ABSTRACT
Compositions, molecular characteristics, and rheological properties of the water-extractable polysaccharides from okra were investigated. The proportions of galacturonic acid and monosaccharides in the refined polysaccharides were 62.47% and 13.47%, respectively. The molecular weight distribution of 717.5 kDa in the crude polysaccharides was removed through a purification process. Okra polysaccharides were pseudo-plastic fluids exhibiting shear thinning behavior. The refined polysaccharides had a higher apparent viscosity (332.15 mL g−1) than the crude polysaccharides (294.69 mL g−1), and exhibited a positive linear correlation with the logarithm of the zero shear viscosity between 1.0% and 4.0%, showed an elastic behavior at a stress of 1 Pa over the frequency range of 1–10 rad/s. When the concentration reduced below 2.0%, the refined polysaccharides showed a predominantly viscous response over the same frequency range. The rheology was greatly affected by their concentrations, indicating water-extractable okra polysaccharides have great potential as a thickening agent in food industry.
Introduction
Okra is widely grown in tropics and subtropics, as well as some other temperate zones around the world. It is rich in human essential nutrients such as polysaccharides, protein, minerals, and vitamins.[Citation1] It was reported that okra in Brazil contains 22.14% protein, 14.01% lipids, and high contents of unsaturated lipids, especially oleic acids and linoleic acids.[Citation2] Besides, okra has high contents of polyphenols such as quercetin derivatives and catechins, which possess great antioxidant activities.[Citation3] Sheu and Lai reported that the hydrolyzed extracts of okra exhibit immunomodulatory effects by activating rat bone marrow hematopoietic cells.[Citation4]
The thick and slimy mucilage of okra can be used in the food and pharmaceutical industries.[Citation5] Due to the high content of polysaccharides, water extracts of okra are often used to thicken soups and stews.[Citation6] Commercially, mucilage polysaccharides, consisting of galactose (Gal), rhamnose (Rha), and galacturonic acid (GalA), are utilized as emulsifiers, diluents, binder, suspending agents, and water retention agents because of their wide functional properties.[Citation7,Citation8] Dried mucilages from okra also show great emulsifying properties at specific conditions.[Citation9] Therefore, okra polysaccharides have great potential as a commercial gelling agent and thickener. In addition, the water-extractable polysaccharides from okra flowers have been used as an immunomodulatory agent in the health food and pharmaceutical therapy.[Citation10]
Rheology is a branch of mechanics to study the deformation of fluid by external forces. Food rheology is used to solve specific problems in food processing. By understanding the rheological behavior, which is associated directly with texture and taste, better processing techniques can be developed to improve food quality. The analysis results of rheological behaviors suggested that the extracted sunflower head pectin was potential thickener for acidic foods and stabilizer for weak acidic to neutral foods.[Citation11] Except as hydrophilic polymer or carrier in pharmaceutical industry, okra extracts are also strong candidates for emulsification in acidic environments with potential applications in acidified food processing.[Citation7,Citation12] Okra polysaccharides have been used as an egg white substitute and fat substitute in chocolate bar cookies and frozen dairy desserts.[Citation13] Alamri et al.[Citation14] revealed that adding okra gum into the starches in rice and sorghum can increase syneresis and decrease gelatinization peak temperature. These semisolid foods with both viscous and elastic property have complicated behaviors, which may lead to different shapes and structures of the foods. Kontogiorgos et al.[Citation15] investigated the rheological properties of okra polysaccharides extracted by different protocols, and found chelating agents are attributed to the structure of the chains.
However, the composition, structure, and property of water-extractable okra polysaccharides have not been reported totally and systematically in previous researches in terms of the convenience and cost-effectiveness. In this paper, crude okra polysaccharides (COPs) were obtained by extraction of the powdered okra by deionized water (dH2O), and were further treated by enzymolysis, precipitation, and dialysis to obtain the refined okra polysaccharides (ROPs). The compositions of the water-extractable polysaccharides were evaluated, including GalA, Rha, arabinose (Ara), xylose (Xyl), mannose (Man), Gal, glucose (Glc), xylan, starch, nitrogen, and ash. The characteristics of okra polysaccharides, such as the molecular weight (Mw), intrinsic viscosity, and rheological properties including fluid types, zero shear viscosity, viscoelasticity, were investigated, which showed that okra polysaccharides may be used as a potential thickening agent in the food industry.
Materials and methods
Materials
Mature okra (Abelmoschus esculentus L. Moench), approximately 10 cm in length, were collected from the Experimental Station of Vegetable Science, Shandong Agricultural University, China, in August 2014. The cultivar of okra used in this research is called “Lujian.” Thermostable α-amylase (Termamyl 120L) was purchased from Novo Nordisk (Copenhagen, Denmark). Protease was purchased from Megazyme (Wicklow, Ireland). Standards of monosaccharides, including Rha, Ara, Xyl, Gal, Man, Glc, and GalA, were purchased from Sigma-Aldrich (St. Louis, MO, USA). M-hydroxydiphenyl was purchased from TCI Development Co., Ltd. (Tokyo, Japan). All other chemicals and solvents were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Extraction and purification of okra polysaccharides
The extraction and purification of okra polysaccharides were performed according to previous methods.[Citation15,Citation16] Fresh okra pods were crosscut into small slices (approximately 1 cm in length), and dried at 50°C in an oven (Shanghai Yiheng Instrument Co., Ltd., Shanghai, China) until the moisture was reduced to below 5.0%. The slices were ground and passed through a 0.25 mm miller sieve (ZKA-WERKE, Germany). The powdered okra contained 20.3% carbohydrate, 7.35% GalA, 3.22% nitrogen, and 6.8% ash.
Okra powder (20 g) was extracted with 300 mL 70% ethanol by stirring at 40°C for 1 h before centrifugation at 5000 rpm for 15 min. The supernatant was discarded, and insoluble residue was subjected to the extraction process again. The ethanol-insoluble fraction (EIS) was obtained by washing the insoluble residue with 95% ethanol and acetone successively, and then dried overnight at 40°C. The EIS (16.9 g) was homogenized in 500 mL dH2O by a high-frequency homogenizer (1000 r/min, 100°C, 1 h). The supernatant was collected by centrifugation at 5000 rpm for 15 min. Similarly, the extraction process was repeated. The combined supernatant was precipitated in ethanol with the final concentration of 60% overnight at 4°C, and subject to centrifuge at 5000 rpm for 15 min. The COPs was obtained by dissolving the precipitate in dH2O and freeze-drying.
The dried COPs were dissolved in dH2O (1:100, w/v), and hydrolyzed by 0.02% protease (≥ 16 U/g) at 60°C for 2 h. The reaction was terminated by increasing the temperature to 95°C, adding 0.02% thermostable α-amylase (≥4000 U/g) (95°C, 30 min), and boiling for 5 min. The supernatant was collected by centrifugation at 5000 rpm for 15 min. The COPs were precipitated by ethanol with a final concentration of 45% overnight at 4°C. The precipitate was collected by centrifugation, and washed with 95% ethanol, followed by re-dissolution in dH2O and dialysis (retaining Mw > 3500 Da) against flowing dH2O for 72 h. The ROPs were obtained by freeze-drying the dialysate.
General methods
Ash and total nitrogen contents were determined with direct method and Kjeldahl method according to the AOAC official methods 923.03 and 920.87, respectively.[Citation17] GalA determination was performed according to the method described by Yuliarti et al.[Citation18] with slight modifications. GalA yield was calculated using the formula as described by Wang et al.[Citation19]
GalA yield was the ratio of GalA content (dry weight) in purified polysaccharide over the GalA content (dry weight) in okra.
Xylan was determined by the method of glacial acetic acid-phloroglucinol.[Citation20] Starch was detected using 0.01 mol/L Lugol’s iodine solution made by dissolving 1.27 g of iodine (I2) and 3.0 g of potassium iodide (KI) in 1 L of distilled water.[Citation21] Briefly, 50 μL of standard iodine solution was added to 4 mL sample solution. The absorbance was measured at 575 nm using an UV-2600 spectrophotometer (Unico, Shanghai, China).
Monosaccharide and molecular weight
The determination of monosaccharides and molecular weight was performed according to our previously developed method.[Citation22] Monosaccharides were measured by gas chromatography (Shimadzu-GC2010, Kyoto, Japan) equipped with a DM-2330 polar column (30 m × 0.32 mm i.d., 0.2 μm, DIKMA, Beijing, China) and a flame ionization detector. Separation temperature was 240°C, injection temperature was 250°C, and detection temperature was 260°C. The carrier gas was N2, the split ratio was 1:14. Molecular weight was determined using high-performance size-exclusion chromatography (HPSEC) on a high-performance liquid chromatography system (Shimadzu, Kyoto, Japan) coupled with a series of TSKgel G3000PWxl column, G4000PWxl column, and G5000PWxl column (300 mm × 7.8 mm i.d.) with a TSK guard column (35 mm × 4.6 mm i.d., Tosoh, Tokyo, Japan). The injection volume of the filtered polysaccharide solution (2.0 g/L) was 20 μL. Elution was carried out at a flow rate of 0.3 mL/min at 35°C with 50 mmol/L sodium nitrate solution containing 0.5 g/L sodium azide as preservative.
Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectroscopic analysis was performed using a Nicolet 5700 FTIR spectrophotometer (Thermo Fisher Scientific, USA) according to the method of Wang et al.[Citation23] with slight modifications. Polysaccharides powder (1 mg) and KBr (190 mg) were incorporated, ground, and pressed into tablets, which were scanned within the range of 4000–400 cm−1.
Intrinsic viscosity
Okra polysaccharides were dispersed in 0.1 mol/L NaCl (0.1%) in sealed glass vials and left overnight under continuous stirring. The fully dissolved polysaccharides were filtered through a 0.45 μm membrane filter (Millipore Co., Milford, MA, USA). Intrinsic viscosity ([η]) was determined by an Oswald capillary tube (Great Wall Glass Co., Tianchang, China) at 20°C and calculated using the methods as described by Kontogiorgos et al.[Citation15] and Chen et al.[Citation24]
Rheology
The rheology was measured on a stress-controlled rheometer (AR2000ex, TA Instruments, USA) with cone–plate geometry.[Citation25] The cone is made of standard ETC steel with a cone diameter of 20 mm and cone angle of 2°. A steady flow process (0.01–1000 s−1) was used to determine the rheological properties at 25°C. For temperature sweeping, the temperature ranged between 10°C and 70°C with the shear stress of 2 Pa. Oscillatory measurements were used to determine the G' and G''. The linear viscoelastic region was determined by a stress sweep (0.1–1000 Pa) at 25°C. Frequency sweep measurements were performed at a given stress (chosen in the linear domain where the amplitude of the deformations is very low) in the frequency region of 0.1–10 rad/s.
Statistical analysis
All data were the average of three parallel determinations. Results were expressed as mean ± standard deviation (SD). Data were analyzed using analysis of variance (DPS 7.05, Hangzhou Ruifeng Information Technology Co., Ltd., Hangzhou, China), and the t-test was used to determine the statistical significance (P < 0.05).
Results and discussion
Chemical compositions
The COPs and ROPs have significantly different compositions, as summarized in , especially for the GalA and ash content, which are the most common parameters to determine the purity of okra polysaccharides. Clearly, ROPs have a significantly higher GalA content (62.47%) compared with the COPs (48.62%). The ash content in the COPs was 10.81%, while the ash content in the ROPs was 5.75%. The increased GalA and decreased ash contents suggested that the purity of the polysaccharides in the ROPs was higher than the COPs.
Table 1. Compositions of okra polysaccharides.
The enzymolysis process during the extraction had significant effects on the compositions of okra polysaccharides. The xylan content was 2.69% in the COPs, which decreased to 1.12% after enzymolysis. By amylorrhexis, the starch content decreased from 0.92% to less than 0.05%, which further indicated that the purity of the ROPs was greatly improved. Macromolecules, such as starch and protein, were degraded to Glc and amino acids, which, along with some free metal ions, were removed through dialysis. The reduction of the xylan content was owing to its low molecular weight. There was still 0.49% nitrogen present in the ROPs, which was likely the nitrogenous groups on polysaccharide chains. Apart from nitrogen, the ash content was still 5.75%. The removal of the metal ions that were chelated to the polysaccharide groups was challenging.
The COPs consisted of 1.43% Rha, 2.15% Ara, 0.48% Xyl, 0.24% Man, 10.60% Gal, and 1.83% Glc. However, no Man and Glc were found in the ROPs, which could also be attributed to the low molecular weight of the oligosaccharides removed by dialysis. The main monosaccharides were Gal (10.66%), Ara (1.07%), Rha (1.34%), and Xyl (0.40%) in the ROPs. Gal was the main monosaccharide in the polysaccharides side chain, accounting for 63.36% and 79.14% of total sugars in the COPs and ROPs, respectively. There was no significant difference in the Gal content before and after purification. The result indicated the Gal could not pass through the dialysis membrane. The compositions and structure of the okra polysaccharides were significantly influenced by the extraction procedure and the ethanol concentration.
As the main polysaccharides extracted from okra, GalA can be used to evaluate the efficiency of extraction.[Citation19] The calculated GalA yields in the COPs and ROPs were 73.1% and 86.0%, respectively. It is generally acknowledged that pectins are acidic heteropolysaccharide including homogalacturonan (HG), rhamnogalacturonan I (RG-I), and rhamnogalacturonan II (RG-II). HG domain is called the “smooth” region, and RG-I and RG-II with side chains are the “hair” region. The main structural elements of okra polysaccharides contained a repeating unit of alternating α-(1–2)-linked rhamnosyl and α-(1–4)-linked GalA residues with a disaccharide side chain of β-(1–4)-linked galactosyl groups attached to O-4 of l-rhamnosyl residues.[Citation13] Therefore, the ratio of Rha and GalA (Rha/GalA) represents the proportion of RG-I. The Rha/GalA ratio decreased from 0.03 in the COPs to 0.02 in the ROPs, which consisted mainly of polygalacturonic acid-rich “smooth” regions, suggesting the COPs had substances with lower molecular weight. It was reported that neutral sugar side chains usually inhibit the formation of gel, because the polysaccharide side chains limit the extension of inter-chain association. Consequently, the higher GalA content and lower monosaccharide proportion suggest the ROPs have excellent gelling and thickening capability.
Molecular weight
The COPs and ROPs had different molecular weight distributions, as shown in . Specifically, the molecular weight distribution of COPs was divided into three sections, whereas it was divided into two sections in the ROPs. The molecular weight distributions of the two sections in the ROPs were 3265 and 36.9 kDa respectively, which was in accordance with the molecular weight of I and III in the COPs. The II (717.5 kDa) in the COPs was likely to be removed through purification. In addition, the proportion of I in the COPs was only 50% of that in the ROPs, and the comparison of peak molecular weight (Mp) was similar with molecular weight distributions between COPs and ROPs, indicating that the purification protocol has a great impact on the composition of okra polysaccharides.
Table 2. Molecular weight of okra polysaccharides.
FTIR analysis
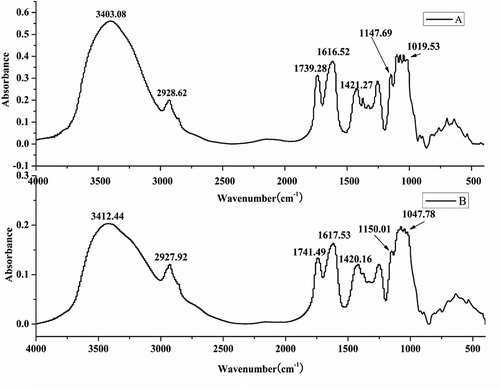
As shown in , the spectrum curve of ROPs (A) was similar to that of COPs (B). The characteristic peaks corresponding to the hydroxyl stretching vibrations are shown in (3403 cm−1) and 1B (3412 cm−1). The band at 2928 cm−1 resulted from C–H stretching vibrations.[Citation26] Strong bands between 1720–1740 and 1600–1620 cm−1 were derived from the ester carbonyl (COOR) groups and carboxylate ion stretching band (COO–), respectively. The band at 1420 cm−1 corresponded to the COH in in-plane bending vibration. The bands between 1200 and 950 cm−1 were the “fingerprints” of polysaccharides, which can be used to distinguish the main chemical groups.[Citation23]
The degree of esterification (DE) is calculated as the ratio of the peak area at 1745 cm−1 (COOR) over the sum of the peak areas of 1745 cm−1 (COOR) and 1645 cm−1 (COO-).[Citation27] As the DE increased, the ester carbonyl groups increased in intensity and band area, while the intensity of the carboxylate stretching band decreased. Compared with commercial pectin standards, the pectin in okra polysaccharides should be low methoxyl.
Intrinsic viscosity
Intrinsic viscosity, [η], gives an index of the total degree of space-occupancy of the macromolecule. Because of the presence of counterions in the polysaccharide solutions to prevent polysaccharides from interacting with each other, [η] was performed at a fixed ionic strength (0.1 mol/L NaCl). The reduced viscosity ηsp/c stands for the viscosity of fluid increase per unit of polymer solution.[Citation26] [η] of the ROPs was 332.15 mL g−1 by extrapolation to zero (). It should be noted that [η] is the volume per unit mass that the polymer occupies in a solution which was related to the molecular weight, conformation, and the property of the solvent.[Citation28] As shown in , [η] of the ROPs (332.15 mL g−1) was higher than that of the COPs (294.69 mL g−1), which is probably due to the improved purity. Besides, intermolecular interactions were strengthened under per unit volume.
Table 3. Intrinsic viscosity of okra polysaccharides.
Steady rheology
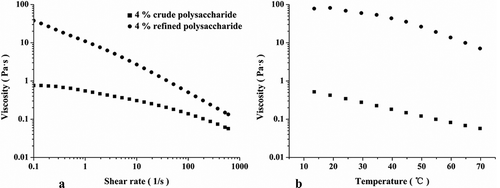
Because of the high content of ash, protein, and oligosaccharides, the apparent viscosity of the COPs () was lower than that of the ROPs. The COPs was not available for oscillatory measurements due to its dilute solution properties. Therefore, both polysaccharides were only compared at the concentration of 4.0%. The apparent viscosity of both solutions prepared with COPs and ROPs decreased gradually with the increase of shear rate, which indicated that okra polysaccharides are pseudo-plastic fluids and exhibit shear-thinning behavior. It was probably because the destruction velocity was greater than the reestablishment of physical crossover when the shear rate reached a specific value. The viscosity of the ROPs was much higher than that of the COPs. The shear thinning region of the samples was fitted with the Ostward–De Waele equation (η = m*γn−1), where m is the consistency index, and n is the flow behavior index, which reflects the degree of deviation between sample fluid and Newtonian fluid.[Citation29] When n = 1, the sample fluid was a Newtonian fluid. When n < 1, the sample fluid exhibited a shear thinning behavior which was a pseudo-plastic fluid. When n > 1, the sample fluid exhibited a shear thickening behavior which was a dilatant fluid. As demonstrated in , the absolute value of the slope in the ROPs was significantly higher than that of the COPs, which implied that n was smaller and the solution deviated from the Newtonian fluid. In other words, the ROPs were more pseudo-plastic.
Temperature sweeping had a similar tendency with the steady shear measurement, higher the temperature, the lower the viscosity. The viscosities decreased with increasing temperature for both cases. This might be because the raised temperature increased the energy dissipation of molecules and decreased the intermolecular interactions, which decreased the interference of the hydrodynamic domain.[Citation30] The apparent viscosity of the ROPs was almost constant when the temperature was below 30°C, which implied that molecules did not achieve the point to weaken the interaction thus destroy the chain–chain entanglement.
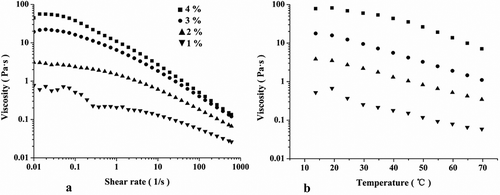
The flow behaviors at different concentrations (1%, 2%, 3%, and 4%) are shown in . As expected, the increased concentrations caused a significant increase of viscosity, which was in agreement with the results by Min et al.[Citation31] The application of a shear to the system causes the destructions of ordered structures, which results in a decreased viscosity as the rate of deformation increases.[Citation30] Obviously, polysaccharides can form ordered structures by chain–chain entanglement. However, shear force can disrupt ordered structures at a high shear rate and contribute to a low viscosity. On the contrary, the low rates of disruption and formation of the polymer chain entanglements were at equilibrium. Okra polysaccharide solutions showed properties of a Newtonian fluid at a low shear rate (). Further increase of the shear rate resulted in the disruption of weak interactions between polysaccharide molecules, which led to lower viscosity. For the diluted polymer systems, the molecules stay away from each other and the chain entanglement between polysaccharide molecules was at minimum. Thus, the viscosity is less shear rate–dependent, and the shear-thinning behavior is not significant.[Citation24] Our results indicate that okra polysaccharides have great potential as a thickening agent in the food industry.
To further investigate the rheological properties of okra polysaccharides, a temperature sweep procedure was performed from 20°C to 70°C (). Clearly, in all cases, the viscosities decreased with the increase of temperature. In addition, the viscosity was almost constant at 20°C. This might be because the slower intermolecular thermal motion led to the chain–chain entanglements, but the formation rate was higher than the force disruption. The results also indicate that the okra polysaccharides could be used to thicken the aqueous samples and stabilize emulsions, foams, and particulate suspensions.
Zero shear viscosity
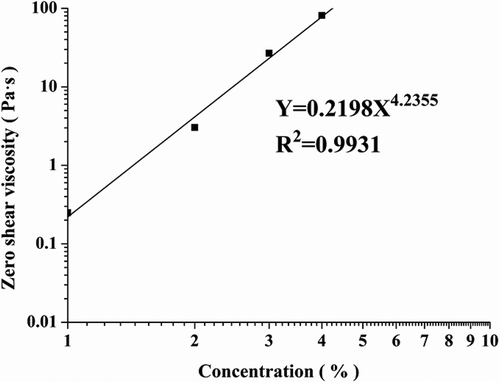
Zero shear viscosity (η0) of the ROPs is shown in A linear relationship was found between the η0 and the concentrations of okra polysaccharides. The entanglement concentration c* means the change of the slope in the polymer solution in the viscosity–concentration curve. At concentrations below c*, the η0 scales as η0 ∞ c1.3, and at concentrations above c*, it scales as η0 ∞ c4.[Citation29] In our study, the calculated scaling exponent was 4.2. No change in the slope of the curves was found in the range of 1%–4%. Therefore, the 1% solution was also above c*.
Oscillatory measurements
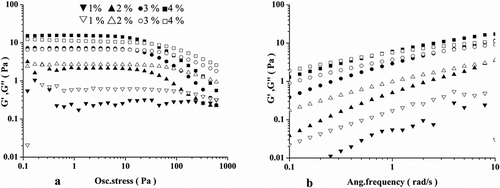
summarizes the changes of G' and G''. The linear viscoelastic region of the okra polysaccharides could be determined using the stress sweep with the deformation values from 0.1 to 1000 Pa. The shear stress value of 1 Pa was chosen, which was within the linear viscoelastic region.[Citation19] Visually, the polysaccharide solution showed elastic property (G' > G'') at a higher concentration (>2%) with deformation values above 10 Pa. When the concentration was lower than 2%, the polysaccharides exhibited a viscous behavior (G' < G'').
To determine the dependence of G' and G'' on the frequency, frequency sweeps were set with a range from 0.1 to 10 rad/s at a stress of 1 Pa. At the lowest concentration (1%), the G' was lower than G'' at all times, indicating a viscous behavior. At the concentration of 2%, the difference between G' and G'' was reduced with increasing frequency, and G' became higher than G'' at 9 rad s−1. Similar changes were also observed at higher concentrations and this cross point was shifted to 2 and 0.8 rad s−1 for 3 % and 4%, respectively. Compared with the samples with lower concentrations, the crossover of 4% solution occurred at much lower frequencies. The results suggest that the okra polysaccharide solution is more solid-like with increasing concentrations. The crossover of G' and G'' provides a good indicator for viscoelastic behavior of the okra polysaccharides.
Conclusion
COPs and ROPs have different compositions, molecular weight distributions, and rheological properties. The II period (717.5 kDa) in the COPs could be removed by enzymolysis and dialysis. The pectin appeared in the okra polysaccharides is a low methoxyl, and the okra polysaccharides were pseudo-plastic fluids. Both COPs and ROPs showed a shear thinning behavior with increasing shear rates at a concentration of 4%. The ROPs behaved more solid-like as a viscous liquid at higher concentrations (>2%). As a new food additive, the water-extractable okra polysaccharides will find more applications in the food industry due to its convenience and cost-effectiveness.
References
- Santos, I.F.; Santos, A.M.P.; Barbosa, U.A.; Lima, J.S.; Santos, D.C.; Matos, G.D. Multivariate Analysis of the Mineral Content of Raw and Cooked Okra (Abelmoschus esculentus L.). Microchemical Journal 2013, 110, 439–443.
- Soares, G.D.F.; Gomes, V.D.; Albuquerque, A.D.; Dantas, M.B.; Rosenhain, R.; De Souza, A.G.; Persunh, D.C.; Gadelha, C.A.D.; Costa, M.J.D.; Gadelha, T.S. Spectroscopic and Thermooxidative Analysis of Organic Okra Oil and Seeds from Abelmoschus esculentus. The Scientific World Journal 2012, 2012, 1–6.
- Saleh, M.A.; El-Gizawy, A.M.; El-Bassiouny, R.E.L.; Ali, H.M. Effects of Anti-Coloring Agents on Blackening Inhibition and Maintaining Physical and Chemical Quality of Fresh-cut Okra during Storage. Annals of Agricultural Science 2013, 58, 239–245.
- Sheu, S.; Lai, M. Composition Analysis and Immuno-modulatory Effect of Okra (Abelmoschus esculentus L.) Extract. 2012, 134, 1906–1911.
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra Extracts in Pharmaceutical and Food Applications. Food Hydrocolloid 2014, 42, 342–347.
- Georgiadis, N.; Ritzoulis, C.; Sioura, G.; Kornezou, P.; Vasiliadou, C.; Tsioptsias, C. Contribution of Okra Extracts to the Stability and Rheology of Oil-in-water Emulsions. Food Hydrocolloids 2011, 25, 991–999.
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra Extracts as Emulsifiers for Acidic Emulsions. Food Research International 2013, 54, 1730–1737.
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and Characterization of Mucilage Polysaccharide for Biomedical Applications. Carbohydrate Polymers 2013, 98, 89–94.
- Temenouga, V.; Charitidis, T.; Avgidou, M.; Karayannakidis, P.D.; Dimopoulou, M.; Kalogianni, E.P.; Panayiotou, C.; Ritzoulis, C. Novel Emulsifiers as Products from Internal Maillard Reactions in Okra Hydrocolloid Mucilage. Food Hydrocolloids 2016, 52, 972–981.
- Zheng, W.; Zhao, T.; Feng, W.; Wang, W.; Zou, Y.; Zheng, D.; Takase, M.; Li, Q.; Wu, H.; Yang, L.; Wu, X. Purification, Characterization and Immunomodulating Activity of a Polysaccharide from Flowers of Abelmoschus esculentus. Carbohydrate Polymers 2014, 106, 335–342.
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Zhang, J. Rheological Properties of Natural Low-Methoxyl Pectin Extracted from Sunflower Head. Food Hydrocolloid 2015, 44, 122–128.
- Emeje, M.; Olaleye, O.; Isimi, C.; Fortunak, J.; Byrn, S.; Kunle, O.; Ofoefule, S. Oral Sustained Release Tablets of Zidovudine Using Binary Blends of Natural and Synthetic Polymers. Biological and Pharmaceutical Bulletin 2010, 33, 1561–1567.
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Characterisation of Cell Wall Polysaccharides from Okra (Abelmoschus esculentus (L.) Moench). Carbohydrate Research. 2009, 344, 1824–1832.
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effect of Okra Gum on the Pasting, Thermal, and Viscous Properties of Rice and Sorghum Starches. Carbohydrate Polymer 2012, 89, 199–207.
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological Characterization of Okra Pectins. Food Hydrocolloids 2012, 29, 356–362.
- Vahid, S.;. Polysaccharide Extraction from Abelmoschus esculentus: Optimization by Response Surface Methodology. Carbohydrate Polymer 2013, 95, 588–597.
- AOAC. Official Methods of Analysis, 18th., United States, Maryland, Gaithersburg: Association of Official Analytical Chemists, Maryland, United States, 2005.
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and Characterisation of Pomace Pectin from Gold Kiwi Fruit (Actinidia chinensis). Food Chemistry 2015, 187, 290–296.
- Wang, X.; Lü, X. Characterization of Pectic Polysaccharides Extracted from Apple Pomace by Hot-Compressed Water. Carbohydrate Polymers 2014, 102, 174–184.
- Douglas, S.G.; A Rapid Method for the Determination of Pentosans in Wheat Flour. Food Chemistry 1981, 7, 139–145.
- Chrastil, J. Improved Colorimetric Determination of Amylose in Starches or Flours. Carbohydrate Research 1987, 159, 154–158.
- Guo, M.M.; Du, J.H.; Zhang, K.L.; Jin, Y.H. Content and Molecular Weight of Water-extractable Arabinoxylans in Wheat Malt and Wheat Malt-based Wort with Different Kolbach Indices. Journal of the Science of Food and Agriculture 2014, 94, 2794–2800.
- Wang, X.; Chen, Q.; Lü, X. Pectin Extracted from Apple Pomace and Citrus Peel by Subcritical Water. Food Hydrocolloids 2014, 38, 129–137.
- Chen, J.; Wu, S.S.; Liang, R.H.; Liu, W.; Liu, C.M.; Shuai, X.X.; Wang, Z.J. The Effect of High Speed Shearing on Disaggregation and Degradation of Pectin from Creeping Fig Seeds. Food Chemistry 2014, 165, 1–8.
- Ping, A.L.; Du, P.P.; Wei, X.L.; Sun, D.Z.; Zhang, J.H.; Liu, J.; Zhong, X.R. Rheological Behavior and Physico-chemical Properties of Cetyltrimethylammonium Salicylate in Aqueous Solutions. Fluid Phase Equilibria 2014, 369, 39–46.
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural Characterization and DPPH· Radical Scavenging Activity of a Polysaccharide from Guara Fruits. Carbohydrate Polymers 2014, 103, 143–147.
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted Heating Extraction of Pectin from Grape Fruit Peel: Optimization and Comparison with the Conventional Method. Food Chemistry 2015, 178, 106–114.
- Liang, R.; Chen, J.; Liu, W.; Liu, C.; Yu, W.; Yuan, M.; Zhou, X. Extraction, Characterization and Spontaneous Gel-forming Property of Pectin from Creeping Fig (Ficus pumila Linn.) Seeds. Carbohydrate Polymer 2012, 87, 76–83.
- Sengkhamparn, N.; Sagis, L.M.C.; Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Physicochemical Properties of Pectins from Okra (Abelmoschus esculentus (L.). Moench). Food Hydrocolloid. 2010, 24, 35–41.
- Chen, J.; Liang, R.; Liu, W.; Luo, S.; Liu, C.; Wu, S.; Wang, Z. Extraction of Pectin from Premna microphylla Turcz Leaves and Its Physicochemical Properties. Carbohydrate Polymer 2014, 102, 376–384.
- Min, B.; Lim, J.; Ko, S.; Lee, K.; Lee, S.H.; Lee, S. Environmentally Friendly Preparation of Pectins from Agricultural Byproducts and Their Structural/Rheological Characterization. Bioresource Technology 2011, 102, 3855–3860.