ABSTRACT
The present study is the first report on essential oil (EO) composition, phytochemicals, and biological potential of Achillea sivasica tested against free radicals, oxidative damage, and tyrosinase enzyme. Gas-Chromatography Flame Ionization Detector (GC-FID) and gas chromatography/mass spectrometry (GC/MS) analyses revealed that β-pinene (11.5%, 9.3%, and 6.7%), β-pinene (7.0%, 3.0%, and 6.9%), 1,8-cineole (18.0%, 22.1%, and 6.7%), and camphor (7.6%, 4.1%, and 9.0%) were the major constituents in the EOs from the herb, flower, and leaves, respectively. Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analysis of the extracts revealed the presence of caffeoylquinic acid derivatives, luteolin, apigenin, patuletin, isorhamnetin, cirsimaritin, and santin. The leaf extracts demonstrated strongest free radical scavenging, cupric reducing, lipid peroxidation inhibition, and antityrosinase activities.
Introduction
The genus Achillea is included in the Anthemideae Cass. tribe of Compositae family and has about 115 taxa. In the Flora of Turkey, the genus Achillea is represented by 6 sections and 49 species (58 taxa), and 24 species of them are endemic with an endemism ratio 49%. An endemic species Achillea sivasica Çelik & Akpulat has recently been described by Çelik and Akpulat.[Citation1]
Achillea species are known in Anatolia with local names as civanperçemi, binbiryaprak otu, ayvadanasi, and kiliç otu. Some representatives are consumed as an infusion for their organoleptic properties. In traditional medicine, the aerial parts of Achillea species are generally used for analgesic effects to eliminate the flatulence of infants, as emenagog, against diarrhoea, and for wound healing.[Citation2–Citation4] The most investigated Achillea species is A. millefolium L. (yarrow) which is cultivated in many countries.[Citation5,Citation6] Monographs on the herb and flowers of A. millefolium are recorded in different pharmacopeias: American Herbal Pharmacopoeia,[Citation7] The British Herbal Pharmacopoeia,[Citation8] European Pharmacopoeia,[Citation9] German Homoeopathic Pharmacopoeia,[Citation10] and WHO Monographs.[Citation11]
Previous phytochemical investigations on different Achillea species reported about monoterpenes, sesquiterpenes, sesquiterpene lactones, essential oils, flavonoids, lignans and caffeoilquinic acid derivatives.[Citation12–Citation22]
A wide range of biological activities of Achillea species were scientifically investigated: antioxidant,[Citation22–Citation26] anti-inflammatory,[Citation19,Citation21] antifungal and antimicrobial,[Citation21,Citation27–Citation30] antinociceptive,[Citation21,Citation31] insecticidal and herbicidal activities.[Citation32,Citation33] The antidiabetic effect of A. santolina L. ethanol–water extract was proposed due to its antioxidant effects.[Citation34] A. crithmifolia Friv. ex Hampe and A. nobilis subsp. neilrechii, A. millefolium subsp. pannonica, A. teretifolia, and A. nobilis subsp. sipylea showed the highest activities on catalase, on superoxide dismutase, on glutation peroxidase, and on lipid peroxidation enzyme systems of leucocytes, respectively. The infusions of Achillea species can be considered as a potential source of effective antioxidants which can prevent the diseases in which lipid peroxidation takes contribution.[Citation24] Antioxidants derived from plants struggle against oxidative stress in the living organism via supporting a balance between oxidants and antioxidants.[Citation35] Today, most of processed foods contain synthetic antioxidant additives for preservation of foods from oxidative decomposition. Actually they are reported to be safe. However, synthetic additives in foods may be harmful with unwanted consequences to our health (cancer, allergy etc.), especially in well-nourished populations.[Citation36] So, there is increasing demand in safe, cheap, and effective antioxidants derived from natural sources with minimum side effects. Therefore, we subjected EOs and the extracts of A. sivasica to investigation in different antioxidant activity tests.
Tyrosinase is the key enzyme in the production of melanin.[Citation37] The central role of tyrosinase in dopamine neurotoxicity as well as in contribution to the neurodegenerative Parkinson’s disease is well-documented.[Citation38] Tyrosinase belongs to copper-containing enzymes that catalyse the hydroxylation of tyrosine to 3,4-dihydroxyl-L-phenilalanin (DOPA) by monophenolase action and oxidation of DOPA to DOPA-quinone which may polymerize to form high-molecular-weight compounds or brown pigments.[Citation39] Also, tyrosinase is responsible for the colour change such as browning of fruits and vegetables. In the literature, there is a report that A. millefolium EO suppressed melanin production by decreasing tyrosinase activity.[Citation40] Therefore, we attempted to test A. sivasica herb, flower, and leaf EOs as well as the extracts towards tyrosinase from mushroom.
Almost no published information about the chemistry and biological activity of A. sivasica is available, despite the local importance of many Achillea species in the traditional health care system of Turkey. Wide biological activities of Achillea species prompted us to investigate A. sivasica for different biological activities. Literature survey revealed only botanical description of endemic A. sivasica which was reported by Çelik & Akpulat.[Citation1] The main goal of the present study is to give a first detailed phytochemical and biological characterization of A. sivasica.
Materials and methods
Reagents
DOPA, Folin–Ciocalteu phenol reagent (FCR), β-carotene, quercetin, butylated hydroxytoluene (BHT), gallic acid (GA), neocouproine (Nc), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ammonium acetate, copper chloride, kojic acid, and tyrosinase from mushroom (EC 1.14.18.1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All solvents were purchased from Sigma Aldrich (Germany) and were of analytical grade.
Plant material
Plant materials (herb, flower, and leaf) of A. sivasica were collected in Sivas: Kangal to Gürün, 8 km, calcareous area, 39°07ˊ56.3ˊˊN, 37°13ˊ48.0ˊˊE, June 5, 2015. The herb was dried under the shade. Botanical identification was performed by Dr. M. Tekin (Cumhuriyet University). Voucher specimen is kept in the Herbarium of Cumhuriyet University, Faculty of Science (CUFH) under herbarium code M. Tekin 1570.
EO isolation
A. sivasica herb, flowers, and leaves were separately subjected to hydrodistillation (3 h) in Clevenger-type apparatus to yield EO.[Citation41] The EOs were stored in conditions reported previously.[Citation42]
Preparation of extracts
A. sivasica herb, flowers, and leaves were powdered and separately subjected to maceration in ethyl acetate, methanol, and water. Extraction of the plant material was performed at room temperature by maceration of the ground drug in the solvent (drug /solvent ratio 1:10) for 24 h with continuous shaking. The ethyl acetate and methanol extracts were dried under vacuum. The aqueous extracts were lyophilized.
Gas chromatographic analyses
The gas chromatography/mass spectrometry (GC/MS) and GC-FID analyses were carried out with an Agilent 5975 GC-MSD and Agilent 6890N GC systems (Agilent, USA; SEM Ltd., Istanbul, Turkey) equipped with the HP-Innowax FSC column (60 m × 0.25 mm id with 0.25 μm film thickness, Agilent, USA) in conditions reported earlier.[Citation42] Identification of the individual compounds was performed as reported previously.[Citation42]
Liquid chromatography-mass spectrometry/mass spectrometry analysis
Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analysis was carried out using an Absciex 3200 Q trap MS/MS detector. Experiments were performed by using LC-MS/MS (20A HPLC system, Shimadzu) coupled to an Applied Biosystems 3200 Q-Trap instrument equipped with an ESI source operating in negative ion mode. GL Science Intersil ODS 250 × 4.6 mm, i.d., 5 µm particle size, and octadecyl silica gel analytical column operating at 40°C have been used for the chromatographic separation. The solvent flow rate was maintained at 0.7 mL/min. Detection was carried out with PDA (photodiode array) detector. The elution gradient consisted of mobile phases (A) acetonitrile:water:formic acid (10:89:1, v/v/v) and (B) acetonitrile:water:formic acid (89:10:1, v/v/v). The composition of B was increased from 10% to 100% in 40 min. Liquid Chromatography- Electrospray Ionization-tandem Mass Spectrometry (LC-ESI-MS/MS) data were collected and processed by Analyst 1.6 software. For enhanced mass scan (EMS), the MS was operated at a mass range of 100–1000 amu. Enhanced product ion spectra were measured from m/z 100 up to m/z 1000. Nitrogen was used as the collision gas, and the collision energy was set at 30. The parameters were as follows: collusion energy spread (CES)-0, declustering potential (DP)-20, entrance potential (EP)-10, curtain gas (CUR)-20, gas source 1 (GS1)-50, gas source 2 (GS2)-50, CAD-medium, Ihe-on, and temperature (TEM)-600. For the Information-Dependent Acquisition (IDA) experiment, the criteria were arranged for ions greater than 100,000 m/z and smaller than 1000 m/z and excluded former target ions after 3.0 occurrence(s) for 3000 s.
Determination of total phenol content
Total phenolic contents of the extracts were determined with FCR by employing the method of Singleton et al.[Citation43] The results were calculated as the average values of gallic acid equivalent (GAE) with standard error mean (SEM). The calculations were performed with the equation y = 1.6575x − 0.0013 (rCitation2 = 0.9986). Analyses were performed in triplicate. Experimental details are available as supplementary material in Section S1.
Determination of total flavonoid content
The total flavonoid content of the extracts was determined spectrophotometrically according to Miliauskas et al.[Citation44] Calculations were performed with the equation y = 1.9673x − 0.0236 (rCitation2 = 0.9997). Analytical characteristic for the applied method are reported as supplementary material in Section S2.
Free radical scavenging activity assay (DPPH test)
The free radical scavenging abilities of A. sivasica EOs and the extracts were measured from the bleaching of DPPH (purple-coloured stable radical) using the method of Brand-Willam et al.[Citation45] with slight modifications. The DPPH solution (0.08 mg/mL, in methanol) was prepared daily and stored in the dark at 4°C. The stock solutions of the EOs (30 mg/mL), the extracts (10 mg/mL), and GA (0.1 mg/mL) were prepared in methanol. 100 μL of the sample (EO/extract/standard) solution and 100 μL DPPH solution were placed by multichannel automatic pipette (Eppendorf Research® plus, Germany) into 96-flat bottom-well plate cells and incubated in the dark for 30 min. The control well contained 100 μL methanol (instead of the sample) mixed with 100 μL of DPPH. The absorbance was recorded at 517 nm. GA was used as positive control. The free radical scavenging activity of the samples was expressed as the percentage of inhibition calculated according to the equation:
where Abscontrol is the absorbance of the control (containing all reagents except the test compound) and Abssample is the absorbance of the sample with added DPPH. The IC50 values were obtained by plotting the DPPH scavenging percentage of each sample against the sample concentration. Data were analysed using the SigmaPlot software (Version 12.0).
Cuprac reducing capacity (CUPRAC) assay
The cupric ion-reducing capacity of EOs and the extracts was determined according to the method of Apak et al.[Citation46] with slight modifications. The EOs and the extract solutions were prepared (30 mg/mL and 10 mg/mL, respectively) in methanol. CuCl2 solution (1.0 × 10−Citation2 M) and ammonium acetate buffer (1.0 M, pH 7.0) were prepared in ultrapure water. Neocuproine (Nc) solution (7.5 × 10−Citation3 M) was prepared in absolute ethanol. Each well contained 55 μL sample solution (EO/extract/standard), 50 μL CuCl2 solution, 50 μL neocuproine solution, and 50 μL NH4Ac buffer. The control well contained the same reagents except the sample (methanol was added). After incubation at 25°C for 30 min, the absorbance at 450 nm of wells was measured using an ELISA microplate reader (Biotek Powerwave XS). Cuprac reducing capacity of EOs and the extracts was expressed as Trolox equivalents (TE) and in mM/g of extract which were obtained from the following standard curves of Trolox: y = 2.1358x + 0.2658 (rCitation2 = 0.999), where y represents the absorbance and x represents the cupric reducing capacity in TE mM/mL. The results are expressed as average TE values with SEM. The analytical characteristics for the applied method are reported as supplementary material in Section S3.
Β-carotene/linoleic acid peroxidation inhibition assay
β-Carotene bleaching test involves hydrogen atom transfer reactions. Here, linoleic acid is the model lipid substrate used in an emulsified form. The assay lies in between methods employing only model substrates (e.g., DPPH) and those using real lipids.[Citation47] Inhibition of lipid peroxidation by A. sivasica EOs and the extracts was measured according to method of Marco[Citation48] with slight modifications. Briefly, β-carotene (5 mg) dissolved in chloroform (5 mL) was added to a flask containing linoleic acid (120 mg) and Tween 20 (1200 mg). The contents of flask were vigorously shaken before chloroform was evaporated under the vacuum. After evaporation, 300 mL of pure water was added and shaken vigorously. BHT (1 mg/mL) was used as the reference antioxidant. 10 μL sample (EO (10 mg/mL)/extract (10 mg/mL)/standard) and 2 mL of β-carotene emulsion were mixed in the deep-well plate. After that, 300 μL of the mixture was placed by 8-channel automatic pipette (Eppendorf Research® plus, Germany) into a 96-well microplate and incubated at 50°C for 2 h. Control was prepared without sample or standards according the same procedure. The rate of β-carotene bleaching was monitored by measuring the absorbance at 15 min periods at 470 nm sing an ELISA microplate reader (Biotek Powerwave XS).[Citation48,Citation49] Analyses were run in triplicate, and the results were expressed as the average of inhibition percentage values (% ±SEM) calculated according to the equation:
where AA is the antioxidant activity, Abs0sample and Abs120sample are the absorbances of the sample at 0 min and 120 min, Abs0control and Abs120control are the absorbances of the control at 0 min and 120 min.
Tyrosinase inhibition assay
Tyrosinase inhibitory activity of A. sivasica EOs and the extracts was assessed using the modified 96-well microplate method reported by Masuda et al.[Citation50] The solutions of EOs and the extracts (10 mg/mL) were prepared in phosphate buffer (pH 6.8). Dimethyl sulfoxide (DMSO) was added for insoluble samples. The experiment was designated as follows: eight wells were used, A (three wells, control), B (one well, blank), C (three wells, sample), and D (one well, blank), which contained the following reaction mixtures: A, 120 μL of a 0.1 M phosphate buffer (pH 6.8) and 40 μL of tyrosinase (33.3 U/mL) in the same buffer; B, 160 μL of the same buffer; C, 80 μL of the same buffer, 40 μL of tyrosinase (33.3 U/mL) in the same buffer, 40 μL of the sample–buffer solution containing DMSO; D, 120 μL of the same buffer and 40 μL of the same amount of the sample solutions containing DMSO. Pipetting was performed with 8-channel automatic pipette (Eppendorf Research® plus, Germany). As a positive control experiment, kojic acid (0.01–0.1 mg/mL in buffer) was used. Quercetin (0.1 mg/mL in buffer) was used as the reference. The contents of each well were mixed and then preincubated at 23°C for 10 min, before 40 μL L-DOPA (2.5 mM) in the same buffer was added. After incubation at 23°C for 15 min, the absorbance at 475 nm of wells was measured using an ELISA microplate reader (Biotek Powerwave XS). The percentage inhibition of the tyrosinase activity (Inh %) was calculated according to the following equation:
Antityrosinase activity of the EOs and the extracts was also expressed as kojic acid equivalents (KAE) in mg/g of extract or EO. Kojic acid dilutions in methanol were prepared to get a calibration curve. Calculations were performed with the equation y = 1014.1x + 11.798 (rCitation2 = 0.9958), where y represents the % inhibition and x represents the concentration in mg/mL. Analyses were run in triplicate and the results were expressed as average values of KAE with SEM. The analytical characteristics for the applied method are reported as supplementary material in Section S4.
Results and discussion
Phytochemical analysis
According to WHO publications, an approximately 80% of the world’s population from developing countries use plant-derived medicines for their primary health care. The remaining 20% of people consider the herbal medicines as alternative approach for health care purposes.[Citation51] Achillea species have an important place among those types of medicines. The biological activity of EOs and polyphenols of Achillea species is well-documented.[Citation24,Citation52–Citation54]
The present work aimed to get further knowledge on phytochemical and biological properties of the EOs and the extracts of endemic A. sivasica species from Turkey. Hydrodistillation of the herb, flowers, and leaves of A. sivasica resulted in greenish oils with specific odour. The extracts with different polarity solvents (methanol, ethyl acetate, and aqueous) were obtained from the herb, flower, and leaves. The EOs and the extract yields are presented in .
Table 1. Essential oils and extract yields of Achillea sivasica.
The GC-FID and GC/MS techniques allow us to determine the qualitative and quantitative profiles of volatiles from the herb, flowers, and leaves of A. sivasica. The list of detected compounds with their relative percentages, relative retention indices (RRIs), and method of identification is given in . The EOs of A. sivasica were characterized with high diversity of volatile constituents classified as mono- and sesquiterpene hydrocarbons and their oxygenated forms. The oxygenated monoterpenes were the most abundant among other groups in the herb, flower, and leaf of A. sivasica EOs representing 33.6%, 41.0%, and 24.2%, respectively. 1,8-Cineole (18.0%, 22.1%. and 6.7%, respectively) and camphor (7.6%, 4.1%, and 9.0%, resp.) were found to be the major constituents of this group. It was followed by the monoterpene hydrocarbons which were detected in the herb, flower, and leaf EOs (23.4%, 15.6%. and 17.9%, respectively) with β-pinene (11.5%, 9.3%, and 6.7%, respectively) and β-pinene (7.0%, 3.0%, and 6.7%, respectively) as major constituents. Oxygenated sesquiterpenes comprised the next noteworthy group in the herb, flower, and leaf EOs (15.9%, 12.9%, and 16.9%, respectively) with a-bisabolol (7.5%, 4.3%, and 6.6%, respectively) as major representative. On the other hand, the sesquiterpene hydrocarbons in the herb, flower, and leaf EOs were found as 9.6%, 8.8%, and 2.7%, respectively, with germacrene D as the main compound (4.8%, 3.5%, and 2.0%). The distribution of major compound classes in A. sivasica EOs is presented in . Only the compounds with the relative amounts higher than 0.3% were listed in . Representative chromatogram of the EO of A. sivasica is presented as supplementary material in Section S5.
Table 2. Chemical composition of Achillea sivasica essential oils obtained from the herb, flower, and leaves.
Figure 1. The distribution of major compound classes in Achillea sivasica herb, flower, and leaf essential oils. OM: oxygenated monoterpenes; MH: monoterpene hydrocarbons; OM: oxygenated sesquiterpenes; SH: sesquiterpene hydrocarbons.
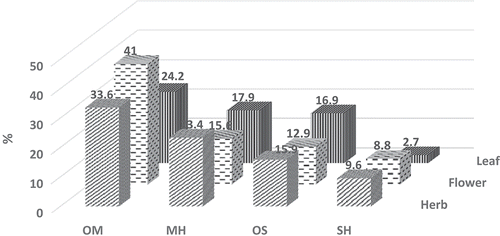
The composition of A. sivasica EO was compared with Achillea species reported earlier. A comparative study revealed that A. sivasica contains several common compounds detected in Achillea species, namely β- and β-pinene, 1,8-cineole, and camphor. A high percentage of β-pinene has earlier been reported for A. odorata L. var. microphylla (Willd.) Willk.,[Citation55] while β-pinene was the main constituent of A. millefolium L. and A. sintenisii Hub.-Mor. oils.[Citation56,Citation57] The oxygenated monoterpenes, 1,8-cineole, and camphor were detected in a number of Achillea oils.[Citation32,Citation52] However, distribution of β-bisabolol was not so common in Achillea oils: A. millefolium ssp. millefolium[Citation58] and A. barrero.[Citation59]
The data about the total phenol contents in A. sivasica extracts are summarized in . The flower (51.1 mg GAEs/g extract) and the leaf (54.7 mg GAEs/g extract) extracts of A. sivasica prepared with methanol had the highest polyphenol contents. The lowest amount of the total phenols and flavonoids was determined in the aqueous extracts. The total phenolic content in the extracts of A. sivasica was determined spectrophotometrically by using FCR. Phenolic compounds react with FCR under basic conditions (pH = 10). In this condition, the phenolic proton dissociates to a phenolate anion, which can reduce FCR.[Citation60] Of course, this method does not give information about nature of phenolic compounds. Although, the results can give tips about the potential of A. sivasica as a possible source of antioxidants.
Table 3. The total phenolic and flavonoid contents in the solvent extracts of Achillea sivasica (mean ± SD).
Total flavonoids in A. sivasica extracts were determined using spectrophotometric method with AlCl3 and the obtained results varied from 3.7 to 31.7 QE mg/g extract. All methanol extracts, herb (31.7 QE mg/g extract) > leaf (31.4 QE mg/g extract) > flower (27.1 QE mg/g extract) possessed highest content of flavonoid compared to ethyl acetate and aqueous extracts ().
Polyphenolic compounds are widespread virtually in many plants and include phenols, phenolic acids, flavonoids, tannins, and lignans.[Citation61] It has recently been proved that herbal flavonoids are absorbed at an extent that may promote an antioxidant effect. They can act as reducing agents, donators of hydrogen, quenchers of singlet oxygen, scavengers of free radicals, and chelators of metals.[Citation35] Both the digested flavonoids and their metabolites may display an in vivo antioxidant activity, which may involve the physiological antioxidants (β-tocopherol and β-carotene).[Citation62] The Folin–Ciocalteu method and aluminium chloride colorimetric method for determination of the total content of phenols and flavonoids, respectively, are rapid and reliable assays, giving basic information about the amounts of phytochemicals. However, these methods do not give a full picture concerning the qualification of phenolic compounds in complex samples. Therefore, the phenolic profile of MeOH, EtOAc, and aqueous extracts of A. sivasica herb were further assessed by LC-MS/MS, and the results are presented in . As can be seen, phenols comprised of flavonoids and phenolic acids, namely 4,5-dicaffeoylquinic acid, 3-caffeoylquinic acid, 5-caffeoylquinic acid, luteolin, apigenin, patuletin, isorhamnetin, hispidulin glucosides, cirsimaritin, and santin. This is the first contribution into the chemical composition of phenolic compounds of A. sivasica. Seventeen phenolic compounds were identified according to mass spectral data.
Table 4. Mass spectral data and putative identification of Achillea sivasica herb extracts components.
The chemical profile of non-volatile constituents detected in A. sivasica was compared with that reported earlier for Achillea species. Flavonoid’s chemical profile was found to be close to that of A. millefolium,[Citation16] A. conferta DC,[Citation63] A. nobilis L.,[Citation64] and A. ageratum.[Citation65] Cafeoylquinic acid derivatives have earlier been characterized in A. collina Becker ex Rchb. [Citation66] and A. millefolium,[Citation67]
Antioxidant activity
A comprehensive review about the multifaceted aspects of antioxidants and the basic kinetic models of inhibited oxidation and the chemical mechanisms of antioxidant capacity assays has recently been published by Huang et al.[Citation60] Among the antioxidant activity measuring tests, DPPH free radical scavenging assay is the most extensive applied one. Foti and co-workers suggested that the reaction between antioxidant compounds and DPPH radicals behaves like an electron transfer reaction.[Citation68] It has earlier been argumented that antioxidants have the ability to act as free radical scavengers (DPPH• and ABTS+• scavenging assays),[Citation69] as reducing agents (cupric ions),[Citation70] or as hydrogen atom donators (inhibition of linoleic acid oxidation).[Citation71] The evaluation of antioxidant capacity of some compounds in EOs and extracts has been the subject of a number studies.[Citation72,Citation73] However, antioxidant potentials of plant products cannot be carried out accurately by only one method.[Citation60] Therefore, we applied several antioxidant assays that would provide a better insight into the true antioxidant potential of EOs and the extracts of A. sivasica. Namely, total phenolic and total flavonoid contents, free radical scavenging activities (DPPH• and ABTS+•), inhibition of lipid peroxidation (β-carotene bleaching assay), and cupric reducing antioxidant capacity (CUPRAC) were carried out.
The leaf and the herb methanol extracts of A. sivasica demonstrated the strongest free radical scavenging activity with IC50 values 0.12 μg/mL and 0.22 μg/mL, respectively (). All the ethyl acetate extracts and EOs were found to be ineffective towards DPPH free radicals. The aqueous extracts displayed relatively lower antioxidant activity in comparison to methanol extracts (). The CUPRAC assay allowed us to measure the total antioxidant potential of EOs and the extracts of A. sivasica. This method is based on the reduction of Cu(II) to Cu(I) by antioxidants present in the sample. A chromogenic reagent, neocuproine (2,9-dimethyl-1,10-phenanthroline), forms a complex with Cu(I), which has a maximum absorbance at 450 nm.[Citation46] Cupric reducing capacity values obtained for A. sivasica varied from 0.22 to 0.94 mM TE (). The leaf methanol extract (0.94 mM TE) and the herb EO demonstrated highest reducing capacity (0.81 mM TE). However, the aqueous extracts showed the lowest reducing capacity values (0.22–0.31 mM TE) ().
Table 5. Biological activities of the essential oils and extracts of Achillea sivasica (mean ± SD).
β-Carotene bleaching assay allowed us to measure the inhibition capacity of antioxidants in protecting the bleaching of β-carotene, a naturally occurring carotenoid, by the hydroperoxides formed due to oxidation of linoleic acid. In this system, the highest inhibition values of linoleic acid oxidation were estimated as 53.9% and 55.8% in the presence of the leaf methanol and ethyl acetate extracts, respectively while for BHT this value was 85.4%. The herb and the flower methanol extracts displayed lower inhibitory activity (49.9% and 41.5%, resp.). All the EOs and the aqueous extracts of A. sivasica were found to be weak inhibitors of lipid peroxidation (). As far as our literature survey could ascertain, there is no previous study on the antioxidant potential of A. sivasica.
Evaluation of antioxidant potent of the tested A. sivasica extracts should be performed by taking into consideration the total phenol and flavonoid contents. As can be seen from , the highest content of phenols was measured in the leaf methanol extract (54.7 ± 2.2 GAE). The highest flavonoid content was detected in the herb (31.7 ± 1.0) and the leaf (31.4 ± 3.9 QE). It is known that the antioxidant potential of natural products is related with phenolic compound content and profile. Also, it may depend on the synergistic actions between EO and phenolic constituents.[Citation74] So, high antioxidant potential of the leaf methanol extract may be supposed to be due to phenols and EO which is extracted with methanol too.
Tyrosinase inhibition activity
Antityrosinase assay is based on inhibition of tyrosinase enzyme from mushroom. Tyrosinase is known to be a key enzyme in melanin biosynthesis as well as in dermatological disorders (age spots, melanoma, and freckles) which arise from excessive accumulation of melanin. Therefore, inhibitors of tyrosinase have become increasingly important for treatment of skin disorders. In our experiments, all the methanol extracts and the flower and the leaf ethyl acetate extracts of A. sivasica demonstrated tyrosinase inhibitory activity which varied from 2.9 to 6.2 mg KAE/g extract. The most active extracts were found to be leaf methanol and ethyl acetate extracts (5.5 mg KAE/g extract and 6.2 mg KAE/g extract, respectively). However, EOs and the aqueous extracts were not effective towards this enzyme (). According to antityrosinase activity values, the leaf methanol and ethyl acetate extracts of A. sivasica might be considered as sources of phytochemicals effective against neurodegenerative (Parkinson’s disease) and skin (melanogenesis) diseases related with activity of tyrosinase. Nowadays, natural inhibitors of tyrosinase are in demand for food industry. It is known that unfavourable enzymatic browning of plant-derived foods by tyrosinase causes a decrease in nutritional quality and economic loss of food products.[Citation75]
Conclusion
The present work is the first investigation of A. sivasica EOs and extracts obtained with different solvents from different plant parts. We carried out phytochemical investigations using GC-FID, GC/MS, LC-MS/MS, and spectrophotometric techniques. Based on the biological activity assays, antioxidant and antityrosinase properties of A. sivasica were revealed. The EOs of A. sivasica oils obtained from the herb, flower, and leaves can be explored as a rich source of valuable volatile constituents such as β-pinene, β-pinene, 1,8-cineole camphor, and β-bisabolol. The extracts of A. sivasica were not rich in polyphenols/flavonoids. However, the activity of natural products is much more related to synergy than to richness of certain groups. In this point of view, the polyvalence effect of numerous secondary constituents, such as polyphenols and terpenoids, must be taken into account.[Citation76] The highest total phenolic and flavonoid contents were determined in the leaf methanol extract. Among the tested extracts for antioxidant and enzyme inhibition activities, methanol extracts of the leaf also demonstrated the highest values. In this context, it should be noted that methanol is the solvent which is able to extract not only polyphenols (hydrophilic fraction), but also terpenoids (lipophilic fraction) from the plant material. In the literature, the polyvalence effect of secondary constituents, such as polyphenols and terpenoids, was reported.[Citation74] Since MeOH extracts of A. sivasica are rich in these two groups of constituents, these compounds can strongly enhance the overall efficacy of these extracts. Probably, lower biological potent of the aqueous extracts, which contain mostly hydrophilic compounds and are poor in lipophilic fraction, can be attributed to the lacking of such type of synergism.
Finally, A. sivasica could be considered as a natural source of active constituents for food supplements and therapeutic applications.
LJFP_A_1308954_Supplementary_data.docx
Download MS Word (80.3 KB)Funding
The authors are gratefully thanks to Anadolu University Scientific Research Department for supporting this research project (BAP №1504S163). All authors of the manuscript declare that they do not have financial/commercial conflicts of interest.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Çelik, N.; Akpulat, A. Achillea sivasica (Asteraceae: Sect. Babounya (DC.) O. Hoffm.), a New Species from Inner Anatolia, Turkey. The Board of Trustees of the Royal Botanic Gardens, Kew 2008, 63, 485–489.
- Fujita, T.; Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional Medicine in Turkey VII. Folk Medicine in Middle and West Black Sea Regions. Economic Botany 1995, 49(4), 406–422.
- Honda, G.; Yesilada, E.; Tabata, M.; Sezik, E.; Fujita, T.; Takeda, Y.; Tanaka, T. Traditional Medicine in Turkey VI. Folk Medicine in West Anatolia: Afyon, Kütahya, Denizli, Mugla, Aydın Provinces. Journal of Etnopharmacology 1996, 5m, 75–87.
- Sezik, E.; Yesilada, E.; Tabata, M.; Honda, G.; Takaishi, Y.; Fujita, T.; Tanaka, T.; Yoshio, T. Traditional Medicine in Turkey. VIII. Folk Medicine in East Anatolia; Erzurum, Erzican, Agri, Kars, Igdir Province. Economic Botany 1997, 51, 195–211.
- Rohloff, J.; Skagen, E.B.; Steen, A.H.; Iversen, T.-H. Production of Yarrow (Achillea millefolium L.) in Norway: Essential Oil Content and Quality. Journal of Agricultural and Food Chemistry 2000, 48(12), 6205–6209.
- Amin, G.; Sourmaghi, M.S.; Azizzadeh, M.; Yassa, N.; Asgari, T. Seasonal Variation of the Essential Oil Composition of Cultivated Yarrow in Tehran-Iran. Journal of Essential Oil Bearing Plants 2008, 11(6), 628–633.
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia; CRC Press, Taylor & Francis Group, Boca Raton: Florida, USA, 2011.
- Association, B.H.M.;. British Herbal Pharmacopoeia, British Herbal Medicine Association, Bournemouth, Dunfermline, United Kingdom, 1996.
- European; Pharmacopoeia, Yarrow, 5th ed.; EDQM, Council of Europe, Strasbourg: France, 2005.
- German Homoeopathic Pharmacopoeia; Medpharm GmbH Scientific Publishers, Birkenwaldstrasse 44, 70191, Stuttgart, Germany. 2003.
- WHO Monographs on Selected Medicinal Plants, 2009; 456 p.
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical Constituents of the Plants in the Genus Achillea. Chem Biodivers 2006, 3(11), 1163–1180.
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A Review on Phytochemistry and Medicinal Properties of the Genus Achillea. Daru 2011, 19(3), 173–186.
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical Analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands). Natural Product Research 2016, 30(8), 912–919.
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MS Identification of Chlorogenic Acids. Journal of Agricultural and Food Chemistry 2003, 51, 2900–2911.
- Dias, M.I.; Barros, L.; Duenas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.; Santos-Buelga, C.; Ferreira, I. Chemical Composition of Wild and Commercial Achillea millefolium L. and Bioactivity of the Methanolic Extract, Infusion and Decoction. Food Chem 2013, 141(4), 4152–4160.
- Ahmed, A.A.; Mahmoud, A.A.; Ali, E.T.; Tzakou, O.; Couladis, M.; Mabry, T.J.; Gáti, T.; Tóth, G. Two Highly Oxygenated Eudesmanes and 10 Lignans from Achillea holosericea. Phytochemistry 2002, 59(8), 851–856.
- Awad, B.; Ibrahim, A.K.; Sand, E.; Wanas, A.S.; Radwan, M.M.; Elsohaly, M.; Ahmed, S.A. Anticancer Metabolites from Achillea fragrantissima. Planta Medica 2015, 81(05), PC13.
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; El-Desoky, A.H.; Soliman, G.A.; Pare, P.W.; Hegazy, M.E.F. Evaluation of the Anti-Inflammatory, Analgesic and Anti-Ulcerogenic Potentials of Achillea fragrantissima (Forssk.). S African Journal Botanic 2015, 98, 122–127.
- Ertas, A.; Boga, M.; Hasimi, N.; Yesil, Y.; Goren, A.C.; Topcu, G.; Kolak, U. Antioxidant, Anticholinesterase, and Antimicrobial Activities and Fatty Acid Constituents of Achillea cappadocica Hausskn. Et Bornm. Turkish Journal of Chemistry 2014, 38(4), 592–599.
- Demirci, B.; Gurbuz, I.; Yeslada, E.; Baser, K.H.C. Characterization and Biological Activity of Achillea teretifolia Willd. and A. nobilis L. Subsp. neilreichii (Kerner) Formanek Essential Oils. Turkish Journal of Biology 2009, 33(2), 129–136.
- Akcin, A.; Akcin, T.A.; Seyis, F.; Coban, A.Y.; Durupinar, B. Antimicrobial and Antioxidant Activity of the Essential Oil of the Turkish Endemic Species Achillea phrygia Boiss. & Bal. Journal of Essential Oil Bearing Plants 2014, 17(2), 219–227.
- Potrich, F.B.; Allemand, A.; Da Silva, L.M.; Dos Santos, A.C.; Baggio, C.H.; Freitas, C.S.; Mendes, D.A.G.B.; Andre, E.; Werner, M.F.D.; Marques, M.C.A. Antiulcerogenic Activity of Hydroalcoholic Extract of Achillea millefolium L.: Involvement of the Antioxidant System. Journal of Ethnopharmacology 2010, 130(1), 85–92.
- Konyalioglu, S.; Karamenderes, C. The Protective Effects of Achillea L. Species Native in Turkey against H2O2-Induced Oxidative Damage in Human Erythrocytes and Leucocytes. Journal of Ethnopharmacology 2005, 102(2), 221–227.
- Kazemi, M.;. Gas Chromatography-Mass Spectrometry Analyses for Detection and Identification of Antioxidant Constituents of Achillea tenuifolia Essential Oil. International Journal of Food Properties 2015, 18(9), 1936–1941.
- Bozin, B.; Mimica-Dukic, N.; Bogavac, M.; Suvajdzic, L.; Simin, N.; Samojlik, I.; Couladis, M. Chemical Composition, Antioxidant and Antibacterial Properties of Achillea collina Becker Ex Heimerl Sl and A. pannonica Scheele Essential Oils. Molecules 2008, 13(9), 2058–2068.
- Bariş, Ö.; Gulluce, M.; Sahin, F.; Ozer, H.; Kilic, H.; Ozkan, H.; Sokmen, M.; Ozbek, T. Biological Activities of the Essential Oil and Methanol Extract of Achillea biebersteinii Afan. (Asteraceae). Turkish Journal of Biology 2006, 30(2), 9.
- Senatore, F.; Napolitano, F.; Arnold, N.A.; Bruno, M.; Herz, W. Composition and Antimicrobial Activity of the Essential Oil of Achillea falcata L. (Asteraceae). Flavour and Fragrance Journal 2005, 20(3), 291–294.
- Maggi, F.; Bramucci, M.; Cecchini, C.; Coman, M.M.; Cresci, A.; Cristalli, G.; Lupidi, G.; Papa, F.; Quassinti, L.; Sagratini, G.; Vittori, S. Composition and Biological Activity of Essential Oil of Achillea ligustica All. (Asteraceae) Naturalized in Central Italy: Ideal Candidate for Anti-Cariogenic Formulations. Fitoterapia 2009, 80(6), 313–319.
- Magiatis, P.; Skaltsounis, A.L.; Chinou, I.; Haroutounian, S.A. Chemical Composition and In-Vitro Antimicrobial Activity of the Essential Oils of Three Greek Achillea Species. Zeitschrift Fur Naturforschung C-A Journal of Biosciences 2002, 57(3–4), 287–290.
- Dadkhah, A.; Fatemi, F.; Alipour, M.; Ghaderi, Z.; Zolfaghari, F.; Razdan, F. Protective Effects of Iranian Achillea wilhelmsii Essential Oil on Acetaminophen-Induced Oxidative Stress in Rat Liver. Pharmaceutical Biology 2015, 53(2), 220–227.
- Polatoglu, K.; Karakoc, O.C.; Goren, N. Phytotoxic, DPPH Scavenging, Insecticidal Activities and Essential Oil Composition of Achillea vermicularis, A. teretifolia and Proposed Chemotypes of A. biebersteinii (Asteraceae). Industrial Crops and Products 2013, 51, 35–45.
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and Herbicidal Properties of Essential Oils and N-Hexane Extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Industrial Crops and Products 2009, 29(2), 562–570.
- Yazdanparast, R.; Ardestani, A.; Jamshidi, S. Experimental Diabetes Treated with Achillea santolina: Effect on Pancreatic Oxidative Parameters. Journal of Ethnopharmacology 2007, 112(1), 13–18.
- Carocho, M.; Ferreira, I.C. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food and Chemical Toxicology 2013, 51, 15–25.
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant Supplements and Mortality. Current Opinion in Clinical Nutrition & Metabolic Care 2014, 17(1), 40–44.
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V. A Second Tyrosinase-Related Protein, TRP-2, Is A Melanogenic Enzyme Termed Dopachrome Tautomerase. The EMBO Journal 1992, 11(2), 519–526.
- Xu, Y.; Stokes, A.H.; Freeman, W.M.; Kumer, S.C.; Vogt, B.A.; Vrana, K.E. Tyrosine Mrna Is Expressed in Human Substantia Nigra. Molecular Brain Research 1997, 45(1), 159–162.
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M. Novel Roles for the Polyphenol Oxidase Enzyme in Secondary Metabolism and the Regulation of Cell Death in Walnut. Plant Physiology 2014, 164(3), 1191–1203.
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The Melanogenesis Alteration Effects of Achillea millefolium L. Essential Oil and Linalyl Acetate: Involvement of Oxidative Stress and the JNK and ERK Signaling Pathways in Melanoma Cells. PLoS ONE 2014, 9(4), e95186. https://doi.org/10.1371/journal.pone.0095186.
- EDQM. Determination of Essential Oils in Vegetable Drugs, European Directorate for the Quality of Medicines, Council of Europe F-67075 Strasbourg Cedex, France, 2005.
- Schepetkin, I.A.; Kushnarenko, S.V.; ÖZek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.C.; Kovrizhina, A.R. Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents. Journal of Agricultural and Food Chemistry 2016, 64(38), 7156–7170.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chemistry 2004, 85(2), 231–237.
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Science and Technology 1995, 28(1), 25–30.
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of Antioxidant Capacity Assays and the CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Assay. Microchim Acta 2008, 160, 413–419.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53(10), 4290–4302.
- Marco, G.J.;. A Rapid Method for Evaluation of Antioxidants. Journal of the American Oil Chemists’ Society 1968, 45(9), 594–598.
- Koşar, M.; Göger, F.; Başer, K.H.C. In Vitro Antioxidant Properties and Phenolic Composition of Salvia halophila Hedge from Turkey. Food Chemistry 2011, 129(2), 374–379.
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among Extracts of Seashore Plants and Identification of Potent Inhibitors from Garcinia subelliptica. Bioscience Biotechnology and Biochemistry 2005, 69(1), 197–201.
- Chivian, E. Biodiversity: Its Importance to Human Health; Center for Health and the Global Environment, Harvard Medical School: Cambridge, MA, 2002.
- Kotan, R.; Cakir, A.; Dadasoglu, F.; Aydin, T.; Cakmakci, R.; Ozer, H.; Kordali, S.; Mete, E.; Dikbas, N. Antibacterial Activities of Essential Oils and Extracts of Turkish Achillea, Satureja and Thymus Species against Plant Pathogenic Bacteria. Journal of the Science of Food and Agriculture 2010, 90(1), 145–160.
- Kundakovic, T.; Dobric, S.; Bokonjic, D.; Dragojevic-Simic, V.; Kilibarda, V.; Kovacevic, N. Anti-Inflammatory and Anti-Ulcer Activity of Achillea alexandri-regis. Pharmazie 2000, 55(11), 866–867.
- Kundakovic, T.; Dukic, N.M.; Kovacevic, N. Free Radical Scavenging Activity of Achillea alexandri-regis Extracts. Fitoterapia 2005, 76(6), 574–576.
- Bekhechi, C.; Bekkara, F.A.; Casanova, J.; Tomi, F. Composition and Antimicrobial Activity of the Essential Oil of Achillea odorata L. Subsp. pectinata (Lamk) Var. microphylla (Willd.) Willk. from Northwestern Algeria. Journal of Essential Oil Research 2011, 23(3), 42–46.
- Agnihotri, V.K.; Lattoo, S.K.; Thappa, R.K.; Kaul, P.; Qazi, G.N.; Dhar, A.K.; Saraf, A.; Kapahi, B.K.; Saxena, R.K.; Agarwal, S.G. Chemical Variability in the Essential Oil Components of Achillea millefolium Agg. from Different Himalayan Habitats (India). Planta Medica 2005, 71(3), 280–283.
- Sokmen, A.; Vardar-Unlu, G.; Polissiou, M.; Daferera, D.; Sokmen, M.; Donmez, E. Antimicrobial Activity of Essential Oil and Methanol Extracts of Achillea sintenisii Hub. Mor. (Asteraceae). Phytotherapy Research 2003, 17(9), 1005–1010.
- Afsharypuor, S.; Asgary, S.; Lockwood, G. Volatile Constituents of Achillea millefolium L. Ssp. millefolium from Iran. Flavour and Fragrance Journal 1996, 11(5), 265–267.
- Barrero, A.F.;. Bisabolene Derivatives and Other Constituents from Achillea odorata. Phytochemistry 1990, 29(10), 3213–3216.
- Huang, D.; Ou, B.; Prior, L. The Chemistry behind Antioxidant Capacity Assays. Journal Agricultural Food Chemistry 2005, 53, 1841–1856.
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annual Review of Nutrition 2002, 22(1), 19–34.
- Pietta, P.-G.;. Flavonoids as Antioxidants. Journal of Natural Products 2000, 63(7), 1035–1042.
- Saeidnia, S.; Yassa, N.; Gohari, A.R.; Shafiee, A. Isolation and Identification of Flavonoid Constituents from of Achillea conferta DC. Journal of Medicinal Plants 2005, 2(14), 12–20.
- Krenn, L.; Miron, A.; Pemp, E.; Petr, U.; Kopp, B. Flavonoids from Achillea nobilis L. Zeitschrift Fur Naturforschung. C, Journal of Biosciences 2003, 58(1–2), 11–16.
- Vieira, L.M.; Kijjoa, A.; Pereira, J.; Gedris, T.E.; Herz, W. Germacranes and Flavonoids from Achillea ageratum. Phytochemistry 1997, 45(1), 111–115.
- Giorgi, A.; Mingozzi, M.; Madeo, M.; Speranza, G.; Cocucci, M. Effect of Nitrogen Starvation on the Phenolic Metabolism and Antioxidant Properties of Yarrow (Achillea collina Becker Ex Rchb.). Food Chemistry 2009, 114(1), 204–211.
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic Compounds from Achillea millefolium L. and Their Bioactivity. Acta Biochimica Polonica 2011, 58(2), 203–209.
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH Radical in Alcoholic Solutions. The Journal of Organic Chemistry 2004, 69(7), 2309–2314.
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH Assay: A Critical Review and Results. Food Chemistry 2012, 130(4), 1036–1043.
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. Journal of Agricultural and Food Chemistry 2004, 52(26), 7970–7981.
- Sánchez-Moreno, C.;. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Science and Technology International 2002, 8(3), 121–137.
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America. Bioresource Technology 2010, 101(12), 4676–4689.
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant Activity of Chemical Components from Sage (Salvia officinalis L.) and Thyme (Thymus vulgaris L.) Measured by the Oil Stability Index Method. Journal of Agricultural and Food Chemistry 2002, 50(7), 1845–1851.
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16(2), 97–110.
- Kubo, I.; Chen, Q.-X.; Nihei, K.-I. Molecular Design of Antibrowning Agents: Antioxidative Tyrosinase Inhibitors. Food Chemistry 2003, 81(2), 241–247.
- Nemeth, E.; Bernath, J. Biological Activities of Yarrow Species (Achillea Spp.). Current Pharmaceutical Design 2008, 14(29), 3151–3167.
- Kaul, P.N.; Rajeswara Rao, B.R.; Singh, K.; Bhattacharya, A.K.; Mallavarapu, G.R.; Ramesh, S. Volatile Constituents of Essential Oils Isolated from Different Parts of Alpinia calcarata Rosc. Journal of Essential Oil Research 2005, 17(1), 7–9.
- Keefover-Ring, K.;. Making Scents of Defense: Do Fecal Shields and Herbivore-Caused Volatiles Match Host Plant Chemical Profiles? Chemoecology 2013, 23(1), 1–11.
- Noorizadeh, H.; Farmany, A.; Noorizadeh, M. Application of GA-PLS and GA-KPLS Calculations for the Prediction of the Retention Indices of Essential Oils. Química Nova 2011, 34(8), 1398–1404.
- Cavaleiro, C.; Pinto, E.; Goncalves, M.; Salgueiro, L. Antifungal Activity of Juniperus Essential Oils against Dermatophyte, Aspergillus and Candida Strains. Journal of Applied Microbiology 2006, 100(6), 1333–1338.
- Asikin, Y.; Fukunaga, H.; Yamano, Y.; Hou, D.X.; Maeda, G.; Wada, K. Effect of Cultivation Line and Peeling on Food Composition, Taste Characteristic, Aroma Profile, and Antioxidant Activity of Shiikuwasha (Citrus depressa Hayata) Juice. Journal of the Science of Food and Agriculture 2014, 94(12), 2384–2392.
- Maggio, A.; Rosselli, S.; Bruno, M.; Spadaro, V.; Raimondo, F.M.; Senatore, F. Chemical Composition of Essential Oil from Italian Populations of Artemisia alba Turra (Asteraceae). Molecules 2012, 17(9), 10232–10241.
- Saraccedil, H.T.; Akın, M.; Huuml, K.; Başer, C. Chemical Composition and Antibacterial Activity of Essential Oils from Different Parts of Some Bupleurum L. Species. African Journal of Microbiology Research 2012, 6(12), 2899–2908.
- Bignell, C.; Dunlop, P.; Brophy, J. Volatile Leaf Oils of Some South‐Western and Southern Australian Species of the Genus Eucalyptus (Series 1). Part XIX. Flavour and Fragrance Journal 1998, 13(2), 131–139.
- Brophy, J.J.; Goldsack, R.J.; Punruckvong, A.; Bean, A.R.; Forster, P.I.; Lepschi, B.J.; Doran, J.C.; Rozefelds, A.C. Leaf Essential Oils of the Genus Leptospermum (Myrtaceae) in Eastern Australia. Part 7. Leptospermum petersonii, L. Liversidgei and Allies. Flavour and Fragrance Journal 2000, 15(5), 342–351.
- Noorizadeh, H.; Farmany, A. Exploration of Linear and Nonlinear Modeling Techniques to Predict of Retention Index of Essential Oils. Journal of the Chinese Chemical Society 2010, 57(6), 1268–1277.
- Demirci, B.; Hüsnü Can Başer, K.; Yıldız, B.; Bahçecioǧlu, Z. Composition of the Essential Oils of Six Endemic Salvia Spp. from Turkey. Flavour and Fragrance Journal 2003, 18(2), 116–121.
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of Chemical Composition, Antimicrobial and Antioxidant Activities of Artemisia Essential Oils. Phytochemistry 2008, 69(8), 1732–1738.
- Viljoen, A.; Van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Başer, H.; Van Wyk, B.-E. Osmitopsis asteriscoides (Asteraceae)-The Antimicrobial Activity and Essential Oil Composition of a Cape-Dutch Remedy. Journal of Ethnopharmacology 2003, 88(2), 137–143.
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Characterisation of Phenolic Compounds in Wild Fruits from Northeastern Portugal. Food Chemistry 2013, 141(4), 3721–3730.
- Valant, K.; Besson, E.; Chopin, J. C-Glycosylflavones from the Genus Achillea. Phytochemistry 1978, 17(12), 2136–2137.
- Dall’Acqua, S.; Bolego, C.; Cignarella, A.; Gaion, R.M.; Innocenti, G. Vasoprotective Activity of Standardized Achillea millefolium Extract. Phytomedicine 2011, 18(12), 1031–1036.
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis Sl Restrain the Native European Flora? Journal of Ecology 2008, 96(5), 993–1001.
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, Characterization and Quantification of Phenolic Compounds in Blueberries and Red and Black Currants by HPLC− DAD− ESI-MS N. Journal of Agricultural and Food Chemistry 2011, 59(8), 4009–4018.
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC–Msn Analysis of the Cis Isomers of Chlorogenic Acids. Food Chemistry 2008, 106(1), 379–385.
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis Leaves as a Natural Source of Bioactive Compounds. International Journal of Molecular Sciences 2014, 15(11), 20585–20606.
- Tuberoso, C.I.G.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessi, M.A.; Pizza, C.; Cabras, P. Flavonoid Characterization and Antioxidant Activity of Hydroalcoholic Extracts from Achillea ligustica All. Journal of Pharmaceutical and Biomedical Analysis 2009, 50(3), 440–448.
