ABSTRACT
Antarctic krill (Euphausia superba) has a very high economic value due to its enormous production, higher protein content, and nutritional value. This study presents a detailed optimization of the analysis of volatile flavor compounds from Antarctic krill by combining headspace solid-phase microextraction (HS-SPME) as the extracting method and gas chromatography/mass spectrometry (GC-MS) as the analysis. An optimal solid-phase microextraction (SPME) fiber was selected, and the microextraction conditions were optimized using the response surface method (RSM). The Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) fiber showed the highest extraction efficiency and was selected for further optimization of extraction temperature, extraction time, and sodium chloride concentration. Forty-two types of volatile substances producing characteristic flavors were detected, seven of which contributed significantly to the flavor of Antarctic krill. Ethers, pyrazine, and aldehyde compounds contribute to the main flavor of Antarctic krill. The compounds 2-ethyl-3, 5-dimethyl-pyrazine, 2, 3, 5-trimethyl-6-ethyl-pyrazine, and 3-ethyl-2, 5-dimethyl- pyrazine produce the roasted flavor and the characteristic nutty odor. Moreover (E, Z)-2, 6-nonadienal adds a cucumber fragrance. The compounds 3-methyl-butanal and 3-methional play an important role in determining the overall flavor.
Introduction
Solid-phase microextraction (SPME) has found widespread application in the delectation of volatile compounds for its versatility and simplicity.[Citation1] Compared with other gas-phase extraction techniques, SPME achieves the simultaneous extraction of multiple substances, and it has been applied in several studies characterizing the volatile substance of fish and shrimp.[Citation2] However, SPME efficiency depends on the type of the fiber coatings of the headspace (HS), i.e. the extraction of nonpolar compounds can be enhanced with polymethylsiloxane fibers but can hardly be conducted with carbowax fibers.[Citation3] In addition, different factors, such as the state between vapor and liquid phases, temperature, sodium chloride addition, and stirring, all influence the properties of the analytes.[Citation4]
With an estimated total biomass for 10–30 million tonnes, and annual catches of 1.17–3.79 tonnes, Antarctic krill is one of the world’s largest animal meat sources.[Citation5] It has a high nutritional value; it is rich in protein (11.9–15.4%) with a biological value proven to be higher than that of milk proteins and other meat proteins, but lower than that of egg proteins.[Citation6,Citation7] Apart from its use as a fish protein powder, Antarctic krill could also be processed into protein supplements, condiment, and functional foods, such as shrimp paste, shrimp surimi, and krill oil, indicating its wide application potential in the aquatic product processing industry.[Citation8] However, aquatic products generally have a fishy odor, which consumers generally reject, restricting their exploitation and utilization. Previous studies of Antarctic krill have mostly concentrated on defluorination, protein recovery, and chitin-degrading enzymes,[Citation9–Citation11] while few researches have focused on the problem of fishy odors of aquatic products.
In this study, the volatile flavor compounds of Antarctic krill were analyzed by SPME coupled with GC-MS. We optimized the extraction parameters, qualitatively determined Antarctic krill volatile flavor compounds by the optimized protocol, and discuss the feasibility of HS-SPME for the extraction of Antarctic krill volatile flavor compounds.
Materials and methods
Materials and chemicals
The Antarctic krill used in this study was provided by CNFC Overseas Fisheries Co., Ltd. and stored at −20°C until use. A stock solution of the internal standard for SPME, 2-methoxy-5-nitro-pyridine, was prepared in methanol at a concentration of 1000 mg/L. Sodium chloride was treated at 550°C for 3 h. The n-alkanes standard (C5-C20) was provided by the Chinese Academy of Sciences. Three SPME fibers (65 μm polydimethylsiloxane/divinylbenzene (65 μm PDMS/DVB), 75 μm CAR/PDMS carboxen/polydimethyl-siloxane (75 μm CAR/PDMS), and 50/30 μm Divinylbenzene/Carboxen/Polydimethylsiloxane (50/30 μm DVB/CAR/PDMS)) for SPME were purchased from Supelco (Bellefonte, PA, USA).
HS-SPME sampling conditions
For HS-SPME analysis, homogenized krill sample (6 g) was transferred into a 15 mL vial, NaCl and 2 μL of internal standard stock solution were added, and the vial was sealed with a Polytetrafluoroethylene (PTFE)-silicone septum and screw cap, and incubated at 65°C for 45 min to equilibrate. To remove any possible contaminants, each of the three fibers tested was conditioned before usage according to the manufacturer’s instructions. Before extraction, each fiber was reconditioned for 10 min at 250°C in the GC injector port. The fiber coating was exposed to the headspace for 10–60 min (depending on the test) at temperatures ranging from 35°C to 85°C. Then the fiber was transferred to an injection port, and desorbed for 5 min at 250°C.
Experimental data were fitted to a second-order polynomial model and regression coefficients were obtained. The generalized second-order polynomial model used in the response surface analysis was as follows:
where Y is the measured response variable; β0 is a constant; βi, βii, and βij are the linear, quadratic, and cross-product regression coefficients, respectively; and Xi and Xj represent the hydrolysis parameters. Mathematical models were evaluated for each response by means of multiple regression analysis. Significant terms in the model for each response were identified by analysis of variance (ANOVA) and significance was determined by the F-statistic calculated from the data.
Gas chromatography/mass spectrometry
The GC-MS system consisted of an Agilent 6890N GC with a 5973N mass selective detector (Agilent Technologies Inc., USA). One microliter of the extract was injected into an HP-5MS column (30 m × 0.32 mm × 0.25 μm, Agilent Inc., USA). The injector temperatures were set at 250°C. Helium was used as a carrier gas at a flow rate of 0.8 mL/min. The oven temperature was isothermal at 40°C for 3 min, increased to 250°C at 5°C/min, and remained isothermal at 250°C for 5 min. The following conditions were used for detection: ion source temperature 230°C; quadrupole temperature 280°C; electron impact ionization with 70 eV energy; mass range 35–350 amu.
Identification and quantitation of volatile components
The volatile compounds were identified on the basis of mass spectra (Wiley/NIST 2008) using Kovats retention index (KI) with references. Based on the retention time of standard n-alkanes, KI for the unknown compounds was calculated using the following:[Citation12]
where X is the retention time, I is an unknown substance, and n-alkanes Z having Z carbon atoms. The pending material X value should be between two adjacent n-alkanes X values, XZ < Xi < XZ+1. C5-C20 n-alkanes (Carbosynth China Ltd., Suzhou, China) were dissolved in dichloromethane at 0.2% and were run under the same chromatographic conditions for calculating the KI of the unknown compounds. KIs were compared with those in the Pherobase (www.pherobase.com), LRI & Odour (www.odour.org.uk), and Flavornet (www.flavornet.org) databases, as well as those reported in the literature for compound identification.[Citation13] For each extraction, 10 μL of 2, 4, 6-trimethyl pyridine (0.948 mg/mL of methanol) as an internal standard was added to the sample.
Estimation of the relative response factors
Solutions containing 10 standard volatile compounds (octanol, hexanal, 2-heptanone, dimethyl disulfide, hexanoic acid ethyl ester, undecane, 2, 6-dimethyl-pyrazine, phenol, indole, and trimethylamine) were used. Five concentrations of each compound were analyzed. The analyses were carried out under the same SPME conditions as that used for sample analysis, using 50/30 μm DVB/CAR/PDMS fiber. Relative response factors were calculated using the following formula:
Relative response factor = (concentration of the standard volatile compound) /(peak area of the standard volatile compound) * (peak area of the internal standard) /(concentration of the internal standard).
Relative response factors used for subsequent calculations were the means of the response factors for the five concentrations tested.
Statistical analysis
Based on the chromatographic peak areas, extraction time and salt addition were optimized to maximize the extraction recovery. Data from three independent experiments were analyzed and expressed as the mean ± SD. Statistical significance was set at p < 0.05. All statistical analyses were processed with SPSS 19.0 (IBM SPSS, Armonk, NY, USA).
Results and discussion
Selection of the fiber coating
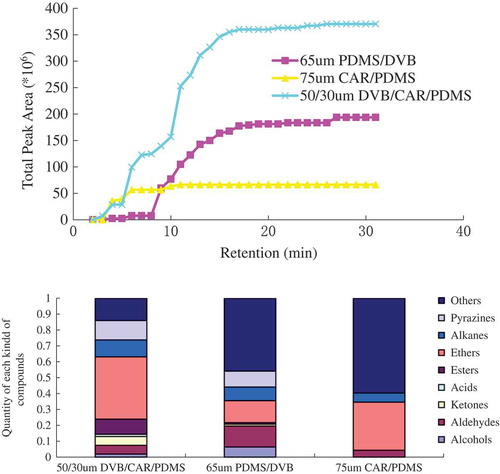
To optimize the HS-SPME technique for the analysis of Antarctic krill volatile compounds, parameters affecting the analysis efficiency, such as fiber coating, HS volume, extraction temperature, extraction time, and desorption conditions, were studied and optimized. The chemical properties of the Antarctic krill volatile compounds determine the optimum type of fiber coating. To select the most appropriate coating for HS-SPME, three commercial SPME fiber coatings were evaluated, including 50/30 μm DVB/CAR/PDMS, 65 μm PDMS/DVB, and 75 μm CAR/PDMS fibers. As shown in , the chromatographic response for the different fibers depended on analyte volatility, retention time, and polarity. At the same retention time, a wider cumulative area indicates higher fiber sensitivity. The 50/30 μm DVB/CAR/PDMS fibers extracted the highest number of compounds, followed by the 65 μm PDMS/DVB fibers. The total compounds extracted with 65 μm PDMS/DVB and 75 μm CAR/PDMS were only 28 and 6, respectively, which were much lower than the number of compounds extracted with 50/30 μm DVB/CAR/PDMS (). This is mainly because of the different polarities of the coatings.[Citation14] DVB/CAR/PDMS fiber enabled the detection of a wide range of volatile compounds extracted from oxidized Atlantic horse mackerel muscle.[Citation15] According to the instructions, DVB/CAR/PDMS is an adsorption-based fiber made of PDMS, which allows the absorption of less polar substances, and CAR and DVB, which are porous coating materials that provide high selectivity and sensitivity for more polar compounds. Bases on the results, DVB/CAR/PDMS was selected as the most appropriate fiber for obtaining the widest composition profile of Antarctic krill volatile compounds, and was used in all further experiments.
Optimization of HS-SPME conditions
The temperature and time of extraction and adsorption that affect the equilibrium are the most important factors for the HS-SPME-GC/MS analysis of flavors. The addition of sodium chloride improves sample extraction efficiency through a salting-out effect. Higher temperatures promote extraction efficiency and induce desorption of the components from the fibers. The response surface method (RSM) was used to optimize the SPME procedure and model equations were proposed. Peak number (Y1) and peak area (Y2) at optimum conditions (temperature 85.0°C, time 55.45 min, NaCl 1.39 g) were 50.9471 and 4.10536 × 108, respectively.
ANOVA of the fitting models demonstrated that the statistical model was significant at a 99% confidence level (p < 0.01). Lack of fit was not significant for all response surface models at a 95% confidence level, which means that all data were in accordance with the model. Meanwhile, R2, adj-R2, and the coefficient of variation (CV) were calculated to check the model feasibility. The R2 values were 0.9654 and 0.9618 for peak number and peak area, respectively, indicating that the variation could be accounted for satisfaction of the data. However, a high value of R2 does not always indicate a good regression model. It is better to use adj-R2 to evaluate the model adequacy. The R2 and adj-R2 values for the model did not significantly differ, indicating that no nonsignificant terms had been included in the model. The CV is the intrasubject standard deviation, expressed as a percentage of the mean, and smaller values of CV indicate better reproducibility.[Citation16,Citation17] The CV values were found to be 15.51 and 19.41 for peak number and peak area, respectively. To compare the predicted peak number and area with actual values, the experiment was performed using optimal conditions. The experimental peak number and area were 51 and 4.2 × 108 (means of three experiments), respectively, which were in good agreement with the predicted values. These results confirmed that the response model was valid and applicable for predicting the experimental results and that RSM is an efficient approach to optimizing the HS-SPME procedure.
Volatile compounds in Antarctic krill as analyzed by HS-SPME-GC-MS
Antarctic krill should have a good taste and flavor, which depend on the composition of volatile compounds. Thus, the identification and quantification of the volatile compounds affecting flavor or off-flavor are considered key for quality control. As shown in , 12 aldehydes and two ketone aromatic compounds were detected. Of these, 2-heptanone, benzaldehyde, nonanal, and decanal were the most abundant ketones and aldehydes. Benzaldehyde is used as an artificial essential oil of almond and typically contained in cherry flavors.[Citation18]
Table 1. Effect of different SPME fibers on extraction efficiency.
Table 2. Volatile components identified in samples by HS-SPME-GC-MS.
Volatile ethers and pyrazine compounds in Antarctic krill
Volatile ethers and pyrazine compounds can contribute to desirable aroma as well as rancid odor, which can be produced by microbial action, lipid autoxidation, or enzymatic reactions.[Citation6,Citation9] As shown in , four ethers and eight pyrazines were identified in Antarctic krill. 3-ethyl-2, 5-dimethyl-pyrazine was the most abundant pyrazine and thus may play a major role in the aroma of Antarctic krill. 2-Ethyl-3, 5-dimethyl-pyrazine, 2, 3, 5-trimethyl-6-ethyl-pyrazine, and 3-ethyl-2, 5-dimethyl-pyrazine were detected with relative contents normalized to the 2-methoxy-5-nitro-pyridine standard of 529%, 228%, and 72%, respectively. Pyrazines are important compounds responsible for food flavor and have been studied principally for their characteristic flavor notes in various foods.[Citation19] They possess unique organoleptic characteristics that can dramatically influence the sensory aspects of food.[Citation20,Citation21] Pyrazines commonly provide a roasted flavor and a nutty and toasted odor.[Citation22,Citation23] Some studies have reported that pyrazines smell like roasted potato,[Citation23] and have been studied principally in virtue of their characteristic flavor notes in various foods.[Citation19] Esters such as hexyl acetate, present in seafood products, are associated with fruity sensory notes.[Citation24,Citation25] As shown in , esters were a minority compared with pyrazines.
Aldehyde compounds in Antarctic krill
Aldehyde compounds detected and identified in Antarctic krill are shown in . Twelve types of aldehydes were detected. With low threshold values, aldehydes are important contributors to the total flavor. Aldehydes are the hydrogen peroxide degradation products of linoleic acid ester and linolenic acid.[Citation26] With a low threshold value, (E, Z)-2, 6-nonadienal, which adds the cucumber fragrance, largely contributes to the overall flavor; it is produced by the degradation of ω-3 polyunsaturated fatty acids. Moreover, (E, Z)-2, 6-nonadienal can be degraded to (Z)-4-heptenal through the inverse-aldol condensation in an aqueous medium, e.g. in vegetable and flax oil. The compound 3-methyl-butanal with an almond, cashew nuts flavor is produced from valine, isoleucine, and leucine amino acid degradation via Strecker degradation, and can be oxidized to carboxylic acid or methyl alcohol, which is reduced to 3-methyl-butanol.[Citation27] The compound 3-methional, generally found in onion and meat, exists in cooked crab, crawfish tail meat, and the baked krill. In summary, Antarctic krill contains mainly cucumber, nut, and meat flavors.
Hydrocarbons in Antarctic krill
Multiple hydrocarbon compounds were detected in the krill, which may be due to lipid autoxidation or the decomposition of carotenoids (astaxanthin) by radicals. However, the hydrocarbon sensory threshold is high and these compounds contribute little to the overall flavor.
Other volatile compounds in Antarctic krill
A large number of other volatile compounds, including amides, pyridines, aromatic hydrocarbons, terpenes, and sulfur compounds, were detected in Antarctic krill, which are also very important for its aroma due to their low threshold values. As an important volatile compound, pyridine is related to the reaction of aldehydes and amines in fish products, as previously reported.[Citation28] As shown in , the level of trimethylamine, which is produced by the degradation of trimethylamine N-oxide (TMAO) and is used as an indicator of fish freshness, was 147%. TMAO, a well-known substance occurring in marine fish, plays an important role in osmoregulation and protein stabilization against denaturation by hydrostatic pressure.[Citation29]
Conclusion
In this study, we developed a method to evaluate the volatiles from Antarctic krill. Comparing three different SPME fibers (65 μm PDMS/DVB, 75 μm CARB/PDMS, and 50/30 μm DVB/CAR/PDMS) on the basis of the number of volatile components extracted, chemical species, and cumulative peak areas, the 50/30 μm DVB/CAR/PDMS gave the best results. Optimal extraction conditions were as follows: extraction time of 55.45 min, extraction temperature of 85.0°C, and addition of 1.39 g sodium chloride. Forty-two aromatic substances were extracted from Antarctic krill, including six esters, 12 aldehydes, three ketones, four ethers, one ester, seven hydrocarbons, five pyrazines, one phenol, and six other compounds. The main flavor of Antarctic krill is determined by ethers, pyrazine, and aldehydes. Ethers can contribute to desirable aroma as well rancid odor. Pyrazine included 2-ethyl-3, 5-dimethyl-pyrazine, 2, 3, 5-trimethyl-6-ethyl-pyrazine, and 3-ethyl-2, 5-dimethyl-pyrazine, providing a roasted flavor and nutty odor. The compound (E, Z)-2, 6-nonadienal, providing a cucumber fragrance, also contributes to the overall flavor. The compounds 3-methyl-butanal and 3-methional play an important role in determining the overall flavor.
Funding
This work received financial supports from the project supported by the State Key Program of National Natural Science of China (Grant 31330060) and the National Natural Science Fund of China (Grant 31571865), which is gratefully acknowledged.
Additional information
Funding
References
- Matsuoka, S.; Yoshimura, K. Recent Trends in Solid Phase Spectrometry: 2003–2009. A Review. Analytica Chimica Acta 2010, 664, 1–18.
- Jaffrèsa, E.; Lalanne, V.; Macé, S.; Cornet, J.; Cardinal, M.; Sérot, T.; Dousset, X.; Joffraud, J.J. Sensory Characteristics of Spoilage and Volatile Compounds Associated with Bacteria Isolated from Cooked and Peeled Tropical Shrimps Using SPME-GC-MS Analysis. International Journal of Food Microbiology 2011, 147, 195–202.
- Garcia-Esteban, M.; Ansorena, D.; Astiasaran, I.; Martin, D.; Ruiz, J. Comparison of Simultaneous Distillation Extraction (SDE) and Solid-Phase Microextraction (SPME) for the Analysis of Volatile Compounds in Dry-cured Ham. Journal of the Science of Food and Agriculture 2004, 84, 1364–1370.
- Rocha, S.; Ramalheira, V.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Headspace Solid Phase Microextraction (SPME) Analysis of Flavor Compounds in Wines. Effect of the Matrix Volatile Composition in the Relative Response Factors in a Wine Model. Journal of Agricultural and Food Chemistry 2001, 44, 5142–5151.
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Jessopp, M.J.; Loeb, V. A Re-Appraisal of the Total Biomass and Annualproduction of Antarctic Krill. Deep-Sea Research 2009, 56, 727–740.
- Wang, L.Z.; Xue, C.H.; Wang, Y.M.; Yang, B. Extraction of Proteins with Low Fluoride Level from Antarctic Krill (Euphausia superba) and Their Composition Analysis. Journal of Agricultural and Food Chemistry 2011, 59, 6108–6112.
- Suzuki, T.; Shibata, N. The Utilization of Antarctic Krill for Human Food. Food Reviews International 1990, 6, 119–147.
- Wang, L.Z.; Xue, C.H.; Xue, Y.; Wang, Y.M.; Li, Z.J. Optimization and Evaluation of a Novel Technique for Hydrolyzing Antarctic Krill (Euphausia superba) Proteins. Food and Bioproducts Processing 2015, 94, 629–636.
- Si, Y.X.; Song, J.J.; Fang, N.Y.; Wang, W.; Wang, Z.J.; Yang, J.M.; Qian, G.Y.; Yin, S.J.; Park, Y.D. Purification, Characterization, and Unfolding Studies of Arginine Kinase from Antarctic Krill International. International Journal of Biological Macromolecules 2014, 67, 426–432.
- Jung, H.R.; Kim, M.A.; Seo, Y.S.; Lee, Y.B.; Chun, B.S.; Kim, S.B. Decreasing Effect of Fluoride Content in Antarctic Krill (Euphausia superba) by Chemical Treatments. International Journal of Food Science & Technology 2013, 48, 1252–1259.
- Chen, Y.C.; Jaczynski, J. Gelation of Protein Recovered from Whole Antarctic Krill (Euphausia superba) by Isoelectric Solubilization/Precipitation as Affected by Functional Additives. Journal of Agricultural and Food Chemistry 2007, 55, 1814–1822.
- Kaseleht, K.; Leitner, E.; Paalme, T. Determining Aroma-Active Compounds in Kama Flour Using SPME-GC-MS and GC-Olfactometry. Flavour and Fragrance Journal 2011, 26, 122–128.
- Xie, W.C.; Yang, X.H.; Zhang, C.H. Determination of Volatile Flavour Compounds of Shrimp Head by Headspace Solid Phase Microextraction-gas Chromatography-mass Spectrometry. Chinese Journal of Analytical Chemistry 2011, 39, 1852–1857.
- Huang, L.F.; Zhou, S.Y. Headspace Solid-phase Microextraction and Gas Chromatography-mass Spectrometry for Rhizoma Atractylodis Macrocephalae Volatile Components Analysis. Chromatography 2007, 25, 43–47.
- Medina, I.; González, M.J.; Iglesiasa, J.; Hedges, N.D. Effect of Hydroxycinnamic Acids on Lipid Oxidation and Protein Changes as Well as Water Holding Capacity in Frozen Minced Horse Mackerel White Muscle. Food Chemistry 2009, 114, 881–888.
- Abdulra’uf, L.B.; Tan, G.H. Chemometric Approach to the Optimization of HS-SPME/GC-MS for the Determination of Multiclass Pesticide Residues in Fruits and Vegetables. Food Chemistry 2015, 177, 267–273.
- Burin, V.M.; Marchand, S.; Revel, G.D.; Bordignon-Luiz, M.T. Development and Validation of Method for Heterocyclic Compounds in Wine: Optimization of HS-SPME Conditions Applying a Response Surface Methodology. Talanta 2013, 117, 87–93.
- Loch, C.; Reusch, H.; Ruge, I.; Godelmann, R.; Pflaum, T.; Kuballa, T.; Schumacher, S.; Lachenmeier, D.W. Benzaldehyde in Cherry Flavour as a Precursor of Benzene Formation in Beverages. Food Chemistry 2016, 206, 74–77.
- Bertrand, E.; Meyer, X.M.; Machado-Maturana, E.; Berdagué, J.L.; Kondjoyan, A. Modelling the Maillard Reaction during the Cooking of a Model Cheese. Food Chemistry 2015, 184, 229–237.
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the Volatile Compounds Useful for the Characterisation of Fresh and Thawed Cultured Gilthead Sea Bream Fish By: Microextraction Gas Chromatography-mass Spectrometry. Food Chemistry 2009, 115, 1473–1478.
- Lee, S.E.; Chung, H.; Kim, Y.S. Effects of Enzymatic Modification of Wheat Protein on the Formation of Pyrazines and Other Volatile Components in the Maillard Reaction. Food Chemistry 2012, 131(4), 1248–1254.
- Lancker, F.V.; Adams, A.; Kimpe, N.D. Formation of Pyrazines in Maillard Model Systems of Lysine-Containing Dipeptides. Journal of Agricultural and Food Chemistry 2010, 58, 2470–2478.
- Senger-Emonnot, P.; Rochard, S.; Pellegrin, F.; George, G.; Fernandez, X.; Lizzani-Cuvelier, L. Odour Active Aroma Compounds of Sea Fig (Microcosmus sulcatus). Food Chemistry 2006, 97, 465–471.
- Zviely, M.; Abushqara, E.; Hodrien, D. Chocarom Pyrazine: A Remarkable Pyrazine for Flavors and Fragrances. Natural Flavors and Fragrances: American Chemical Society 2005, 908, 189–202.
- Coogan, R.C.; Wills, R.B.H. Flavour Changes in Asian White Radish (Raphanus sativus) Produced by Different Methods of Drying and Salting. International Journal of Food Properties 2008, 11, 253–257.
- Hu Hou, H.; Zhao, X.; Li, B.F.; Li, P.L.; Zhang, Z.H.; Shao, X.M.; Pang, W.J.; Qu, X. Solid-Phase Microextraction Method for the Determination of Volatile Compounds in Hydrolysates of Alaska Pollock Frame. International Journal of Food Properties 2013, 16, 790–802.
- Peinado, I.; Koutsidis, G.; Ames, J. Production of Seafood Flavour Formulations from Enzymatic Hydrolysates of Fish By-Products. LWT-Food Science and Technology 2016, 66, 444–452.
- Peinado, I.; Miles, W.; Koutsidis, G. Odour Characteristics of Seafood Flavour Formulations Produced with Fish By-Products Incorporating EPA, DHA and Fish Oil. Food Chemistry 2016, 212, 612–619.
- Yancey, P.H.;. Organic Osmolytes as Compatible, Metabolic and Counteracting Cytoprotectants in High Osmolarity and Other Stresses. The Journal of Experimental Biology 2005, 208, 2819–2830.