ABSTRACT
Purple basil was used as powder or water extract forms in the manufacture of yogurt for possible contribution on its taste and flavour of yogurt. The volatiles were extracted by solid-phase micro-extraction and then determined by gas chromatography-mass spectrometry (GC-MS) system. Effects of the addition of purple basil as powder or water extract form on the volatile composition and sensory characteristics during 3 weeks of storage at 4°C were evaluated. Forty-nine compounds were identified in the volatile composition of yogurt samples, including 12 ketones and aldehydes, 9 esters, 7 acids, 8 alcohols, 9 terpenes, and 4 miscellaneous compounds. Acetoin, ethyl acetate, hexanoic acid, acetic acid, 1-hexenol, 3-hexen-1-ol, 2-ethylhexenol, dL-limonene, and linalool were most frequently identified volatiles in the yogurt samples. The level of linalool, which was main volatile in basil, was higher in powder basil flavoured yogurt samples than those in extracted basil flavoured yogurt samples. Principal component analysis of the GC-MS data showed that use of basil as powder or water extract form significantly changed the volatile profile of yogurt during storage. Also, sensory scores for basil-free yogurt were higher than yogurt samples manufactured by incorporating basil. In conclusion, use of basil enhanced the volatile composition of yogurt, and basil-flavoured yogurt may be offered for consumers as an alternative type of flavoured yogurt.
Introduction
Yogurt is a dairy product obtained by fermenting milk of two species including Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus.[Citation1] It is among the most common fresh dairy products consumed around the world, and its acceptability by the consumer is largely depended on its sensory properties.[Citation2] Yogurt consumption tends to gradually increase in the world. Although plain yogurt consumption is high in Balkans, the Middle East, South Asia, North Africa, and Arab countries, flavoured yogurt consumption is high in Europe and North America because of non-preferrable natural acidic taste of plain yogurt.[Citation3] Development of dairy products, especially yogurt, with new flavours provides an advantageous option for consumers.[Citation4] Several authors stated that popularity of yogurt increases by the addition of different sources of fruit flavourings.[Citation5,Citation6] Ye et al[Citation7] showed that sensory evaluation in terms of appearance and flavour scores of hickory-black soybean yogurt had no significant difference from cow milk yogurt.[Citation7]
Flavour in yogurt is formed by action of yogurt starter bacteria and originated from biochemical changes in carbohydrates, lipids, and proteins. The flavour in the final product is characterised by the correct balance of natural flavour constituents present in milk and generated by the metabolism of yogurt starter bacteria.[Citation8] Starter cultures are primarily responsible for the production of the flavour compounds which contribute to the aroma of yogurt. These compounds may be divided into four main categories including non-volatile acids (lactic, pyruvic, oxalic, or succinic), volatile acids (formic, acetic, propionic, or butyric), carbonyl compounds (acetaldehyde, acetone, acetoin, or diacetyl), and miscellaneous compounds (certain amino acids and/or constituents formed by thermal degradation of protein, fat, or lactose).[Citation9]
The Ocimum genus (Lamiaceae) consists of annual and perennial herbs and scrubs native to the tropical and subtropical regions of Asia, Africa, and Central South America. While the Ocimum genus includes more than 150 species, it is thought that only 65 species of Ocimum may be available in the literature.[Citation10] Ocimum basilicum or Basil is called as ‘feslegen’ or ‘reyhan’ in Turkish.[Citation11,Citation12] It is an aromatic plant that is used extensively to add a distinctive aroma and flavour for food or meals[Citation10,Citation13] and widely used to enhance the flavour of foods such as salads, pasta, tomato products, vegetables, pizza meat, soups, marine foods, confectioneries, and other products.[Citation11,Citation14,Citation15] The major compounds for the typical basil aroma are 1,8-cineole, methyl cinnamate, methyl chavicol, and linalool.[Citation16,Citation17] Fresh basil is also consumed with yogurt in Turkey. The main objectives of this study were to develop a dairy product with a new flavour, understand any positive contribution for yogurt flavour from extract or powder form of basil, and determine consumer panel responses to basil flavoured yogurt during storage.
Materials and methods
Basil samples
Purple basil (Ocimum bacilicum L.) samples averaging 1 kg were cultivated from Arapgir (39° 02ʹ N, 38° 29ʹ E) county of Malatya (Turkey) with growing areas between June and September. Purple basils were used in yogurt production either shade-dried (powder form) or water extract. For preparation of water, dried basil was mixed with water at 1:10 (for sample A) or 1:25 (for sample B) and then centrifuged at 4°C at 2,000 rpm for 10 min. The mixture was rested for 12 h at 70°C and stored in a cold room before use.
Yogurt manufacture
Two replicate trials were carried out in yogurt production. Raw cow’s milk (25 L) was pasteurised at 85°C for 5 min and cooled to 47°C. The milk was divided into five parts. One part (5 L of milk) was used as control (sample K). The rests were as given in . After blending of milk with basil extract and powder, the mixtures were separately homogenised using an Ultra Turrax blender (IKA, Werk, Germany) at 14,000 rpm until all ingredients were mixed. Samples were inoculated with yogurt starter culture consisting of a mixture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus at a level of 3% (w/v), dispersed into plastic cups, ca. 200 g, and incubated at 43 ± 1°C until pH 4.7. Following incubation, all samples were kept at room temperature (21°C) for about 30 min and then immediately moved to a cold room. The yogurt samples were stored at 4°C for 21 d and sampled at 1, 7, 14, or 21 d of storage.
Table 1. Experimental design for yogurt samples.
Determination of volatiles in yogurt samples
Frozen yogurt samples were placed immediately in glass bottles in a freezer at −20°C. A duplicate 3.0 g portion of the sample was then placed in a 15 mL vial (Supelco, Bellefonte, PA, USA), followed by 10 μL of internal standard containing 81 mg/kg of 2-methyl-3-heptanone and 2-methylpentanoic acid in methanol (Sigma-Aldrich Co., St. Louis, MO, USA), and allowed to equilibrate at 40°C for 30 min. Extraction of volatiles was carried out using Head Space Solid Phase Micro Extraction (HS-SPME) technique. Extraction of volatiles was carried out using 2 cm 50/30 μm divinilybenzene-carboxen-polydimethylsiloxane (DVB/CAR/PDMS) fibre (Supelco, Bellefonte, PA, USA), and the volatile compounds were separated on a DB-Wax column (60 m × 0.25 mm × 0.25 μm; J&W Scientific, Folsom, CA, USA).[Citation18] The fibre was positioned at 3.0 scale units in each run. Desorption of the extracted volatiles was carried out using a Shimadzu GC-2010 gas chromatography—QP-2010 mass spectrometry system (Shimadzu Corporation, Kyoto, Japan) and run in splitless mode. During desorption, the fibre remained in the injector for 2 min at a temperature of 250°C, with helium as the carrier gas at a flow rate of 1.0 mL/min. The oven was held at 40°C for 2 min (desorption period), and then the temperature was increased at the rate 5°C per min to 70°C, where it was held for 1 min. The temperature was then raised at 10°C per min to 240°C, to give a run time of 30 min. The compounds were calculated as given in the method Kovats[Citation19], and the data were expressed as µg/kg of cheese.
Sensory analysis
Sensory analysis of the yogurt samples was evaluated after 1, 7, 14, and 21 d of storage by eight graders (from the permanent staff of the Department of Food Engineering, Inonu University, Turkey) who were experienced yogurt tasters. They evaluated the yogurt samples for colour, appearance, structure and consistency, serum separation, sourness, odour intensity, taste and general acceptability (scale 1 to 5). Samples were removed from the refrigerator about 1 h before evaluation and kept at room temperature. Approximately 400 g of yogurt was presented to each panel member. Water and bread were also provided to the panelists to rinse their mouths between samples. Graders were requested to rank the samples in order to their overall quality or general acceptability.
Statistical analysis
The data were analysed using the SPSS 16.0 statistical software programme (SPSS Inc., Chicago, IL, USA) for windows. All analyses were performed at two times, and results were given as mean ± standard deviation (SD). To compare the significant differences of the mean values at P < 0.05, Duncan’s multiple-range tests were used. Data from volatile components were also analysed using multivariate statistical techniques for easy interpretation of the data from GC-MS.
Results and discussion
Volatile composition
Fourty-nine compounds were identified in the volatile fractions of yogurt samples, including 12 ketones and aldehydes, 9 esters, 7 acids, 8 alcohols, 9 terpenes, and 4 miscellaneous compounds. The compounds identified from the yogurts are listed by chemical groups in –. The largest volatile compounds group was ketones and aldehydes. A total of 12 ketones and aldehydes were identified in yogurt samples. Acetoin with creamy and butter-like flavour was identified in all samples. Acetoin is readily converted from diacetyl by diacetyl reductase and so, its flavour is weaker than diacetly.[Citation8] The concentration of acetoin was higher than acetaldehyde and diacetyl. Guler and Park[Citation20] analysed yogurt sold in Hatay province (Turkey) and reported that the amounts of acetoin in their samples changed from 3.63 to 12.79 µg/100 g. These values were higher than the findings of the present study for the concentration of acetoin. Acetaldehyde, which is characterised as green apple or nutty flavour, is known as a key compound for typical flavour of yogurt.[Citation8] The level of acetaldehyde in the samples was significantly influenced by addition of purple basil at the beginning of storage. While the concentration of acetaldehyde was 2.8 mg/kg in control sample, it changed from 1.0 to 1.6 mg/kg in basil-added samples (P < 0.05). As expected, the concentration of acetaldehyde decreased by storage.[Citation5,Citation21] Diacetyl is another carbonyl compound produced by the action of yogurt starter bacteria and contributed to the yogurt flavour.[Citation1,Citation22] The concentration of diacetyl was higher in basil-added yogurt samples compared with control sample (). On the other hand, no significant differences were observed between the samples in terms of hexenal and 2-heptanone.
Table 2. Ketones and aldehydes in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
Table 3. Esters in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
Table 4. Acids in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
Table 5. Alcohols in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
Table 6. Terpenes in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
Table 7. Miscellaneous compounds in control (K) and purple basil flavoured (A, B, C, and D) yogurt samples (µg/kg yogurt).
A total of 10 esters were identified in the samples (), and ethyl acetate was the predominant ester for all samples. Ethyl esters with sweet and fruity notes are originated from enzymatic or chemical esterification of acids with ethanol.[Citation8,Citation23] Use of purple basil (extract or powder form) did not change the concentrations of some esters including methyl propanoate, methyl butanoate, isobutyl isobutanoate, butyl isobutanoate, and butyl butanoate. On the other hand, the highest concentrations of methyl cinnamate were identified in sample C manufactured using higher amount of basil powder. Methyl cinnamate is originated from basil and is a dominant volatile component of basil.[Citation13,Citation17] So, as expected, this volatile compound is not identified in control yogurt samples. In general, the concentration of esters in yogurt samples were considerably low level (<1 mg/kg) and not influenced by use of purple basil with exception of methyl cinnamate. Normally, esters are found in yogurt at low concentrations[Citation8] or appeared in salted yogurt after 30 days of storage.[Citation24]
Due to an acidic taste of yogurt, some volatile carboxylic acids are present and contribute to the flavour of yogurt. Acidity is a crucial factor for flavour acceptance of yogurt and maintained near pH 4.4.[Citation8] The concentrations of carboxylic acids in yogurt samples were significantly higher than other chemical groups (e.g., aldehydes, esters, alcohols), and fluctuations were observed in their amounts during storage (). Acetic acid was one of the main acids in yogurt samples, and significant differences were observed between samples in terms of its concentration. Samples of C containing 1% of basil powder exhibited the highest values of acetic acid between all samples during storage. Butanoic, hexanoic, and octanoic acids were also determined in all samples at substantial levels (near the concentration of acetic acid), and the concentration of these acids was almost maintained during storage. It was reported that while hexanoic acid is responsible for sour note, acetic acid is responsible for vinegar and acid notes.[Citation25] The concentration of hexanoic acid at the first day of storage in K and B samples was 29.3 µg/kg and 41.2 µg/kg, respectively. Our results were lower than those reported by Guler and Gursoy-Balcı,[Citation21] for hexanoic and acetic acid in yogurt samples with amounts of 1.2 to 2.1 µg/g.[Citation21]
Alcohol is one of the most important volatile groups contibuted in yogurt flavour. The most abundant alcohols identified in yogurt samples were 1-hexenol, 3-hexen-1-ol (Z), and 2-ethylhexanol. Higher concentrations of ethanol and 1-hexanol were identified in the D samples compared with others. 3-Hexen-1-ol, benzene methanol, and benzene ethanol did not identify in control (K) sample (). Ethanol content was found in the range of 1.2–5.1 mg/kg[Citation26] and 2.86–9.15.[Citation20] The samples of C and D contained higher levels of alcohols among the samples, especially in ethanol, 1-hexanol, 1-octanol, and benzene methanol. Use of basil (in particular, powder form) caused an increase in the concentration of alcohols ().
Terpenes are of plant origin and transformed into milk and milk products by feeding[Citation27] or addition of plant material to the dairy product for certain purposes. In this study, the milk was converted to yogurt after addition of basil at water extract or powder forms. So, it can be said that almost all terpenes identified were basil originated. A total of nine terpenes were identified in yogurt samples. Significant differences in concentration of terpenes were observed between the samples. The results showed that use of basil (especially powder form) significantly increased the concentration of some compounds including β-myrcene, dl-limonene, and carvone. 1,8-Cineole, linalool oxide cis, and linalool which are originated from basil, regardless of agricultural and environmental conditions,[Citation13,Citation17] did not identify in control sample. The availability of these compounds implies the presence of basil in yogurt, and the samples were separated by multivariate statistical analysis (see ). Linalool, 1,8-cineole, linalool oxide cis, and α-terpineol were not detected in control sample, while the concentration of these compounds were dramatically high in basil (especially powder) added yogurt samples. So, these results imply that the above compounds are originated from basil and strongly contributed to the yogurt flavour.
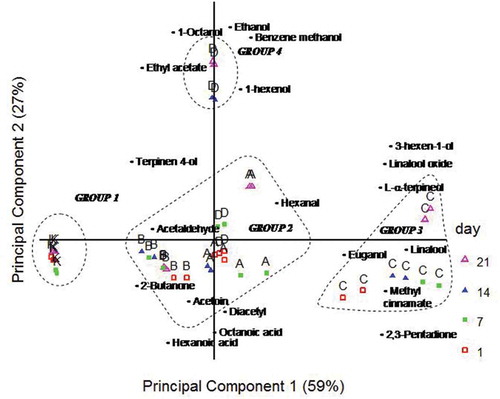
Figure 1. Bi-plots of principal components 1 and 2 showing the sample scores and variable loadings from principal component analysis of the volatile data for control (K) and purple basil flavoured (A, B, C, and D) yogurt samples after 1, 7, 14 and 21 days of storage.
A total of four miscellaneous compounds including p-xylene, styrene, methyleugenol, and eugenol were identified in yogurt samples. Methyl euganol and euganol were determined in basil-added samples at different levels and not determined in control (K) samples (). The levels of methyl euganol and euganol in sample C were higher than other samples. Moreover, the concentrations of these compounds were increased by storage, probably due to release of them in yogurt matrix by time. The levels of p-xylene and styrene were almost the same in all samples (P > 0.05).
Principal component analysis (PCA) was performed on volatile data from GC-MS analysis to determine the effects of the addition of purple basil. The obtained PCA plot is shown in . Principal component (PC) 1 and PC 2 explained 86% of the variance (). There were four different groups, and the samples were positioned based on the concentrations of volatiles. Control sample (K) was positioned at the left side of PC1, and all K yogurt samples were located together regardless of storage/sampling time. Sample C (group 3) was positioned in the positive side of PC1. Significant differences were observed between fresh and 21-day-old yogurts of this group. Sample C is associated with the higher levels of linalool, euganol, methyl cinnamate, linalool oxide, L-α-terpineol (which are originated from purple basil), 3-hexen-1-ol, and 2,3-pentadione ( and –) when compared to other samples. The levels are of these compounds significantly changed by storage, and the same age-related differences were observed for sample D, that is, fresh sample centred, while 14- or 21-day-old yogurt samples are located in the positive side of PC2 (Group 4). Higher levels of ethanol, ethyl acetate, and benzene methanol distinguished the sample D from other yogurts, as shown in . The most important separation was observed for K (basil-free) sample. The K sample is positioned in the right side of PC1 regardless of storage time. According to these results, PCA was a powerful tool to distinguish the samples based on treatment (use of basil in yogurt production) and in less extent storage time.
Sensory analysis
shows the sensory scores of eight panelists for the control and basil flavoured yogurts depending on colour, appearance, consistency, serum separation, sourness, odour intensity, taste, and general acceptability (on scale 1 to 5). As shown in , K sample was the most popular yogurt in terms of colour and appearance scores during storage. Yogurts made from basil extract (A and B) were much preferred than those made from powder of basil (C and D). The differences in colour value of all yogurts were not statistically significant (P > 0.05) during storage. Consistency is one of the most important criteria for set-type yogurt.[Citation1] Consistency scores were higher in the K and B samples compared with other samples (P < 0.05). In general, yogurt samples made with basil extract (A and B) had more acceptability than the yogurts made with basil powder (C and D). No significant differences were observed in terms of serum separation between the samples, and addition of basil at powder or extract form did not exhibit any changes in the serum separation. Sourness scores for yogurt samples were higher at 14th days of storage than other sampling days. Furthermore, use of basil in yogurt production significantly influenced the odour and taste scores (P < 0.05). The K, A, and C samples had higher odour intensity scores during storage (with exception of the first day). Finally, while the panelists preferred K yogurt at the beginning of storage, the scores of basil-flavoured samples (especially A and B yogurts) gradually increased during storage.
Table 8. Sensory scores obtained by sensory evaluation for control (K) and purple basil flavoured (A, B, C, and D) yogurt samples.
Conclusions
Volatile compounds and sensory analysis of basil flavoured yogurt samples were determined after 1, 7, 14, and 21 days of storage. Forty-nine compounds were identified in the volatile fractions of yogurt samples, including 12 ketones and aldehydes, 9 esters, 7 acids, 8 alcohols, 9 terpenes, and 4 miscellaneous compounds. Acetoin, ethyl acetate, hexanoic acid, acetic acid, 1-hexenol, 3-hexen-1-ol, 2-ethylhexenol, dL-limonene, and linalool were the most abundant volatile compounds identified in yogurts. Linalool amount in powder basil flavoured yogurt samples (C and D) was higher than extract basil flavoured yogurt samples (A and B). cis-Linalool oxide and α-terpineol, which were originated from basil, were observed in all samples of yogurt with exception of K sample. Sensory evaluation for the samples showed that the control sample received higher scores than those of basil-flavoured samples, probably due to extraordinary flavour and appearance of basil-flavoured yogurts. However, the assessors pointed out that the basil flavouring may be good alternative for flavoured yogurts.
References
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology; DC: Woodhead Publishing Ltd: Boca Raton Boston New York Washington, 2007; pp. 608–645.
- Saint-Eve, A.; Levy, C.; Martin, N.; Souchon, I. Influence of Proteins on the Perception of Flavored Stirred Yogurts. Journal of Dairy Science 2006, 89, 922–933.
- Ozer, B. Yoğurt Bilimi Ve Teknolojisi; SIDAS: İzmir, Turkey, 2006; 488.
- Boeneke, C.A.; Aryana, K.J. Effect of Folic Acid Fortification on the Characteristics of Lemon Yogurt. LWT - Food Science and Technology 2008, 41, 1335–1343.
- McGregor, J.U.; White, C.H. Effect of Sweeteners on Major Volatile Compounds and Flavor of Yogurt. Journal of Dairy Science 1987, 70, 1828–1834.
- Barnes, D.L.; Harper, S.J.; Bodyfelt, F.W.; NcDaniel, N.R. Correlation of Descriptive and Consumer Panel Flavor Ratings For. Journal of Dairy Science 1991, 74, 2089–2099.
- Ye, M.; Ren, L.; Wu, Y.; Wang, Y.; Liu, Y. Quality Characteristics and Antioxidant Activity of Hickory-Black Soybean Yogurt. LWT - Food Science and Technology 2013, 51, 314–318.
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Critical Reviews in Food Science and Nutrition 2010, 50, 938–950.
- Tamime, A.Y.; Robinson, R.K. Yoghurt Science and Technology; Woodhead Publishing: London, UK, 1999; 622.
- Labra, M.; Miele, M.; Ledda, B.; Grassi, F.; Mazzei, M.; Sala, F. Morphological Characterization, Essential Oil Composition and DNA Genotyping of Ocimum basilicum L. Cultivars. Plant Science 2004, 167, 725–731.
- Chalchat, J.C.; Ozcan, M.M. Comparative Essential Oil Composition of Flowers, Leaves and Stems of Basil (Ocimum basilicum L.). Used as Herb. Food Chemistry 2008, 110, 501–503.
- Giachino, R.R.A.; Sonmez, C.; Tonk, F.A.; Bayram, E.; Yuce, S.; Telci, I.; Furan, M.A. RAPD and Essential Oil Characterization of Turkish Basil (Ocimum basilicum L.). Plant Systematics and Evolution 2014, 300, 1779–1791.
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of Volatile Components in Basil (Ocimum basilicum L.) and Thyme Leaves (Thymus vulgaris L.) and Their Antioxidant Properties. Food Chemistry 2005, 91, 131–137.
- Ghasemi Pirbalouti, A.;. Diversity in Chemical Composition and Yield of Essential Oil from Two Iranian Landraces of Sweet Basil. Genetika 2014, 46, 419–426.
- Díaz-Maroto, M.C.; Palomo, E.S.; Castro, L.; Viñas, M.A.G.; Pérez-Coello, M.S. Changes Produced in the Aroma Compounds and Structural Integrity of Basil (Ocimum basilicum L) during Drying. Journal of the Science of Food and Agriculture 2004, 84, 2070–2076.
- Barbieri, S.; Elustondo, M.; Urbicain, M. Retention of Aroma Compounds in Basil Dried with Low Pressure Superheated Steam. Journal of Food Engineering 2004, 65, 109–115.
- Klimánkova, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma Profiles of Five Basil (Ocimum basilicum L.) Cultivars Grown under Conventional and Organic Conditions. Food Chemistry 2008, 107, 464–472.
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley-VCH: New York: NY, USA, 1997; 264.
- Kovats, E. Gas-Chromatographische Charakterisierung Organischer Verbindungen. Teil 1: Retentionsindices Aliphatischer Halogenide, Alkohole, Aldehyde Und Ketone. Helvetica Chimica Acta 1958, 41, 1915–1932.
- Guler, Z.; Park, Y.W. Characteristics of Physico-Chemical Properties, Volatile Compounds and Free Fatty Acid Profiles of Commercial Set-Type Turkish Yoghurts. Open Journal of Animal Sciences 2011, 1, 1–9.
- Guler, Z.; Gursoy-Balcı, A.C. Evaluation of Volatile Compounds and Free Fatty Acids in Set Types Yogurts Made of Ewes’, Goats’ Milk and Their Mixture Using Two Different Commercial Starter Cultures during Refrigerated Storage. Food Chemistry 2011, 127, 1065–1071.
- Beshkova, D.; Simova, E.; Frengova, G.; Simov, Z. Production of Flavor Compounds by Yogurt Starter Cultures. Journal of Industrial Microbiology and Biotechnology 1998, 20, 180–186.
- Molimard, P.; Spinnler, H.E. Dairy Foods Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. Journal of Dairy Science 1996, 79, 169–184.
- Guler, Z. Changes in Salted Yogurt during Storage. International Journal of Food Science and Technology 2007, 42, 235–245.
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheese during Ripening: A Review. Lait 2000, 80, 293–324.
- Ott, A.; Germond, J.-C.; Baumgartner, M.; Chaintreau, A. Aroma Comparisons of Traditional and Mild Yogurts: Headspace Gas Chromatography Quantification of Volatiles and Origin of Α-Diketones. Journal of Agricultural and Food Chemistry 1999, 47, 2379–2385.
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as Determined by Gas Chromatography-Olfactometry. International Dairy Journal 2002, 12, 959–984.
