ABSTRACT
Heterocyclic aromatic amines (HCAs) are mutagenic compounds formed when foods are cooked at high temperatures. The objective of this study was to examine the efficiency of the grape seed extract (GSE) on the formation of HACs in beef and chicken meatballs cooked by four different cooking techniques (oven roasting, pan cooking, charcoal-barbecue, and deep-fat frying). Six HCAs; 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 9H-pyrido[3,4-b]indole (norharman), and 1-methyl-9H-pyrido[3,4-b]indole (harman) were measured. In addition, cooking losses, total phenolics, and radical-scavenging activities were determined. In the beef meatballs, the highest inhibitory effects were 69% for norharman, 65% for IQ, 65% for PhIP, and 59% for MeIQx, while in chicken meatballs were 73% for PhIP, 52% for IQ, 37% for MeIQx, and 31% for norharman. Results of this study suggested that addition of GSE can be an important factor in decreasing the levels of total HCAs in charcoal-barbecued beef meatballs (65%) and oven roasted chicken meatballs (37%).
Introduction
Our diet is highly associated with the occurrence of some diseases, such as cancer, hypertension, and heart attacks. The incidence of cancer also depends upon the style of the diet that we have. People cook meat to increase its safety and palatability; however, heat-processing temperatures favor reactions between compounds inherent in meat products yielding genotoxic substances.[Citation1] Heterocyclic aromatic amines (HCAs) are potent mutagens at ng/g levels in cooked foods and play an important role in the etiology of human cancer.[Citation2] The International Agency for Research on Cancer (IARC) regards some of the HCAs as possible human carcinogens 2-amino-3,4-dimethyl-imidazo[4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethyl-imidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)) and one as a probable human carcinogens, 2-amino-3-methylimidazo[4,5-f]quinoline (IQ).[Citation3] According to the IARC monograph reported in the year 2015, processed meat was classified as carcinogenic to humans (Group 1). It was estimated that every 50 g portion of processed meat eaten daily increases the risk of colorectal cancer by about 18%.[Citation2] These substances, with the exception of the β-carbolines norharman and harman, have been found potent mutagenic in the Ames test with Salmonella typhimurium TA 98 after metabolic activation, and HCAs are over 100 fold more mutagenic than aflatoxin B1 and over 2,000 fold more mutagenic than benzo[a]pyrene.[Citation4,Citation5] Some HCAs have been shown to be carcinogenic in long-term animal studies on rodents and primates.[Citation6] More than 20 HCAs have been isolated and characterized from heated protein-rich foods (meat products).[Citation7] Their possible formation even during ordinary cooking processes implies frequent exposure by the general public. A publication of the World Cancer Research Fund and the American Institute for Cancer Research in 2007[Citation8] highlighted risks associated with consumption of red and processed meats in colorectal cancer.
Generally, HCAs are formed as a result of the Maillard reaction of creatine, carbohydrates and free amino acids at temperatures of 150–250 °C (cooking, roasting, baking etc.).[Citation9] The concentrations of HCAs can be dependent on meat type, cooking time, pH, water activity, purine and pyrimidine bases and their nucleosides, the time of ripening and muscle type, heat and mass transfer, lipids, lipid oxidation, and antioxidants.[Citation10–Citation14] It is a well-known fact that the formation of HCAs is closely related to temperature during cooking and cooking techniques, such as pan frying, barbecuing and deep-fat frying. An increase in temperature causes more abundant formation of HCAs.[Citation15–Citation19] Conductive and convective heat transfer from the heating equipment to the surface of the product, e.g., by air convection or metal conduction, plays a substantial role.[Citation20] During cooking, the amounts of precursors at the meat surface may be enhanced by the transport of water and water-soluble precursors from the inner parts of the meat. This mass transport is essential for the formation of HCAs. A high cooking loss has been found to be related to the formation of large amounts of HCAs.[Citation17] It was reported that beef and chicken contained different types and amounts of HCAs.[Citation10,Citation16,Citation21] In addition, it was found that HCAs produced during the grilling of beef may be bonded to food matrix.[Citation22] During digestion in human digestive track bound HCAs may be released and cause cancer.
Some studies have shown that the concentrations of HCAs can be reduced by addition of compounds with an antioxidant potential.[Citation6,Citation10,Citation17,Citation23] The addition of natural products containing antioxidants that may act as free radical scavengers, such as polyphenols, reduces the amount of HCAs in the heat-processed meat.[Citation23–Citation34] Kikugawa et al.[Citation24] reported that the scavenging effect of antioxidants on pyrazine cation radicals that participate in the formation of HCAs has been demonstrated by a decrease in electron spin resonance signals in the presence of antioxidants. Among these additives, the effect of tea,[Citation23,Citation25] red wine,[Citation26,Citation27] olive oil,[Citation17] garlic,[Citation28] hibiscus extract,[Citation29] black pepper,[Citation30] pomegranate seed extract,[Citation10] and others has been demonstrated. Natural extracts usually contain a mixture of various types of phytochemicals, which likely exhibit different mechanisms of interaction with regard to the inhibition of HCA formation. The hypothesis for their action is that these inhibitors act against the free radicals generated during HCA formation, preventing the mutagens formation through radical quenchers. On the other hand, phenolic compounds are known to exert both anti- and pro-oxidative effects depending on their concentrations and interactions with other food components during cooking.[Citation26]
Grape seed extract (GSE) is a good source of anthocyanidins and anthoxanthins, and a large amount of phenolic compounds (phenoldienones, epicatechin, epigallocatechin, epigallocatechingallate, ferulic acid, caffeic acid, p-coumaric acid, resveratrol, kaempferol, quercetin, and myricetin).[Citation33] Natural extract components are very heat stable and active at wide pH ranges.[Citation35,Citation36] Although GSE has been investigated for their inhibitory effect on the formation of HCAs in cooked ground beef and fried beef patties,[Citation6,Citation31,Citation33] to our knowledge there is no information with regard to the inhibitory effect of GSE on the formation of HCAs in traditional beef (red meat) and chicken (white meat) meatballs cooked by different cooking techniques.
Meatball is consumed nearly in all regions of Turkey and plays an important role in the nutrition of many people. In Turkey meatball is produced by using ground meat, breadcrumbs, minced onion, salt, spices, possibly eggs, and some food filling materials. After mixing the ingredients with meat, they are rolled into small balls. It can be prepared by various cooking procedures (pan cooking, oven roasting, charcoal-barbecue, and deep-fat frying), and thus the formation of HCAs might be expected at various levels.[Citation10] Poultry meat is sometimes referred to as white meat, in contrast to red meat, for example, beef and pork. Macro components such as free amino acids, sugars, and creatine are not markedly different in beef and poultry muscles, suggesting that some other components and difference in pH values are responsible for the different rates and types of mutagens formed.[Citation10,Citation16,Citation21] Therefore, the objective of this study was to investigate the effects of the GSE on the formation of HCAs in traditional beef and chicken meatballs cooked by different techniques.
Materials and methods
Chemicals
All chemicals and solvents used were of HPLC or analytical-reagent grade, and water was purified using the Milli-Q gradient A10 system (Millipore, Billerica, MA, USA). All the solutions were filtered through a 0.45 µm filter before being injected into the HPLC instrument. The HCA compounds studied were as follows: (1) 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), (2) 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), (3) 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), (4) 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), and (5) 1-methyl-9H-pyrido[3,4-b]indole (harman) were purchased from Toronto Research Chemicals (Toronto, Canada). The 9H-pyrido[3,4-b]indole (norharman) was supplied from Sigma (Steinheim, Germany). For the solid-phase extraction (SPE), an Oasis MCX cartridge (3 cmCitation3/60 mg, Waters, Milford, Massachusetts, USA) and an Accubond C18 cartridge (3 cmCitation3/200 mg, Agilent Technologies, Santa Clara, CA, USA) were used. Zinc sulfate heptahydrate was purchased from Riedel-de Haen (Seeize, Germany). HPLC-grade methanol and acetonitrile, acetic acid, potassium ferrocyanide trihydrate, ammonia (25%), sodium carbonate, and hydrochloric acid (37%) were all purchased from Merck (Darmstadt, Germany). DPPH (1,1-Diphenyl-2-picryl-hydrazyl radical), Folin-Ciocalteu, and gallic acid (Sigma, Steinheim, Germany) were also used.
Stock standard HCA solutions of 100 mg/l in methanol were prepared separately and used for further dilutions. Standard solutions of 0.1 mg/l, 0.2 mg/l, 0.5 mg/l, 1 mg/l, 5 mg/l, and 10 mg/l in methanol were prepared for both calibration and standard addition purposes. Standard solutions were filtered through a 0.45 µm filter before being injected into the HPLC system.
Determination of cooking loss and total phenolics
Cooking loss was measured as the difference between the weights of meatballs before and after cooking.[Citation10] The total amount of phenolic compounds (TPCs) was determined using Folin–Ciocalteu reagent[Citation37] and expressed in gallic-acid equivalents (GAEs). The dry extract and meatballs were diluted in methanol (2000 times for extract and 10 times for meatballs) and 0.5 ml of this solution was treated with 2.5 ml of Folin–Ciocalteu reagent and 2 ml of 7.5% (w/v) Na2CO3 solution. After 30 min of incubation, the optical density was measured at 760 nm, using a ultraviolet–visible (UV/Vis) spectrophotometer (Cary 50, Varian, UK). Results were calculated using a gallic-acid calibration curve. Measurements were made in duplicate.
Determination of radical-scavenging activity using DPPH method
The radical-scavenging activities (RSAs) of the extract and meatballs were measured using the DPPH radical-scavenging assay method.[Citation37] The dry extract and meatballs were dissolved in methanol. Five milliliters of a 0.1 mM methanolic solution of DPPH was added to the tubes containing 0.5 ml of diluted extract (2000 times) or meatballs (10 times) and shaken vigorously. The tubes were allowed to stand at 27 °C for 20 min. The control was prepared as above without any extract or meat samples using methanol only. Changes in the absorbance of the samples were measured at 517 nm. The RSA was calculated using the following formula:
Meatball preparation and cooking conditions
The chicken breast and beef meat muscle were purchased from a local market in Izmir, Turkey. The meat was first minced through both an 8.0-mm orifice plate and afterwards a 3.5-mm grinder plate. The chicken and beef meats were minced with tallow to adjust the fat content to 25%, separately. After that, the meat was stored at –18°C. The same chicken and beef meats were used in the study. Prior to preparing meatballs, minced meat was thawed in a refrigerator at 4°C for 12–24 hour. To give a good structure to the meatballs, unsalted breadcrumbs were added as 20% (w/w). Meatball mix was divided into two equal parts. The one part was control and the other part was for GSE treatment. GSE (MNC USA) was added to meatball mix as 0.5% (w/w). The meatballs were prepared using plastic molds having diameter of 5.0 cm and height of 1.3 cm, and the weight of a meatball was about 30 g.
The cooking techniques most commonly used in Turkey were pan cooking, oven roasting, charcoal-barbecue, and deep-fat frying. For deep-fat frying, fresh sunflower oil was used. When the temperature of oil increased to 150°C, the meatballs were fried for 5 min in a commercial stainless steel deep-fat fryer. The pan-cooking process was carried out with a commercial pan (metal), which was preheated until the surface temperature was 180°C and then, the meatballs were fried for 8 min per side without fat or oil. For the charcoal-barbecue, approximately 1 kg of oak wood charcoal was placed in the bottom of a steel container and 100 ml of kerosene was poured onto charcoal to start the fire. When all the flames had subsided, the charcoal was leveled by raking. The meatballs were then cooked over the charcoal for 10 min per side. The distance between the meatballs and the charcoal was about 8 cm. The surface temperatures of the charcoal and the meatballs were about 380°C and 280°C, respectively. For the oven roasting, the meatballs were placed in an oven for 27 min at 180°C. Temperatures were measured by a laser infrared thermometer (Hongtai HT866, Guangdong, China). The meatballs were turned once during the total cooking time. All cooking experiments were performed in duplicate and for every replicate three meatballs were used for each process. After the cooking processes the meatballs were cooled to room temperature. Then, the cooked meatballs were homogenized using a kitchen blender to produce a uniform sample. The meatball samples were stored at −18 °C. Prior to analysis, samples were thawed in a refrigerator at 4°C for 12–24 h.
Extraction and analysis of heterocyclic aromatic amines
Extraction and analysis of HCAs were performed according to the method developed by Özdestan et al.[Citation38] Sample preparation was as follows: four grams of sample was homogenized in 25 ml 0.2 M HCl and suspension was shaken for 1 h. Then, the SPE clean-up was applied to eliminate matrix interferences in the sample. An Accubond C18 cartridge and an Oasis MCX cartridge were used consecutively to eliminate impurities. The eluate was gently evaporated under a stream of nitrogen and the residue was dissolved in 0.5 ml of methanol. Chromatographic analyses of IQ, MeIQx, and 4,8-DiMeIQx were realized by HPLC equipped with a diode array detector (DAD). The chromatographic column was BDS Hypersil C18 (5 µm particle size, 150 mm × 4.6 mm i.d. Thermo Scientific, USA). IQ was determined at 254 nm, MeIQx, and 4,8-DiMeIQx were measured at 263 nm. The injection volume was 20 μl. For PhIP, norharman, and harman, chromatographic separations and determinations were accomplished by HPLC using a fluorescence detector operating at 340 nm and 420 nm as excitation and emission wavelengths, respectively. The injection volume was 15 μl. HCA analyses were realized in duplicate.
Apparatus
Chromatographic analyses of HCAs were performed by using an Agilent 1200 liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a DAD, a fluorescence detector, a quaternary elution pump and auto sampler injection system, and a temperature-controlled column oven. For the sample preparation, an Eppendorf centrifuge 5804 (Hamburg, Germany) and IKA T25 Ultra-Turrax homogenizer (24,000 rpm, Staufen, Germany) were used. Determinations of total phenolics and RSA were realized by a UV–Vis spectrophotometer (Cary 50, Varian, UK).
Statistical analyses
The results were analyzed using an SPSS 16.0 statistics package program (IBM Corp., Armonk, NY, USA). Significant differences between experimental means were calculated by one-way ANOVA with a Duncan post-hoc test. A paired t-test was used for the comparison of control and GSE-containing samples in terms of RSA, total phenolics, and weight loss values. Pearson’s correlation test was applied to find relationships among individual HCA contents, and between HCAs and RSA, total phenolics, and weight loss contents.
Results and discussion
Weight loss
Cooking losses of beef and chicken meatballs after cooked by four different cooking techniques are presented in . There was no significant difference between the controls and the GSE-containing meatball samples in accordance with related literature (P > 0.05).[Citation6] The percentage of cooking loss (water and fat) varied with the cooking technique (P < 0.05). For beef meatballs, the least percent loss was found as approximately 32% in pan-cooked samples. The meatballs cooked by other techniques had similar weight losses as 50%. Pan-cooked chicken meatballs had the lowest value, while the charcoal-barbecued had the highest value. There were no correlations between the weight losses and total or individual HCA formation (P > 0.05) due to other variables such as meat type and cooking techniques. Several reports have indicated that weight loss may result in increased HCA formation.[Citation15,Citation39] This has been explained by increased transport of water-soluble precursors to the surface where the reactions occur.
Table 1. The weight losses, total phenolics (GAE) and radical-scavenging activity (RSA) of meatballs cooked by different techniques (mean ± standard error)a.
Total phenolics and the radical-scavenging activity
TPC of the GSE was observed as 220.0 mg of GAE in 1 g of extract. Total phenolic concentrations of the cooked beef and chicken meatballs are presented in . The concentrations of total phenolics in the control beef meatballs varied from 201.2 (pan cooked) to 329.9 mg GAE/kg (oven roasted), while beef meatballs with GSE varied from 353.6 (pan cooked) to 471.8 mg GAE/kg (oven roasted). Also, chicken meatballs contained 230.5 (deep-fat fried) to 388.7 mg GAE/kg (charcoal-barbecued) in controls and 382.1 (deep-fat fried) to 502.9 mg GAE/kg (oven roasted) in chicken meatballs with GSE. The differences in TPC values among the tested samples depend on the cooking techniques used. Total phenolic contents of beef and chicken meatballs with GSE were found significantly higher than those for controls (P < 0.05). The higher TPC contents of GSE-containing samples were seen to be effective on HCA contents of meatballs. In the report of Cheng et al.[Citation31], total phenolic contents of grape seed, apple, elderberry, and pineapple extracts were evaluated for their effects on HCA formation in fried beef patties and a positive correlation was observed between TPC values and inhibition of HCA formation. Ahn and Grün[Citation33] demonstrated the effect of GSE, pine bark extract, and oleoresin rosemary on the formation of HCAs and found that inhibition rates for polar HCAs increased with increasing natural extract concentration. In the current study, no correlation was observed between total phenolic contents of eight GSE-containing treatments and total or individual HCA formations (P > 0.05) because of the other variables such as meat type and cooking techniques. These results are in accordance with the previous studies that phenolic compounds are affected by temperature which depends on cooking techniques. Kim et al.[Citation36] reported that TPC in grape seeds and their extracts was significantly increased by heat treatment.
RSA (%) of chicken and beef meatballs is shown in . The differences in RSA values among the samples depend on the cooking techniques used. RSA values of beef and chicken meatballs with GSE were significantly higher than those for controls (P < 0.05). The higher RSA values of GSE-containing samples were found to be effective on HCA contents of meatballs. A good positive correlation was observed between the concentrations of total phenolics and RSA values (P < 0.05). No correlation was observed between RSA per cents and total or individual HCA formations (P > 0.05) due to the differences in meat type and cooking techniques and other variables. There are contradictory reports about the effect of antioxidants on the formation of HCAs. Gibis and Weiss[Citation6] reported that a correlation was observed between inhibition of HCA formation and Trolox equivalents. On the other hand, Damašius et al.[Citation32] found no correlation between the antioxidant activity of extracts and the formation of HCAs. Busquets et al.[Citation26] reported that for the shortest marinating time and the greater antioxidant capacity, higher amount of PhIP was formed. In contrast, long marinating times with lowest antioxidant properties caused a high inhibition of PhIP.
Effects of grape seed extract on the formation of HCAs in the meatballs
HPLC chromatograms of IQ and MeIQx are shown in . shows chromatogram of PhIP, norharman, and harman. Detection limits for PhIP, norharman, harman, IQ, MeIQx, and 4,8-DiMeIQx were 0.04, 0.65, 0.26, 1.25, 0.86, and 1.40 ng/g, respectively.[Citation38] The recoveries for the analyses of six HCAs were between 69% and 88%. HCA contents of the meatballs are presented in .
Table 2. Heterocyclic aromatic amines (HCAs) contents of meatballs (ng/g) after cooked by four different techniques (mean ± standard error).
Figure 1. HPLC chromatograms of IQ and MeIQx in the beef meatball sample cooked by charcoal-barbecue. Peak identification: (1) IQ and (2) MeIQx (with diode array detector at 254 and 263 nm).
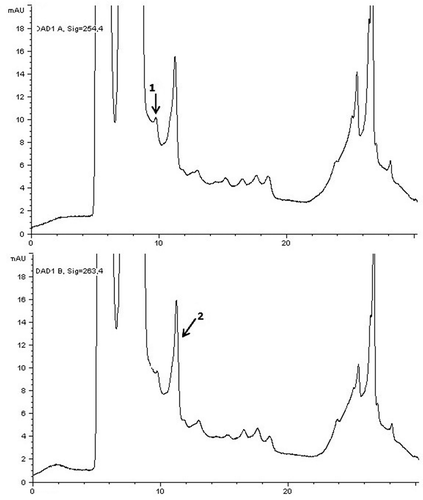
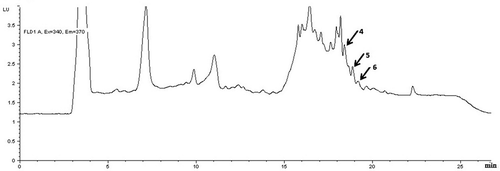
Figure 2. HPLC chromatogram of PhIP, Norharman and Harman in the beef meatball sample cooked by charcoal-barbecue. Peak identification: (4) PhIP, (5) Norharman, and (6) Harman (with fluorescence detector).
As can be seen from , PhIP, norharman, harman, IQ, and MeIQx were detected in meatball samples cooked by four different techniques. In beef meatballs, the maximum level of PhIP was 1.23 ng/g in controlled group cooked by charcoal-barbecue. The inhibitory effect of GSE on PhIP formation was nearly 60% for all cooking techniques except the oven roasting. For the oven roasting, there was no significant difference between control and GSE-containing sample (P > 0.05). In the chicken meatballs, the maximum level of PhIP was 1.92 ng/g in controlled group cooked by oven roasting. The inhibitory effects of GSE were 73, 68, 63, and 30% for pan cooked, oven roasted, charcoal-barbecued, and deep-fat fried chicken meatballs, respectively. Busquets et al.[Citation26] found 72 ng/g PhIP in fried chicken. In contrast, Oz et al.[Citation40] not detected PhIP in chicken chops cooked by different methods. PhIP values reported by Liao et al.[Citation41] were between 0.04 ng/g and 31.06 ng/g for chicken breast cooked by different methods. In the current study, PhIP contents of chicken meatballs were found lower than those reported by Busquets et al.[Citation26] and Liao et al.[Citation41] Ingredients such as minced onion and spices in meatballs may inhibit HCA formation. Total amount of PhIP in controlled-group beef meatballs was 3.60 ng/g and decreased to 1.72 ng/g in GSE-containing samples with 52% inhibition. Controlled-group chicken meatballs had a value of 3.84 ng/g and after the treatment with GSE contained 1.35 ng/g as a result of 65% inhibition. Consequently, chicken samples contained higher amount of PhIP before treatment with GSE and it was seen that GSE is a potent inhibitor for PhIP formation especially for chicken meatballs. Our results are also in agreement with others reporting that GSE significantly reduced the formation of polar HCAs. Gibis and Weiss[Citation6] demonstrated that PhIP level was reduced from 0.3 ng/g to <0.02 ng/g in the marinated beef patties containing GSE (0.8%) fried at 230°C. Cheng et al.[Citation31] reported that grape seed, apple, and elderberry extracts added by 0.1% reduced the level of PhIP by 72%, 69%, and 45% in beef patties fried at 210 °C, respectively, while Ahn and Grün[Citation33] found the addition of 1.0% GSE lowered PhIP content of ground beef cooked at 200 °C from 12.13 ng/g to 4.40 ng/g. A large number of studies have demonstrated the effect of other natural polyphenolic substances on HCA formation. Oz and Kaya[Citation30] reported that the inhibitory effect of black pepper on the formation of PhIP in beef meatballs cooked at 225 °C was up to 100%. The inhibition effect of red wine and beer on PhIP in pan fried beef was studied by Melo et al.[Citation27] and it was reported that reduction rate was 88% for both of them. Similar results, 83–88% inhibition, were found by Busquets et al.[Citation26] using three different types of red wine in fried chicken. In contrast, Damašius et al.[Citation32] found that the addition of 0.5% of basil extract increased the concentration of PhIP in cooked beef almost three times compared with the control sample. Cheng et al.[Citation31] studied the effects of fruit extracts on the formation of HCAs in fried beef patties and showed the presence of various phenolics with different effects. Chlorogenic acid, although effective against the formation MeIQx, significantly enhanced the formation of PhIP.
Norharman and harman are co-mutagenic β-carbolines. They have been shown to enhance the mutagenicity of other mutagenic compounds.[Citation6] Norharman was detected in all tested samples and the maximum concentration in beef meatballs was 6.87 ng/g for control group cooked with charcoal-barbecue. GSE had practically no effect on the concentration of norharman with the exception of charcoal-barbecued meatballs with 69% inhibition. The highest content of norharman in chicken meatballs was 11.47 ng/g for charcoal-barbecued control sample. The maximum inhibition was found as 31% for charcoal-barbecued chicken meatballs. The addition of GSE in beef meatballs was more effective than in chicken meatballs. These findings are in accordance with those in literature reporting some of the phenolics or other antioxidants may reduce the formation of norharman but others may enhance. Some of them may not have any significant effect. Gibis and Weiss[Citation6] reported that no significant differences were found, when GSE was added in fried beef patties at the level of 0.2–0.8%. In another study, formation of norharman decreased from 2.01 ng/g to 0.72 ng/g in fried beef patties and from 5.55 ng/g to 5.16 ng/g in cooked ground beef after the addition of 1.0% GSE.[Citation33] Busquets et al.[Citation26] studied the effects of three different red wines on the formation of HCAs in fried chicken breast and reported that one of them reduced the formation of norharman, the second enhanced the formation and the other had no effect when marinating was carried out for long periods.
Beef and chicken meatballs did not contain harman when cooked by deep-fat frying. The highest amount of harman (3.42 ng/g) was detected in the control group of chicken meatballs cooked by charcoal-barbecue. It was not observed any significant inhibition of harman caused by GSE in beef and chicken meatballs (P > 0.05). In contrast, harman concentration increased approximately twice with the addition of GSE for the oven roasted chicken meatballs. The same trend was seen in the reports of other researchers. Gibis and Weiss[Citation6] examined the effect of 0.8% GSE on harman concentration in fried beef patties and established an increase from 1.1 ng/g to 1.7 ng/g but a decrease with 0.6% rosemary extract. Ahn and Grün[Citation33] found that the amount of harman increased in the presence of 1.0% GSE from 10.88 ng/g to 109.14 ng/g in ground beef cooked at 200°C and from 2.99 ng/g to 210.76 ng/g in pan fried beef patties fried at 210 °C, but decreased with 1.0% oleoresin rosemary. In another study, all of the red wines tested in fried chicken cooked at 220 °C enhanced the formation of harman especially when marinating was carried out for long periods.[Citation26] The increase of harman concentration in GSE-containing samples and red wine-treated samples may be caused by pro-oxidants from GSE and red wine. According to the findings from Cheng et al.,[Citation31] both anti- and pro-oxidative effects can be exhibited by phenolic compounds.
As seen in , the most predominant HCA detected in the meatballs was IQ. It was detected in all beef meatballs and the maximum concentration was found up to 303.06 ng/g in control group cooked by charcoal-barbecue. When GSE was added to charcoal-barbecued beef meatballs, IQ formation was inhibited by 65%. In the chicken meatballs, the maximum IQ concentration was found as 58.79 ng/g in the control group cooked by oven roasting. There was a significant decrease, 52% in IQ content for GSE-containing chicken meatballs oven roasted. In contrast, IQ concentration significantly increased with the addition of GSE in the pan-cooked chicken meatballs. Ahn and Grün[Citation33] reported that 1.0% GSE caused a decrease in IQ content by 64%, from 3.22 ng/g to 1.15 ng/g in ground beef samples cooked at 200 °C. Oz and Kaya[Citation30] found that the reduction of black paper on the formation of IQ in beef meatball fried at 175 °C was up to 34%, from 1.40 ng/g to 0.93 ng/g. Oz et al.[Citation40] determined IQ contents of chicken and fish cooked by different techniques and found values between not detected (nd) and 17.84 ng/g. According to the report of Liao et al.,[Citation41] IQ contents of chicken breast cooked by different methods were between nd and 1.76 ng/g. Gibis and Weiss[Citation6] examined but did not find IQ in beef patties fried at 230 °C. In the current study, IQ contents of meatballs were found significantly higher than those in the literature.[Citation6,Citation30,Citation33,Citation40,Citation41] This increase in IQ contents is difficult to explain but might be caused by differences in the kind of meat, recipes of meat preparation, ingredients used in meatball formulation, and co-elution and peak interference.
Although MeIQx was not detected in pan-cooked control group beef meatball, it was found in all the remaining beef meatballs with the highest concentration of 35.21 ng/g. The inhibitory effects of GSE on MeIQx were 49% and 59% for oven roasted and charcoal-barbecued beef meatballs, respectively. Surprisingly, MeIQx was not detected in chicken meatballs cooked by charcoal-barbecue (). This fact suggested that high temperature may cause the degradation of the formed MeIQx which is in agreement with the result obtained by other authors from kinetic studies.[Citation20] In chicken meatballs, the highest concentration of 111.62 ng/g MeIQx was found in deep-fat fried control sample, and inhibited by 37% by GSE addition. In contrast, GSE caused an increase in MeIQx content approximately 6 times in pan-cooked samples. These contradictory results are in accordance with related previous studies. It was suggested that pro- or anti-oxidant effects of phenolic compounds may depend on the concentration of the used marinades or on the specificity of the interactions with individual HCA.[Citation26,Citation34] In contrast to model studies with pure phenolic acids, marinades with GSE may contain not only antioxidants, but also other components, such as hexose and pentose that could possibly enhance or inhibit the formation of HCAs.[Citation6] According to the results of Ahn and Grün,[Citation33] the formation of MeIQx was effectively reduced from 22.65 ng/g to 8.62 ng/g and 6.90 ng/g by additions of 0.5% and 1.0% of GSE, respectively. Cheng et al.[Citation31] reported that 0.1% grape seed and apple extracts reduced the formation of MeIQx in beef patties fried at 210 °C by 67% and 59%, respectively. Also, Gibis and Weiss[Citation6] reported that GSE at 0.2, 0.4, 0.6, and 0.8% reduced the amount of MeIQx in fried beef patties from 0.9 ng/g to 0.5, 0.4, 0.3, and 0.3 ng/g, respectively.
4,8-DiMeIQx was not detected in beef and chicken meatballs with and without GSE, cooked by four different techniques. This result is in agreement with those in the literature. Keşkekoğlu and Üren[Citation10] could not detect 4,8-DiMeIQx in beef and chicken meatballs with and without pomegranate seed extract. Also Gibis and Weiss[Citation6] examined but did not find 4,8-DiMeIQx in beef patties fried at 230 °C. Oz and Kaya[Citation30] were unable to determine 4,8-DiMeIQx in beef meatballs fried at 175 °C and 200 °C, but 3.35 ng/g of 4,8-DiMeIQx was detected in samples cooked at 225°C. Cheng et al.[Citation31] found 0.95 ng/g of 4,8-DiMeIQx in beef patties fried at 210°C and this value decreased to 0.32 ng/g with 0.1% GSE. Ahn and Grün[Citation33] found that 1.74 ng/g of 4,8-DiMeIQx in ground beef cooked at 200°C decreased below the detection limit with the addition of 1.0% GSE.
Total HCAs (IQ, MeIQx, 4,8-DiMeIQx, PhIP, norharman, and harman) of meatballs as IQ are tabulated in . In beef meatballs, the highest amount of total HCAs was observed up to 346.42 ng/g in the control group of the charcoal-barbecued samples. The high concentration of total HCAs in charcoal-barbecued sample may be the result of the rapid and direct heating (open flame) on the surface of the meat at a high temperature. This finding is in accordance with the result of Liao et al.[Citation41] which showed that chicken breast cooked by charcoal grilling contained the highest content of total HCAs among the different cooking methods. When GSE was added the total amount of HCAs was most effectively (65%) reduced in beef meatballs cooked by charcoal-barbecue. In the chicken meatballs, the highest total HCA formation, 114.67 ng/g was observed in the control group cooked by deep-fat fraying. GSE addition reduced total HCA formation by 37%, 23%, and 33% when cooked by oven roasting, charcoal-barbecue, and deep-fat frying, respectively. Several studies have found that, natural extracts effectively inhibited HCA formation and reduced the mutagenicity of cooked meats.[Citation23,Citation25–Citation33] The inhibition of HCA formation in cooked meat by natural extracts may be related to the antioxidant properties of the polyphenolic compounds. Since the polyphenolic antioxidants can be expected to scavenge free radicals producing HCAs in the Maillard reaction.[Citation42] Because of the high phenolic content and antioxidant capacity,[Citation6] GSE was found to be effective against the HCA formation in the meatballs, especially cooked by charcoal-barbecue. On the other hand, GSE and other polyphenolic compounds may increase specific HCA formation in some cases.
Conclusion
Because the connection between consumption of dietary carcinogens and cancer risks in human has been established, it is necessary to explore effective inhibitors that can prevent the formation of HCAs in cooked meat. The use of antioxidants can be a possible approach to reduce the HCA formation during cooking, because the free radical reactions producing HCAs are inhibited by the radical scavenging effect of antioxidants. In recent years, there is a growing interest in the health benefits of natural extracts containing polyphenols. As GSE contains a large amount of phenolic compounds, marinating of meats with GSE is a useful treatment prior to cooking. In the current study, application of GSE influenced the concentration of HCAs in cooked beef and chicken meatballs; however, this effect depended both on the meat type (red or white) and cooking techniques. The highest inhibitory effect of GSE on the formation of total HCAs was observed in charcoal-barbecued beef meatballs, by 65%, which is the common method of meatball cooking. In the chicken meatballs 37% inhibition was observed for oven roasted samples. This study is the first to report the inhibitory activities of GSE on the formation HCAs in traditional meatballs cooked by four different cooking techniques (oven roasting, pan cooking, charcoal-barbecue, and deep-fat frying). Because this approach may be applicable to various meat systems, the food safety concerns associated with the dietary intake of HCAs from cooked meats could be significantly decreased by adding natural extracts such as GSE. Therefore, the results of the current study would be useful in designing an effective system to minimize HCA formation in cooked meats and consequently to reduce dietary exposure to genotoxic heterocyclic amines.
References
- Khan, M.R.; Bertus, L.M.; Busquets, R.; Puignou, L. Mutagenic Heterocyclic Amine Content in Thermally Processed Offal Products. Food Chemistry, 2009, 112, 838–843.
- IARC. Monographs Programme on the Carcinogenicity of the Consumption of Red Meat and Processed Meat. World Health Organisation, International Agency for Research on Cancer, Press Release No 240: Lyon, France, 2015.
- Cheng, K.W.; Chen, F.; Wang, M. Heterocyclic Amines: Chemistry and Health. Molecular Nutrition Food Research, 2006, 50, 1150–1170.
- Knize, M.G.; Sinha, R.; Rothman, N.; Brown, E.D.; Salmon, C.P. Heterocyclic Amine Content in Fast-Food Meat Products. Food Chemical Toxicology, 1995, 33, 545–551.
- Stavric, B.;. Biological Significance of Trace Levels of Mutagenic Heterocyclic Aromatic Amines in Human Diet: A Critical Review. Food Chemical Toxicology, 1994, 32, 977–994.
- Gibis, M.; Weiss, J. Antioxidant Capacity and Inhibitory Effect of Grape Seed and Rosemary Extract in Marinades on the Formation of Heterocyclic Amines in Fried Beef Patties. Food Chemistry, 2012, 134, 766–774.
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic Amines: Mutagens/Carcinogens Produced during Cooking of Meat and Fish. Cancer Science, 2004, 95(4), 290–299.
- WCRF/AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research AICR: Washington, DC, 2007; 382 pp.
- Oz, F.; Kaban, G.; Kaya, M. Effects of Cooking Methods on the Formation of Heterocyclic Aromatic Amines of Two Different Species Trout. Food Chemistry, 2007, 104(1), 67–72.
- Keşkekoğlu, H.; Üren, A. Inhibitory Effects of Pomegranate Seed Extract on the Formation of Heterocyclic Aromatic Amines in Beef and Chicken Meatballs after Cooking by Four Different Methods. Meat Science, 2014, 96, 1446–1451.
- Szterk, A.; Roszko, M.; Malek, K.; Kurek, M.; Zbieć, M.; Waszkiewicz-Robak, B. Profiles and Concentrations of Heterocyclic Aromatic Amines Formed in Beef during Various Heat Treatments Depend on the Time of Ripening and Muscle Type. Meat Science, 2012, 92, 587–595.
- Szterk, A.; Waszkiewicz-Robak, B. Influence of Selected Quality Factors of Beef on the Profile and the Quantity of Heterocyclic Aromatic Amines during Processing at High Temperature. Meat Science, 2014, 96, 1177–1184.
- Szterk, A.; Jesionkowska, K. Influence of the Cold Storage Time of Raw Beef Meat and Grilling Parameters on Sensory Quality and Content of Heterocyclic Aromatic Amines. LWT – Food Science and Technology, 2015, 61, 299–308.
- Szterk, A.;. Heterocyclic Aromatic Amines in Grilled Beef: The Influence of Free Amino Acids, Nitrogenous Bases, Nucleosides, Protein and Glucose on HAAs Content. Journal of Food Composition and Analysis, 2015, 40, 39–46.
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, Formation and Occurrence of Genotoxic Heterocyclic Amines Identified in Model Systems and Cooked Foods. Zeitschrift für Lebensmittel-Untersuchung und -Forschung A, 1998, 207, 419–427.
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Pork, Beef and Chicken Barbecued at Home by Danish Consumers. Meat Science, 2013, 93, 85–91.
- Persson, E.; Graziani, G.; Ferracane, R.; Fogliano, V.; Skog, K. Influence of Antioxidants in Virgin Olive Oil on the Formation of Heterocyclic Amines in Fried Beef Burgers. Food and Chemical Toxicology, 2003, 41, 1587–1597.
- Louis, E.D.; Zheng, W.; Jiang, W.; Bogen, K.T.; Keating, G.A. Quantification of the Neurotoxic β-Carboline Harman in Barbecued/Grilled Meat Samples and Correlation with Level of Doneness. Journal of Toxicology and Environmental Health, Part A, 2007, 70, 1014–1019.
- Ahn, J.; Grün, I.U. Heterocyclic Amines: Kinetics of Formation of Polar and Nonpolar Heterocyclic Amines as a Function of Time and Temperature. Journal of Food Science, 2005, 70, 173–179.
- Arvidsson, P.; Van Boekel, M.A.J.S.; Skog, K.; Jagerstad, M. Kinetics of Formation of Polar Heterocyclic Amines in a Meat Model System. Journal of Food Science, 1997, 62, 911–916.
- Klassen, R.D.; Lewis, D.; Lau, B.P.-Y.; Sen, N.P. Heterocyclic Aromatic Amines in Cooked Hamburgers and Chicken Obtained from Local Fast Food Outlets in the Ottawa Region. Food Research International, 2002, 35, 837–847.
- Szterk, A.;. Chemical State of Heterocyclic Aromatic Amines in Grilled Beef: Evaluation by in Vitro Digestion Model and Comparison of Alkaline Hydrolysis and Organic Solvent for Extraction. Food and Chemical Toxicology, 2013, 62, 653–660.
- Quelhas, I.; Petisca, C.; Viegas, O.; Melo, A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of Green Tea Marinades on the Formation of Heterocyclic Aromatic Amines and Sensory Quality of Pan-Fried Beef. Food Chemistry, 2010, 122, 98–104.
- Kikugawa, K.; Kato, T.; Hiramoto, K.; Takada, C.; Tanaka, M.; Maeda, Y.; Ishihara, T. Participation of the Pyrazine Cation Radical in the Formation of Mutagens in the Reaction of Glucose/Glycine/Creatinine. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 1999, 444(1), 133–144.
- Weisburger, J.H.; Veliath, E.; Larios, E.; Pittman, B.; Zang, E.; Hora, Y. Tea Polyphenols Inhibit the Formation of Mutagens during the Cooking of Meat. Mutation Research, 2002, 516(1–2), 19–22.
- Busquets, R.; Puignou, L.; Galceran, M.T.; Skog, K. Effect of Red Wine Marinades on the Formation of Heterocyclic Amines in Fried Chicken Breast. Journal of Agricultural and Food Chemistry, 2006, 54, 8376–8384.
- Melo, A.; Viegas, O.; Petisca, C.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of Beer/Red Wine Marinades on the Formation of Heterocyclic Aromatic Amines in Pan-Fried Beef. Journal of Agricultural and Food Chemistry, 2008, 56, 10625–10632.
- Gibis, M.;. Effect of Oil Marinades with Garlic, Onion, and Lemon Juice on the Formation of Heterocyclic Aromatic Amines in Fried Beef Patties. Journal of Agricultural and Food Chemistry, 2007, 55, 10240–10247.
- Gibis, M.; Weiss, J. Inhibitory Effect of Marinades with Hibiscus Extracts on Formation of Heterocyclic Aromatic Amines and Sensory Quality of Fried Beef Patties. Meat Science, 2010, 85, 735–742.
- Oz, F.; Kaya, M. The Inhibitory Effect of Black Pepper on Formation of Heterocyclic Aromatic Amines in High-Fat Meatball. Food Control, 2011, 22, 596–600.
- Cheng, K.W.; Wu, Q.; Zheng, Z.P.; Peng, X.; Simon, J.E.; Chen, F.; Wang, M. Inhibitory Effect of Fruit Extracts on the Formation of Heterocyclic Amines. Journal of Agricultural and Food Chemistry, 2007, 55, 10359–10365.
- Damašius, J.; Venskutonis, P.R.; Ferracane, R.; Fogliano, V. Assessment of the Influence of Some Spice Extracts on the Formation of Heterocyclic Amines in Meat. Food Chemistry, 2011, 126, 149–156.
- Ahn, J.; Grün, I.U. Heterocyclic Amines: 2. Inhibitory Effects of Natural Extracts on the Formation of Polar and Nonpolar Heterocyclic Amines in Cooked Beef. Journal of Food Science, 2005, 70, 263–268.
- Lan, C.M.; Kao, T.H.; Chen, B.H. Effects of Heating Time and Antioxidants on the Formation of Heterocyclic Amines in Marinated Foods. Journal of Chromatography B, 2004, 802, 27–37.
- Hongpattarakere, T.; Johnson, E.A. Natural Antimicrobial Components Isolated from Yerba Mate (Ilex Paraguariensis). Food Research Institute. University of Wisconsin-Madison Annual Rep, 1999, 11, 39.
- Kim, S.Y.; Jeong, S.M.; Park, W.P.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of Heating Conditions of Grape Seeds on the Antioxidant Activity of Grape Seed Extracts. Food Chemistry, 2006, 97, 472–479.
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (punica granatum) Peel and Seed Extracts Using in Vitro Models. Journal of Agricultural and Food Chemistry, 2002, 50, 81–86.
- Özdestan, Ö.; Kaçar, E.; Keşkekoğlu, H.; Üren, A. Development of a New Extraction Method for Heterocyclic Aromatic Amines Determination in Cooked Meatballs. Food Analytical Methods, 2014, 7, 116–126.
- Persson, E.; Sjöholm, I.; Skog, K. Effect of High Water-Holding Capacity on the Formation of Heterocyclic Amines in Fried Beefburgers. Journal of Agricultural and Food Chemistry, 2003, 51, 4472–4477.
- Oz, F.; Kaban, G.; Kaya, M. Effects of Cooking Methods and Levels on Formation of Heterocyclic Aromatic Amines in Chicken and Fish with Oasis Extraction Method. LWT – Food Science and Technology, 2010, 43, 1345–1350.
- Liao, G.Z.; Wang, G.Y.; Xu, X.L.; Zhou, G.H. Effect of Cooking Methods on the Formation of Heterocyclic Aromatic Amines in Chicken and Duck Breast. Meat Science, 2010, 85, 149–154.
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. Journal of Agricultural and Food Chemistry, 2001, 49, 5165–5170.
