation was affected by several factors such as food matrix, pretreatment and processing method. For example, the degradation rate of β-carotene of dehydrated carrots was higher in unblanched carrots compared with blanched samples when they were stored at the same temperature, which also showed that the enzymes in fruits and vegetables could affect the kinetic parameters.[Citation38] The degradation rate of total carotenoids and β-carotene in apricot by microwave heating was higher than that by hot-air drying.[Citation8]
Degradation kinetics of visual colour
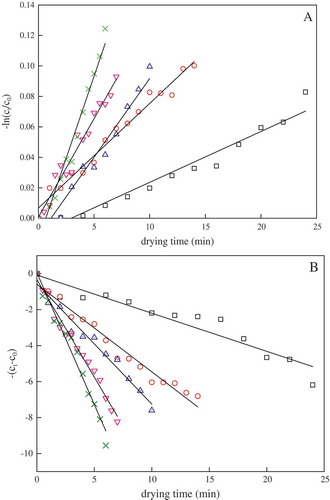
With the increase of drying power and time, pumpkin slices become darker. This corresponds to a decrease in the L* value of the colour scale (). At the end of drying, the Hunter L* value of the pumpkin slice decreased exponentially from the initial values of 80.11–73.74, 72.50, 72.54, 71.85 and 70.61 at 4, 5, 6, 7 and 8 W/g, respectively. However, the changes of a* and b* values were not obvious with the increase of microwave power and time. This phenomenon can be attributed to the fact that β-carotene and α-carotene, the most abundant pigments present in the pumpkin, are yellowish carotenoids, and their degradation produced a higher impact in L* compared with the a* and b* values. However, Ahmed et al.[Citation39] observed that apart from the Hunter b* value, both L* and a* values also decreased with time at a given temperature as the mango puree turned brown. Chutintrasri and Noomhorm[Citation40] and Saxena et al.[Citation17] both found that the Hunter L* and b* values decreased and the a* value increased during heat-treated pineapple puree at 70–110ºC and hot-air drying for jackfruit bulb slices. The different results may be due to the changes in the yellow and red colour of the pumpkin slices, which may be caused not only by the variation of carotenoids content but also by a series of complex Maillard reactions in the drying process.[Citation19] The total colour difference ΔE, which is a combination of the L*, a* and b* values, as given by Eq. (1), is a colorimetric parameter extensively used to characterize the variation of colour in pumpkins during processing. The results presented in this work suggest that the values of ΔE increased during dry processing ().
In the current study, it was observed that the first-order kinetic model fitted well to L* (). In all cases, a significant (p < 0.05) linear regression with the coefficient of determination values (R2) ranging between 0.9513 and 0.9783 was obtained whereas the RMSE values were less than 0.00006. The same order of reaction was found by Chutintrasri and Noomhorm[Citation40] in pineapple puree and by Avila and Silva[Citation41] in peach puree. In addition, it was found that the degradation of ΔE value followed zero-order reaction kinetics with R2 values of 0.9027–0.9806, which is in agreement with previous reports.[Citation17,Citation40]
Relationship between visual colour and carotenoids content
The decrease in Hunter L* value was correlated with the carotenoids concentration of pumpkin slices during drying. The relationship between visual colour and carotenoid concentration has been found to be well described using the linear equation (Eq.7):
where ka and kb are the coefficients, ‘X’ represents Hunter L* value and ‘Y’ represents lutein, α-carotene and β-carotene concentrations (μg/g DW), respectively. The regression equation and the R2 of Eq. (7) are presented in . From the table, there was a certain correlation between lutein, α-carotene and β-carotene concentration and the L* value, with the R2 ranging between 0.8697 and 0.9701, which showed that the fitting degree of the one-dimensional linear regression equation was better. These results suggested that as the carotenoids degradation occurred with processing, the visual colour of the product got affected. Correlations between visual colour coordinates with the carotenoids content are widely discussed in the literature. Ahmed et al.[Citation42] found that the combination of the Hunter a* × b* value adequately represented thermal colour change. Correlation of the Hunter a* × b* values was also reported to agree with the total carotenoids and lycopene contents in heated watermelon juice by Sharma et al.[Citation43]. Saxena et al.[Citation17] described a good relationship of the total carotenoids content and the Hunter L* × b* value and suggested that the combination of Hunter L* × b* value could represent the colour change adequately. Thus, determination of the L* value can be a good predictor of lutein, α-carotene and β-carotene losses during the drying of pumpkin slices in our study.
Table 3. Correlation of brightness value L*and carotenoids content in pumpkin at different microwave powers.
Conclusions
In this paper, the degradation kinetics of lutein, α-carotene, β-carotene and colour degradation in pumpkin slices were studied during MVD. It was observed that the first-order reaction agreed well with the degradation of lutein, α-carotene, β-carotene and Hunter L* values, with the reaction rate constants in the range of 0.0391–0.0756, 0.0353–0.0650, 0.0363–0.0686 and 0.0033–0.0215 min−1, respectively, while the degradation of ΔE values followed zero-order reaction kinetics and the reaction rate constant was in the range of 0.2119–1.4652 min−1. The lutein, α-carotene and β-carotene were correlated with the Hunter L* value, and thus could predict the carotenoids degradation in the pumpkin during drying.
LJFP_A_1306553_Supplementary_Figure.docx
Download MS Word (117.3 KB)Funding
This work was supported by Grants-in-Aid for scientific research from the National Natural Science Foundation of China (No. 31301534), the special fund for agro-scientific research in the public interest (No. 201503142-5), the basal research fund for the Jiangsu Academy of Agricultural Sciences (major cultivation project No. ZX (15)1008) and this project for Key Laboratory of Horticultural Crop Genetic Improvement of Jiangsu Province (2014019).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Ferriol, M.; Picó, B. Pumpkin and Winter Squash. In Handbook of plant breeding, vegetables I; Eds.; Prohens, J.; Nuez, F.; Springer: New York, USA, 2008; 317–349.
- Seo, J.S.; Burri, B.J.; Quan, Z.; Neidlinger, T.R. Extraction and Chromatography of Carotenoids from Pumpkin. Journal of Chromatography A 2005, 1073, 371–375.
- De Carvalho, L.M.J.; Gomes, P.B.; De Oliveira Godoy, R.L.; Pacheco, S.; Do Monte, P.H.F.; De Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.N.; Vieira, A.C.R.A.; Ramos, S.R.R. Total Carotenoid Content, Α-Carotene and Β-Carotene, of Landrace Pumpkins (Cucurbita moschata Duch): A Preliminary Study. Food Research International 2012, 47, 337–340.
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid Content in Different Varieties of Pumpkins. Journal of Food Composition and Analysis 2002, 15, 633–638.
- Sommerburg, O.; Keunen, J.E.; Bird, A.C.; Van Kuijk, F.J. Fruits and Vegetables that are Sources for Lutein and Zeaxanthin: The Macular Pigment in Human Eyes. British Journal of Ophthalmology 1998, 82, 907–910.
- Adaramoye, O.A.; Achem, J.; Akintayo, O.O.; Fafunso, M.A. Hypolipidemic Effect of Telfairia occidentalis (Fluted Pumpkin) in Rats Fed a Cholesterol-Rich Diet. Journal of Medicinal Food 2007, 10, 330–336.
- Chen, B.H.; Huang, J.H. Degradation and Isomerization of Chlorophyll a and Β-Carotene as Affected by Various Heating and Illumination Treatments. Food Chemistry 1998, 62, 299–307.
- Dutta, D.; Dutta, A.; Raychaudhuri, U.; Chakraborty, R. Rheological Characteristics and Thermal Degradation Kinetics of Beta-Carotene in Pumpkin Puree. Journal of Food Engineering 2006, 76, 538–546.
- Prakash, S.; Jha, S.K.; Datta, N. Performance Evaluation of Blanched Carrots Dried by Three Different Driers. Journal of Food Engineering 2004, 62, 305–313.
- Therdthai, N.; Zhou, W. Characterization of Microwave-Vacuum Drying and Hot Air Drying of Mint Leaves (Mentha cordifolia Opiz Ex Fresen). Journal of Food Engineering 2009, 91, 482–489.
- Cui, Z.W.; Xu, S.Y.; Sun, D.W. Effect of Microwave-Vacuum Drying on the Carotenoids Retention of Carrot Slices and Chlorophyll Retention of Chinese Chive Leaves. Drying Technology 2004, 22, 563–575.
- Bechoff, A.; Westby, A.; Owori, C.; Menya, G.; Dhuique‐Mayer, C.; Dufour, D.; Tomlins, K. Effect of Drying and Storage on the Degradation of Total Carotenoids in Orange‐Fleshed Sweetpotato Cultivars. Journal of the Science of Food and Agriculture 2010, 90, 622–629.
- Demiray, E.; Tulek, Y. Degradation Kinetics of Β-Carotene in Carrot Slices during Convective Drying. International Journal of Food Properties 2016. doi:10.1080/10942912.2016.1147460
- Demiray, E.; Tulek, Y.; Yilmaz, Y. Degradation Kinetics of Lycopene, Β-Carotene and Ascorbic Acid in Tomatoes during Hot Air Drying. LWT-Food Science and Technology 2013, 50, 172–176.
- Hadjal, T.; Dhuique-Mayer, C.; Madani, K.; Dornier, M.; Achir, N. Thermal Degradation Kinetics of Xanthophylls from Blood Orange in Model and Real Food Systems. Food Chemistry 2013, 138, 2442–2450.
- Suvarnakuta, P.; Devahastin, S.; Mujumdar, A.S. Drying Kinetics and Β‐Carotene Degradation in Carrot Undergoing Different Drying Processes. Journal of Food Science 2005, 70, s520–s526.
- Saxena, A.; Maity, T.; Raju, P.S.; Bawa, A.S. Degradation Kinetics of Colour and Total Carotenoids in Jackfruit (Artocarpus heterophyllus) Bulb Slices during Hot Air Drying. Food and Bioprocess Technology 2012, 5, 672–679.
- Albanese, D.; Cinquanta, L.; Cuccurullo, G.; Di Matteo, M. Effects of Microwave and Hot‐Air Drying Methods on Colour, Β‐Carotene and Radical Scavenging Activity of Apricots. International Journal of Food Science & Technology 2013, 48, 1327–1333.
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of Non-Enzymatic Browning and Antioxidant Capacity in Processed Foods. Trends in Food Science & Technology 2000, 11, 340–346.
- Song, J.F.; Wang, X.P.; Li, D.J.; Meng, L.L.; Liu, C.Q. Degradation of Carotenoids in Pumpkin (Cucurbita maxima L.) Slices as Influenced by Microwave-Vacuum Drying. International Journal of Food Properties 2016. doi:10.1080/10942912.2016.1212875
- Kao, T.H.; Loh, C.H.; Inbaraj, B.S.; Chen, B. H. Determination of Carotenoids in Taraxacum formosanum by HPLC–DAD–APCI-MS and Preparation by Column Chromatography. Journal of Pharmaceutical and Biomedical Analysis 2012, 66, 144–153.
- Li, D.; Xiao, Y.; Zhang, Z.; Liu, C. Analysis of (All-E)-Lutein and Its (Z)-Isomers during Illumination in a Model System. Journal of Pharmaceutical and Biomedical Analysis 2014, 100, 33–39.
- Garcia, C.C.; Mauro, M.A.; Kimura, M. Kinetics of Osmotic Dehydration and Air-Drying of Pumpkins (Cucurbita moschata). Journal of Food Engineering 2007, 82, 284–291.
- Doymaz, I.; The Kinetics of Forced Convective Air-Drying of Pumpkin Slices. Journal of Food Engineering 2007, 79, 243–248.
- Song, J.F.; Li, D.J.; Pang, H.L.; Liu, C.Q. Effect of Ultrasonic Waves on the Stability of All-Trans Lutein and Its Degradation Kinetics. Ultrasonics Sonochemistry 2015, 27, 602–608.
- Kurz, C.; Carle, R.; Schieber, A. HPLC-DAD-MS N Characterisation of Carotenoids from Apricots and Pumpkins for the Evaluation of Fruit Product Authenticity. Food Chemistry 2008, 110, 522–530.
- Rivera, S.; Canela, R. Influence of Sample Processing on the Analysis of Carotenoids in Maize. Molecules 2012, 17, 11255–11268.
- Fratianni, A.; Albanese, D.; Mignogna, R.; Cinquanta, L.; Panfili, G.; Di Matteo, M. Degradation of Carotenoids in Apricot (Prunus armeniaca L.) during Drying Process. Plant Foods for Human Nutrition 2013, 68, 241–246.
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal Degradation Kinetics of Lutein, Β-Carotene and Β-Cryptoxanthin in Virgin Olive Oils. Journal of Food Composition and Analysis 2011, 24, 811–820.
- Subagio, A.; Wakaki, H.; Morita, N. Stability of Lutein and Its Myristate Esters. Bioscience, Biotechnology, and Biochemistry 1999, 63, 1784–1786.
- Henry, L.K.; Catignani, G.L.; Schwartz, S.J. Oxidative Degradation Kinetics of Lycopene, Lutein, and 9-Cis and All-Trans Β-Carotene. Journal of the American Oil Chemists’ Society 1998, 75, 823–829.
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of Carotenoids in Orange Juice during Microwave Heating. LWT-Food Science and Technology 2010, 43, 867–871.
- Dueik, V.; Robert, P.; Bouchon, P. Vacuum Frying Reduces Oil Uptake and Improves the Quality Parameters of Carrot Crisps. Food Chemistry 2010, 119, 1143–1149.
- Provesi, J.G.; Amante, E.R. Chapter 9–Carotenoids in Pumpkin and Impact of Processing Treatments and Storage. Processing and Impact on Active Components in Food 2015, 71–80.
- Aman, R.; Schieber, A.; Carle, R. Effects of Heating and Illumination on Trans-Cis Isomerization and Degradation of Β-Carotene and Lutein in Isolated Spinach Chloroplasts. Journal of Agricultural and Food Chemistry 2005, 53, 9512–9518.
- Lemmens, L.; De Vleeschouwer, K.; Moelants, K.R.; Colle, I.J.; Van Loey, A.M.; Hendrickx, M.E. Β-Carotene Isomerization Kinetics during Thermal Treatments of Carrot Puree. Journal of Agricultural and Food Chemistry 2010, 58, 6816–6824.
- Zepka, L.Q.; Mercadante, A.Z. Degradation Compounds of Carotenoids Formed during Heating of a Simulated Cashew Apple Juice. Food Chemistry 2009, 117, 28–34.
- Koca, N.; Burdurlu, H.S.; Karadeniz, F. Kinetics of Colour Changes in Dehydrated Carrots. Journal of Food Engineering 2007, 78, 449–455.
- Ahmed, J.; Shivhare, U.S.; Kaur, M. Thermal Colour Degradation Kinetics of Mango Puree. International Journal of Food Properties 2002, 5, 359–366.
- Chutintrasri, B.; Noomhorm, A. Color Degradation Kinetics of Pineapple Puree during Thermal Processing. LWT-Food Science and Technology 2007, 40, 300–306.
- Avila, I.M.L.B.; Silva, C.L.M. Modelling Kinetics of Thermal Degradation of Colour in Peach Puree. Journal of Food Engineering 1999, 39, 161–166.
- Ahmed, J.; Shivhare, U.S.; Sandhu, K.S. Thermal Degradation Kinetics of Carotenoids and Visual Color of Papaya Puree. Journal of Food Science 2002, 67, 2692–2695.
- Sharma, R.; Kaur, D.; Oberoi, D.P.S.; Sogi, D.S. Thermal Degradation Kinetics of Pigments and Visual Color in Watermelon Juice. International Journal of Food Properties 2008, 11, 439–449.