ABSTRACT
Oven dried cut stigmas of Crocus sativus L. cultivated in Eskisehir and Safranbolu regions of Turkey were subjected to micro distillation to extract steam volatiles. They were then analysed by Gas Chromatography and Gas Chromatography/Mass Spectrometry, simultaneously. The colour compounds were extracted with 80% methanol and analysed by High Pressure Liquid Chromatography HPLC/DAD and HPLC/MS/MS. The activities of the extracts of saffron against 2,2ʹ-azinobis-3-ethylbenzothiazoline-6-sulphonic acid free radical (ABTS●+) were investigated using on-line (HPLC-ABTS●+) and off-line methods. Safranal (62.1 and 49.3%) and α-isophorone (10.0 and 16.3%) were found as the characteristic aromatic volatiles in the Eskisehir and Safranbolu samples, respectively. Crude saffron extracts showed high total antioxidant activity against ABTS●+ radical.
Introduction
Saffron, the most valuable and expensive industrial crop in the world, is derived from the flowers of Crocus sativus (Iridaceae). The flower has three stigmas, which are the distal ends of the plant’s carpels. Together with its style, the stalk connecting the stigmas to the rest of the plant is often dried and used in cooking as a seasoning and colouring agent. Because of its high price, saffron is considerably vulnerable to adulteration when it is traded in powder form.[Citation1,Citation2]
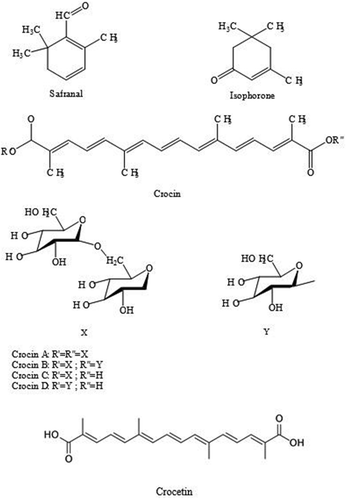
Saffron is characterised by a bitter taste and an iodoform- or hay-like fragrance, which are caused by the chemicals picrocrocin and safranal. Saffron also contains a carotenoid dye, crocin that gives food a rich golden-yellow hue. These traits make saffron a much-sought ingredient in many foods worldwide. Saffron also has medicinal applications.[Citation3,Citation4]
Saffron contains more than 150 volatile and aroma-yielding compounds. Safranal is one of the main components of saffron essential oil. This compound is formed in saffron by the hydrolysis of picrocrocin during drying and storage and is responsible for the aroma of saffron essential oil.[Citation5] Crocin derivatives are biosynthesised in plant cells. During flowering, drying, storage, and extraction, these compounds tend to naturally be degraded in the stigmata cells.[Citation1] Saffron also has many nonvolatile active components, belonging to carotenoids, including zeaxanthin, lycopene, and various α- and β-carotenes. However, saffron’s golden yellow-orange colour is primarily due to α-crocin. α-Crocin is trans-crocetin di-(β-D-gentiobiosyl) ester (systematic (IUPAC) name: 8,8-diapo-8,8-carotenoic acid). This indicates that the underlying saffron aroma is a digentiobiose ester of the carotenoid crocetin. Crocins themselves are a series of hydrophilic carotenoids that are either monoglycosyl or diglycosyl polyene esters of crocetin.[Citation6–Citation9] In addition, crocetin is a conjugated polyene dicarboxylic acid that is hydrophobic, and thus oil-soluble. When crocetin is esterified with gentiobiose sugar, a water-soluble derivative α-crocin is formed. α-Crocin may comprise more than 10% of dry saffron mass. The two esterified gentiobioses make α-crocin ideal for colouring water-based (non-fatty) foods such as rice dishes.[Citation6–Citation9]
Previous research into the aroma of C. sativus has been related on the composition and identification of the volatile components of saffron using Gas Chromatography (GC/FID) and Gas Chromatography/Mass Spectrometry (GC/MS).[Citation1,Citation2,Citation10–Citation12] Non-volatile compounds of saffron such as crocins and flavonoids were analysed using HPLC.[Citation6–Citation9,Citation13]
The aim of the present work was to study the aroma and colouring components of saffron. Samples SE and SS were derived from cultivations of Crocus sativus in Eskisehir (SE) and Safranbolu (SS), respectively. The volatiles of saffron obtained by micro distillation were analysed by GC and GC/MS, simultaneously. The colour compounds of saffron were extracted with 80% methanol and analysed by reversed phase High Pressure Liquid Chromatography Mass Spectrometry (HPLC/MS/MS). The antioxidant compounds of the extracts of saffron against 2,2ʹ-azinobis-3-ethylbenzothiazoline-6-sulphonic acid radical (ABTS●+) free radical were investigated using on-line (HPLC-ABTS●+) and off-line methods.
Materials and methods
Plant material and reagents
Cut stigmas from fresh flowers of Crocus sativus cultivated in Eskisehir and Safranbolu regions in Turkey were collected in June 2005. Chromatographic standards were purchased from Sigma Chemical Company. Ultra-pure water was used throughout and was prepared using a Millipore Milli-RO 12 plus system (Millipore Corp., MA, USA). All remaining reagents were of the highest purity available and obtained from the Sigma Chemical Company (St. Louis, MO, USA).
Isolation and identification of the volatile components
The volatiles in saffron were extracted by micro distillation using Eppendorf Micro Distiller® (Germany) with 10 mL distilled water.[Citation14,Citation15] Dried cut stigmas from flowers of saffron (125–200 mg) were placed in a sample vial together with 10 ml of water. NaCl (2.5 g) and water (0.5 ml) were placed in the collecting vial. n-Hexane (300 µL) was added to the collecting vial to trap volatile components. Sample vials were heated to 108°C at a rate of 20°C/min, kept at 108°C for 90 min, heated to 112°C at a rate of 20°C/min, and kept at this temperature for 30 min. Finally, the samples were subjected to a post-run for 6 min under the same conditions. Collecting vials were cooled to −1°C during distillation. After the distillation was completed, the organic layer in the collection vial was injected into the GC/MS instrument. The GC analysis was carried out using an Agilent 6890N GC system. FID detector temperature was 300°C. To obtain the same elution order with GC/MS, simultaneous auto injection was done on a duplicate of the same column applying the same operational conditions. Relative percentage amounts of the separated compounds were calculated from FID chromatograms.
The GC-MS analysis was carried out with an Agilent 5975 GC-MSD system. Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with helium as carrier gas (0.8 mL/min). GC oven temperature was kept at 60°C for 10 min and programmed to 220°C at a rate of 4°C/min and kept constant at 220°C for 10 min and then programmed to 240°C at a rate of 1°C/min. Split ratio was adjusted at 40:1. The injector temperature was set at 250°C. Mass spectra were recorded at 70 eV. Mass range was from m/z 35 to 450.
Identification of components
Identification of the essential oil components was carried out by comparison of their relative retention times with those of standards or by comparison of their relative retention index (RRI) to series of n-alkanes. Computer matching against commercial (Wiley GC/MS Library, Adams Library, MassFinder 2.1 Library)[Citation16,Citation17] and in-house ‘Başer Library of Essential Oil Constituents’ built up by genuine compounds and components of known oils as well as MS literature data[Citation18–Citation20] were used for the identification.
Antioxidant capacity of saffron
The standard TEAC assay described by Papandreou et al.[Citation7] was used. This assay assesses the capacity of a compound to scavenge the stable ABTS●+ radical, in comparison to the antioxidant activity of Trolox, a water-soluble form of vitamin E. The blue-green ABTS●+ was produced through the reaction of 7 mM ABTS with 2.5 mM sodium persulfate (Na2S2O8) (final concentration) in the dark at room temperature for 12–16 h before use. The concentrated ABTS●+ solution was diluted with ethanol to a final absorbance of 0.8–0.7 at 734 nm. A 10 µL portion of sample/Trolox/standard was added to 990 µL of ABTS●+ solution, and the reaction in absorbance was measured 1-min intervals for 30 min. Trolox equivalent antioxidant capacity of samples was calculated using the calibration curve of Trolox.
Identification of antioxidant compounds in saffron
The antioxidant compounds in saffron were determined using on-line HPLC-ABTS●+ and HPTLC after ABTS●+ derivatisation.
HPLC-ABTS●± derivatisation
A Discovery C18 column (Supelco, 25 cm × 4.6 mm × 5 μm) was used for all analyses. A linear gradient of methanol (10–100%) in water:acetonitrile (85:15 (v/v) was used as a mobile phase with a flow rate of 1.0 mL/min for 60 min at room temperature. Injection volume was 20 mL, and three injections were done. Crocins were detected at 440 nm, and the other phenolics were at 280 nm 7. 2 mM ABTS●+ containing 3.5 mM sodium persulfate was prepared as described in ‘antioxidant capacity of saffron. ABTS●+ reagent was prepared by diluting the stock 8-fold in 0.1 M phosphate buffer at pH 8 (19). After separation of compounds was carried out as described above, eluent derivatised with diluted ABTS●+ reagent at a flow rate of 0.5 mL/min. After mixing by pass through a 3 m × 0.25 mm loop maintained at room temperature, absorbance was monitored at 734 nm.
HPTLC-ABTS●±
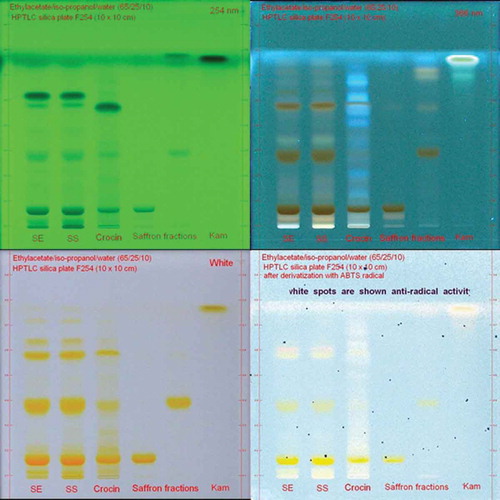
TLC experiments were performed using CAMAG system. A HPTLC Silica F254 (10 cm × 10 cm) glass plate was used. The plate was developed in ethylacetate/iso-propanol/water (65/25/10) mixture at 70% humidity for 80 mm. After drying, the plate was scanned at 254 and 366 nm under white light. The plate was derivatised with concentrated ABTS●+ reagent (7 mM) prepared as above and scanned under white light. Active compounds were bleached the blue-green colour of ABTS●+ radical.
LC/MS/MS analysis
Experiments were performed with a Shimadzu 20A HPLC system on an Applied Biosystems 3200 Q-Trap hybrid triple quadruple/linear ion trap MS/MS instrument equipped with a Turbo-V ESI ion source in the negative mode. Separations were performed on a Supelco Discovery C18 column (25 cm × 4.6 mm × 5 µm) at room temperature at a flow rate of 1 mL/min. Detection was carried out at 280, 440, and 734 nm. A linear gradient elution was carried out using methanol (0.1% formic acid) (10–100%) in water (15% of acetonitrile+ %0.1 formic acid). The HPLC system was connected directly to a 3200 Q TRAP (AB Sciex, Toronto, Canada). Analyst 1.5 software was used for data acquisition. Although the same chromatographic conditions were used as described above, retention time shift was observed in LC-MS/MS analysis due to the formic acid content. Two different scan type were used: 1) For Multiple Reaction Monitoring (MRM), the MS was operated in negative polarity at a scan rate of 4000 Da/s. MRM conditions and fragmentation were used as described previously, using with 3200 Q trap, reported by Verma et al.[Citation21] except for crocin A, which was identified directly from standard Crocin A.
Also Enhanced Mass Scan (MS) and daughter (MS-MS) spectra were measured from m/z 100 up to m/z 1200. Collision-induced fragmentation experiments were performed in ion trap using nitrogen as the collision gas. The parameters were as follows: Collision Energy (CE) −30, Declustering Potential (DP)-20, Entrance Potential (EP)-10, Curtain gas (CUR)-20, Gas Source 1 (GS1)-50, Gas Source 2 (GS2)-50, Temperature (TEM)-550, İon spray voltage (IS) −4500. According to LC-MS/MS analysis, seven compounds were tentatively identified basing on their mass characteristics, except for crocin A which was also identified in comparison with commercial standard.
Results and discussion
Analysis of the volatiles in extracts from saffron was performed by GC and GC/MS
C. sativus samples collected from two different areas of Turkey [Eskisehir (SE) and Safranbolu (SS)] were subjected to micro distillation to extract the volatiles. They were analysed by GC and GC/MS systems, simultaneously. The results of analysis are reported in . Twenty six components were characterised representing 92.1% and 90.0% of the SE and SS samples, respectively. Safranal is the main component in both samples. The analysis revealed the main characteristic components to be 2,6,6-trimethyl-1,3-cyclohexadien-1-carboxaldehyde, namely safranal (64.1% and 49.3%), 3,5,5-trimethyl-2-cyclohexen-1-one, namely α-Isophorone (10.4% and 16.3%), 3,5,5-trimethyl-3-cyclohexen-1-one, namely β-Isophorone (6.4% and 8.4%) in SE and SS, respectively. Results are similar to previously reported studies of saffron.[Citation22] The results presented in show some qualitative differences among the SE and SS. The structures of major compounds are shown in .
Table 1. The volatile compositions of saffron samples cultivated in Eskisehir (SE) and Safranbolu (SS).
In previously work, Zarghami and Heinz reported oxidative breakdown of safranal under UV light resulting in the formation of isoprene-derived compounds.[Citation10] Tarantilis and Polissiou earlier reported the effect of distillation techniques on the composition of the essential oil of saffron. In this work, it was reported that steam distillation method was not suitable for identifying the volatile constituents of saffron due to the formation of breakdown compounds of carotenoids which are not normally present in the original aroma of saffron.[Citation12] Our results demonstrate that micro distillation and the subsequent GC/MS analysis can safely be used to investigate the composition of the saffron volatiles since micro distillation is a milder method of distillation than conventional steam or water distillation.[Citation23]
Evaluating the scavenging activity of saffron extracts against ABTS•+
A number of assays are available for screening the antioxidant activity of pure compounds, food constituents, and body fluids, but few of these methods are applicable to lipophilic substances such as carotenoids and food extracts containing them. The ABTS●+ radical cation has been used to screen the relative radical-scavenging abilities of flavonoids and phenolics through their properties as electron- or H-donating agents. The stable green radical cation shows maximum absorptivity at 734 nm, and the absorbance is decreased with antioxidants during the reaction period depending on the reducing properties. The basis of the method is the generation of a long-lived specific ABTS●+ radical cation chromophore and the relative abilities of antioxidants to quench the radical in relation to that of Trolox, a vitamin E analog. The method is carried out as a decolourisation assay, appropriate for lipophilic (and aqueous) systems in which the antioxidant is added to the preformed radical cation produced on the one-electron oxidation of ABTS●+ and is applied to the monitoring of lipophilic antioxidants such as carotenoids and lipophilic extracts of nutritional components.[Citation24]
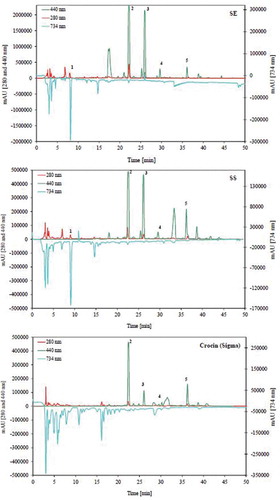
Antioxidant capacity of saffron samples was investigated by ABTS●+ radical scavenging activity test using Trolox as standard, and the results are given in as Trolox equivalent total antioxidant capacity. Saffron samples, their TLC fractions, and some standards were analysed. As seen in , crude saffron extracts have high total antioxidant activity against ABTS●+ radical. However, crocin fractions separated from crude extracts by preparative TLC showed weak antiradical activity. The saffron extracts and fractions were also investigated by HPTLC and HPLC after sequential ABTS●+ derivatisation in order to find responsible compounds for the antiradical activity. HPTLC results are shown in . Crocin purchased from Sigma was also analysed by HPLC-ABTS●+ and HPTLC-ABTS●+ methods, and the results are given in and . Saffron extracts, fractions, and crocin from Sigma were developed on a HPTLC silica F254 plate using ethylacetate/iso-propanol/water (65/25/10) mixture as mobile phase. The plate was investigated at 254 nm, 366 nm, and white light after derivatisation with ABTS●+. Crocin derivatives showed a weak activity. After HPLC and TLC analyses, many compounds were detected in crocin from Sigma. These compounds except for crocin showed antiradical activity ( and ). As seen on the HPLC chromatogram at 440 nm, main peak, crocin, showed the weakest activity.
Table 2. Trolox equivalent total antioxidant capacity of saffron.
Figure 3. HPLC-ABTS+● chromatograms of saffron samples and crocin (Sigma) (SE, saffron from Eskişehir; SS, saffron from Safranbolu; 1, picrocrocin; 2, crocins; 3, crocetin; 4, trans-crocetin mono (β-D-gentiobiosyl) ester; 5, trans-crocetin di (β-D-gentiobiosyl) ester).
After LC-MS/MS analysis, the deprotonated ions were collected using MS/MS detection. The results are given in . According to LC-MS/MS analysis, seven compounds were tentatively identified using with their mass characteristics, except for crocin A which was also identified in comparison with commercial standard. All compounds can be identified after MRM analysis. Crocin and crocetin derivatives (picrocrocin; crocins; crocetin; trans-crocetin mono (β-D-gentiobiosyl) ester; trans-crocetin di (β-D-gentiobiosyl) ester) were detected in saffron extracts using m/z data collected from literature. After both HPLC and LC-MS/MS analyses, kaempferol was not detected in both saffron samples, according to UV spectra and MS data.
Table 3. LC-MS/MS data of extracts of saffron.
Conclusion
In conclusion, crude saffron extracts have shown strong antiradical activity against ABTS●+ radical. Oxidation of foods reduces the shelf life of foods, and free radicals may cause a variety of human diseases. As synthetic antioxidants are known to have many side effects, no wonder why antioxidant-rich saffron has been used for centuries to preserve food in a tasteful fashion.
References
- D’Auria, M.; Mauriello, G.; Rana, G.L. Volatile Organic Compounds of Saffron. Flavour and Fragrance Journal 2004, 19, 17.
- Elefherios, A.P.; Moschos, G.; Gholap, A.; Bongirwar, D. Assessing Saffron (Crocus sativus L.) Adulteration with Plant-Derived Adulterants by Diffuse Reflectance Infrared Fourier Transform Spectroscopy Coupled with Chemometrics. Talanta 2017, 162, 558.
- Li, N.; Lin, G.; Kwan, Y.-W.; Min, Z.-D. Simultaneous Quantification of Five Major Biologically Active Ingredients of Saffron by High-Performance Liquid Chromatography. Journal of Chromatography A 1999, 849, 349.
- Lozano, P.; Castellar, M.; Simancas, M.; Iborra, J. A Quantitative High-Performance Liquid Chromatographic Method to Analyse Commercial Saffron Crocus sativus L. Products. Journal of Chromatography A 1999, 830, 477.
- D’Auria, M.; Mauriello, G.; Racioppi, R.; Rana, G.L. Use of SPME–GC–MS in the Study of Time Evolution of the Constituents of Saffron Aroma: Modifications of the Composition during Storage. Journal of Chromatographic Science 2006, 44, 18.
- Assimopoulou, A.; Sinakos, Z.; Papageorgiou, V. Radical Scavenging Activity of Crocus sativus L. Extract and Its Bioactive Constituents. Phytotherapy Research 2005, 19, 997.
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory Activity on Amyloid-Β Aggregation and Antioxidant Properties of Crocus sativus Stigmas Extract and Its Crocin Constituents. Journal of Agricultural and Food Chemistry 2006, 54, 8762.
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC Quantification of Major Active Components from 11 Different Saffron Crocus sativus L. Sources. Food Chemistry 2007, 100, 1126.
- Kanakis, C.D.; Tarantilis, P.A.; Tajmir-Riahi, H.A.; Polissiou, M.G. Crocetin, Dimethylcrocetin, and Safranal Bind Human Serum Albumin: Stability and Antioxidative Properties. Journal of Agricultural and Food Chemistry 2007, 55, 970.
- Zarghami, N.; Heinz, D. Monoterpene Aldehydes and Isophorone-Related Compounds of Saffron. Phytochemistry 1971, 10, 2755.
- Rodel, W.; Petrzika, M. Analysis of the Volatile Components of Saffron. Journal of High Resolution Chromatography 1991, 14, 771.
- Tarantilis, P.A.; Polissiou, M.G. Isolation and Identification of the Aroma Constituents of Saffron Crocus sativa. Journal of Agricultural and Food Chemistry 1997, 45, 459.
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the Flavonoid Fraction in Saffron Spice by LC/DAD/MS/MS: Comparative Study of Samples from Different Geographical Origins. Food Chemistry 2007, 100, 445.
- Briechle, R.; Dammertz, W.; Guth, R.; Volmer, W. Bestimmung Von Ätherischen Ölen in Drogen. GIT Laboratory Fachz 1997, 41, 749.
- Baser, K.; Demirci, B.; Demirci, F.; Kirimer, N.; Hedge, I. Microdistilasyon as a Useful Tool for the Analysis of Minute Amounts of Aromatic Plant Materials. Chemistry of Natural Compounds 2001, 37, 336.
- McLafferty, F.W.S. The Wiley/NBS Registry of Mass Spectral Data; Journal Wiley and Sons, New York, USA, 1989.
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and related constituents of essential oils. MassFinder 3. Hamburg, Germany: Hochmuth Scientific Consulting.
- Jemings, W.; Shibamoto, T. Quantitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary GC; Academic Press: New York, 1980.
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB-Verlag, Hamburg, Germany, 1998.
- Bacis, E. Boelens Aroma Chemical Information Service; Huizen, The Netherlands, 1999.
- Verma, R.S.; Middha, D. Analysis of Saffron Crocus sativus L. Stigma Components by LC–MS–MS. Chromatographia 2010, 71, 117.
- Urbani, E.; Blasi, F.; Chiesi, C.; Maurizi, A.; Cossignani, L. Characterization of Volatile Fraction of Saffron from Central Italy (Cascia, Umbria). International Journal of Food Properties 2015, 18, 2223.
- Ozek, T.; Demirci, B.; Baser, K.H.C. Comparative Study of the Essential Oils of Heracleum sphondylium Ssp. Ternatum Obtained by Micro-And Hydro-Distillation Methods. Chemistry of Natural Compounds 2002, 38, 48.
- Pellegrin, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of Dietary Carotenoids and Carotenoid-Rich Fruit Extracts for Antioxidant Activities Applying the 2, 2ʹ-Azobis 3-Ethylenebenzothiazoline-6-Sulfonic Acid Radical Cation Decolorization Assay. Methods in Enzymology 1998, 299, 379.