ABSTRACT
Germinating seeds tend to release a variety of bioactive compounds into the surrounding medium and their compositions vary depending on the type of seed and the temperature of the medium. During imbibition, green gram seeds exuded polyphenols and proteins, and their contents were higher at 45°C (warm seed) compared with that obtained from seeds soaked at room temperature. The total exudate from warm seeds also showed higher antioxidant activity compared with that of room-temperature exudate. Hence, the ethanol extract of the warm seed exudate was characterized for its health beneficial effects. Phenolic acids like gallic, ferulic, and sinapic acids, and flavonoids like vitexin and isovitexin were identified in the extract of the exudate. Among these, vitexin content was the highest compared to other bioactive compounds. DPPH radical scavenging activity and reducing power of the extract were comparable to those of butylated hydroxyanisole (BHA). Ethanol extract of warm seed exudate showed acetylcholinesterase (AChE) inhibition with an IC50 value of 9.6 µg GAE/ml and 72% inhibition of platelet aggregation at 0.11 mg GAE/ml concentration. High MW protein (>150kDa) of the exudate showed 83% inhibition of platelet aggregation at a concentration of 1 mg/ml. Low MW proteins of 29 kDa and 12 kDa showed 50% AChE inhibition at 8 μg/ml and 6.82 μg/ml concentrations, respectively.
Introduction
Green gram (Vigna radiata) belongs to the Leguminosae family and it is an important pulse crop. In India, it is widely consumed in the form of cooked whole grain, dhal, and flour. Green gram soup is another popular dish in India, China, and other Asian countries.[Citation1] Nowadays, sprouts are also consumed throughout the world for their health benefits.[Citation2] Germinated seeds are consumed in raw or cooked form. The first step in the germination process involves soaking the seeds for 4–24 h in water for imbibition. The next step is to drain water and allow the water-imbibed seeds to germinate for a period of 1–5 days. During imbibition, the influx of water into the cells of dry seeds causes cracks in the seed coat, which causes the leakage of the endogenous substances of the seed into the water used for soaking. This soaked water is called seed exudate.[Citation3] Seed exudates promote legume–rhizobia symbiosis.[Citation4] During imbibition, seeds tend to release a variety of bioactive compounds like polyphenols, peptides, and proteins in addition to sugars and sugar acids into the surrounding medium, and their content may be varied depending on the temperature of the medium.
Palavalli et al.[Citation5] reported that imbibition of soybean seeds in warm water released large amounts of proteins into the surrounding media. Hartwig and Phillips[Citation6] reported that flavonoids of alfalfa (Medicago sativa L.) seed coat were exuded during the first 4 h of imbibition and these flavonoids were reported to have an important role in the symbiotic process. Several reports indicated the presence of biologically active proteins in the exudates. Some of the proteins that were released in the exudates during the imbibition of Cicer arietinum (L.) seeds inhibited the growth of plant pathogens.[Citation7] The Bowman-Birk protease inhibitor released from the germinating soybean seeds showed a putative anticarcinogenic property.[Citation5] The exuded proteins from soybean seeds showed anti-nematode activity.[Citation8] Exudates from imbibed cowpea (Vigna unguiculata) seeds had antifungal proteins such as chitinase. They also contained lipid transfer proteins.[Citation9] Seed exudates may find applications in treating neurological disorders as well as risk factors in cardiovascular diseases.
Alzheimer’s disease, the most common form of dementia, is a progressive age-related disorder that is characterized by the degeneration of neurological function. Due to increase in acetylcholinesterase (AChE) activity during the progression of the disease, reduction in acetylcholine (ACh) levels in the brain was observed. AChE, which is located on the post-synaptic membrane, terminates the signal transmission by hydrolyzing ACh. Therefore, AChE inhibitors (AChEIs) have been gaining importance to increase the ACh levels and to restore deficient cholinergic neurotransmission.[Citation10] Drugs like tacrine, donepezil, and rivastigmine are the major cholinesterase inhibitors, but they are reported to have side effects. Therefore, there is a need for new AChEIs from natural sources, which are free from dose-limiting side effects. Several studies have shown that vitexin and isovitexin exhibit neuroactive properties. Isovitexin was the most potent inhibitor of AChE.[Citation10,Citation11] Cao et al.[Citation12] reported the presence of vitexin and isovitexin in green gram seed and its seed coat.
The development of cardiovascular diseases such as acute myocardial infarction, cerebrovascular diseases, and peripheral arterial thrombosis is related to the interaction process of atherosclerotic lesions and thrombus formation.[Citation13] Platelets adhere to endothelial cells and contribute to the recruitment of leukocytes involved in local vascular inflammation and thrombosis formation. Several synthetic drugs such as aspirin, ticlopidine, clopidogrel, and triflusal are used to inhibit platelet aggregation. However, it has been reported that these synthetic drugs are associated with several adverse side effects.[Citation14,Citation15] Therefore, the development of safe, alternative therapeutic agents with platelet aggregation inhibitory property is crucial. It has been recognized that dietary components have the potential to modulate platelet aggregation and thus reduce the levels of specific risk factors for cardiovascular disease.[Citation16–Citation18] Plant-derived active principles are being investigated in detail as an alternative to synthetic antiplatelet compounds, because they are a rich source of bioactive compounds with excellent pharmacological actions.[Citation15] However, no studies are reported on the inhibition of platelet aggregation by the proteins of seed exudates.
There are few reports with respect to the exudation of bioactive compounds from different legume seeds. However, no reports are available on bioactive compounds from green gram exudates. The food products prepared from germinated green gram are popular all over the world, but the exudate obtained has not been exploited for their health benefits. In the present study, protein and polyphenol contents in the exudates of imbibed green gram seeds that were soaked in water at room temperature as well as at warm condition were determined, and evaluated for their antioxidant, AChE inhibition, and platelet aggregation inhibition properties.
Materials and methods
Chemicals
Bovine serum albumin (BSA), sodium dodecyl sulphate (SDS), β-mercaptoethanol, gallic acid, ferulic acid, sinapic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), adenosine diphosphate (ADP), 5,5-dithio-bis-(2-nitrobenzoic acid), acetylthiocholine iodide (AChI), AChE from Electrophorus electricus (electric eel), vitexin, and isovitexin were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Sephadex G-100 was obtained from Pharmacia, Uppsala, Sweden. Sodium hypochlorite (4%) solution was obtained from Himedia, Bangalore, India. All other chemicals used were of analytical grade.
Materials
Green gram (Vigna radiata (L.) Wilckzek; Shining Moong variety) seeds were obtained from the local market in Mysore, Karnataka, India.
Preparation of samples
Preparation of total seed exudates from seeds soaked at different temperatures
Exudate was prepared from green gram seeds using the method described by Rose et al.[Citation19] with the following modifications. Green gram seeds (10 g) having no cracks or other injuries on the seed surface were sterilized for 5 min in 20 ml of 1% sodium hypochlorite and rinsed five times with water. The disinfected seeds were taken in two sterile flasks containing 30 ml of water and were kept for exudation at two temperatures viz., room temperature (27±2°C) and warm temperature (45°C) on a rotary shaker overnight. The exudates were collected and filtered using Whatman No.1 paper to remove particulate material, and this filtrate was designated as ‘total seed exudate’. This total seed exudate solution was lyophilized and the powder obtained was stored at 4°C until further use.
Preparation of ethanol extract from warm seed exudate
To 1 g of warm seed exudate powder, 25 ml of aqueous ethanol (80%) was added, stirred for 1 h on a magnetic stirrer, and filtered using Whatman No 1 filter paper. The residue obtained was re-extracted with the same volume of aqueous ethanol and filtered. Both the filtrates were pooled, and used for the estimation of total polyphenols, and identification of phenolic acids and flavonoids. It was also used for the evaluation of selected biological properties.
Preparation of protein fractions from warm seed exudate
Warm seed exudates were dialyzed overnight against water using 3.2 kDa cutoff dialysis membranes. The protein content in the dialysate was determined according to the method described by Bradford.[Citation20] The dialysate was lyophilized and the powder obtained was stored at 4°C until further use.
Determination of phytochemical contents in the total seed exudate
Determination of total polyphenol content
The total polyphenol content was determined using Folin–Ciocalteu reagent according to the method described by Singleton and Rossi.[Citation21] To 50 µl of total exudate/ethanol extract of the exudate, 2.95 ml of distilled water, 250 µl of Folin–Ciocalteu reagent solution, and 7% Na2CO3 (750 µl) were added, mixed using a vortex mixer, and incubated for 8 min at room temperature. To this reaction mixture, 1 ml of distilled water was added and the mixture was allowed to stand for 2 h at room temperature and the absorbance of the color developed was measured at 765 nm using a UV-visible spectrophotometer (Shimadzu UV-1601 PC, Japan). Gallic acid was used as standard and the results were expressed as mg gallic acid equivalents (GAE)/g of exudate.
Determination of flavonoid content
Flavonoid content in the exudates was determined using a colorimetric method described by Heimler et al.[Citation22] The total seed exudate or (+)-catechin standard solution (250 μl) was mixed with 1.25 ml of distilled water and to this 75 µl of 5% NaNO2 solution was added. After 6 min of incubation, 150 µl of a 10% AlCl3 solution was added, allowed to stand for 5 min at room temperature, and then 500 µl of 1M NaOH was added. The mixture was made up to 2.5 ml with distilled water and mixed. The absorbance was measured immediately against the blank (the above reaction mixture without the sample) at 510 nm using a UV-visible spectrophotometer. The results were expressed as milligram of catechin equivalents (mg CAE)/g of the sample.
Determination of the condensed tannin content
Condensed tannin content was determined according to the method described by Broadhurst and Jones[Citation23] with some modifications. To 50 µl of the appropriately diluted green gram seed exudate, 3 ml of a 4% vanillin (dissolved in methanol) solution and 1.5 ml of concentrated hydrochloric acid were added. The mixture was kept for 15 min, and the absorption was measured at 500 nm against methanol as a blank. The amount of condensed tannin was calculated and expressed as mg CAE/g of sample using the calibration curve generated with (+)-catechin.
HPLC analysis and quantification of phenolic acids and flavonoids
Phenolic acids analysis
HPLC analysis of phenolic acids in the ethanol extract of warm seed exudate was carried out using an analytical C18 column (Luna 5 µm, 100 Å, 250 × 4.6 mm) using high-performance liquid chromatograph equipped with a diode array detector (Agilent Technologies) according to the method described by Kim et al.[Citation24] The mobile phase consisted of two solvents, solvent A containing 2% acetic acid in water (v/v) and solvent B acetonitrile (100%). The flow rate was 1 ml/min for a total run time of 70 min and the gradient program was as follows: 100% A to 85% A in 30 min, 85% A to 50% A in 20 min, 50% A to 0% A in 5 min, and 0% A to 100% A in 5 min. The sample injection volume was 20 µl and the peaks were monitored simultaneously at 280 nm (for benzoic acid derivatives) and 320 nm (for cinnamic acid derivatives). All the samples were filtered through a 0.45 µm syringe filter before their injection. The peaks were identified by comparing the retention times with the appropriate phenolic acid standards.
Flavonoids analysis
Vitexin and isovitexin in the ethanol extract of exudate were identified and quantified by separation on an analytical C18 column (Luna 5µm, 100 Å, 250 × 4.6 mm) using high-performance liquid chromatograph equipped with a diode array detector (Agilent Technologies) according to the method described by Li et al.[Citation1] The mobile phase consisted of solvent A: 1% acetic acid in water (v/v) and solvent B: 1% acetic acid in methanol. The gradient program of the mobile phase was as follows: 10–35% B (10 min), 35–42% B (15 min), 42–75% B (10 min), 75% B (5 min), 75–10% B (5 min), and 10% B (5 min). The flow rate was 1.0 ml/min and the injection volume of the sample was 10 µl. Elution of vitexin and isovitexin was monitored at 337 nm. The peaks were identified by comparing the retention times with vitexin and isovitexin standards.
Antioxidant assays
Measurement of DPPH radical scavenging activity
DPPH radical scavenging activity of total seed exudate and ethanol extract was determined according to the method described by Brand-Williams et al.[Citation25] The sample solution/butylated hydroxyanisole (BHA) was made up to 200 μl with methanol, and 1 ml of 100 µM DPPH radical solution dissolved in methanol was added. The mixture was shaken vigorously and left in the dark at room temperature for 20 min. The absorbance of the resulting solution was measured at 517 nm. The control contained methanol and DPPH radical solution without sample/BHA. The capacity to scavenge the DPPH radical was calculated using the following equation:
where As is absorbance of the sample; Ao is absorbance of the control. The half-inhibition concentration (IC50) was defined as the amount of sample (mg GAE/ml) required for 50% DPPH radical scavenging activity and calculated from the plots (plotted percentage of DPPH radical scavenging vs. concentration of sample/BHA).
Determination of reducing power
The reducing power capacity of the total seed exudates and their ethanol extract or BHAs (synthetic standard) was determined according to the method described by Yen and Chen.[Citation26] The sample/BHA was made up to 500 μl with 0.2 M sodium phosphate buffer (pH 6.6) and mixed with 1 ml of potassium ferricyanide (0.1%) and the mixture was incubated at 50°C for 20 min. Five hundred microliters of trichloroacetic acid (10%) was added to the reaction mixture and centrifuged at 3000 g for 10 min. The supernatant obtained was mixed with an equal volume of distilled water and to this 300 μl of 1% ferric chloride was added. The absorbance of the resulting solution was measured at 700 nm. Increase in absorbance of the reaction mixture indicates the increased reducing power.
Determination of AChE inhibition
AChE enzyme activity and its inhibition by total seed exudates, ethanol extract, and protein fractions were determined using the method described by Ingkaninan et al.[Citation27] To the reaction mixture containing 500 µl of 3 mM 5,5′-dithiobis(2-nitrobenzoic acid (DTNB), 100 µl of AChI (Substrate; 15 mM), 250 µl of Tris–HCl buffer (50 mM, pH 8), an appropriate amount of inhibitor (total exudate/ethanol extract /proteins) was added. To this, 25 µl of AChE enzyme was added (0.28 U ml−1) and the reaction was monitored for 3 min at 405 nm. Enzyme inhibition was determined after subtracting the absorbance of the control (absence of inhibitors) from the sample (presence of inhibitors) absorbance and the results were expressed as % of inhibition.
Determination of inhibition of platelet aggregation
Platelet-rich plasma (PRP) was obtained from rat blood by centrifuging at 89 g for 10 min at room temperature. Aggregation of PRP was performed at 37°C using a computerized dual-channel Chrono-Log Aggregometer (Chrono-Log Corporation, Havertown, PA) as previously described by Maheswaraiah et al.[Citation18] Platelet suspension (0.45 ml) (adjusted to 1.5×108 platelets) was pre-incubated with antagonists viz., seed exudate protein and its protein fractions at 250, 500, and 1000 µg/ml concentrations or ethanol extract of exudate at 27.2, 54.4, and 108.8 μg GAE/ml concentrations for 5 min at 37°C. Platelet aggregation was induced by adding ADP as the agonist (40 µg/ml). Platelet aggregation was measured as an increase in light transmission for 5 min and the data were expressed as percent inhibition of aggregation.
SDS- PAGE analysis of seed exudate proteins
Seed exudate proteins were analysed by SDS-polyacrylamide electrophoresis (SDS-PAGE) according to the method described by Laemmli.[Citation28] The protein samples were solubilized in sample buffer containing 62.5 mM Tris-HCl, pH 6.8, 15% glycerol, 2% SDS, and 5% β-mercaptoethanol. The sample and protein standard mixture were separated on 10% separating gel at 50 V. The protein bands were stained with Coomassie brilliant blue R-250 (0.1%), which was dissolved in 40% methanol and 10% acetic acid and destained with a solution containing 10% methanol, 10% acetic acid, and 80% water.
Fractionation of proteins by gel permeation chromatography
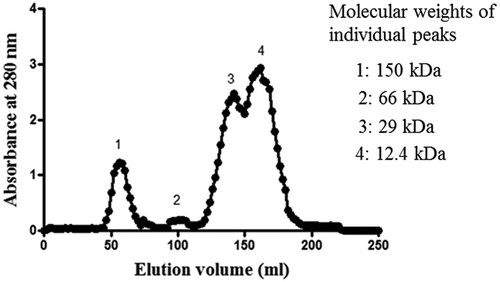
The seed exudate proteins (10 mg) were separated on Sephadex G-100 column (145 cm × 1.27 cm) using 50 mM sodium phosphate buffer (pH 7.0) as the eluent at a flow rate of 25 ml/h and 2 ml fractions were collected. Absorbance of all the fractions was read at 280 nm. Protein fractions in each peak were pooled and lyophilized for further analysis. The column was calibrated with standard proteins like alcohol dehydrogenase (150 kDa), BSA (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa) and the approximate molecular weights of the peaks were determined using the standard plot. The protein content in each pooled fraction was determined according to the method described by Bradford[Citation20] using BSA as a standard.
Statistical analysis
All analyses were carried out in triplicate and data were reported as a mean ± SD. The data were analysed by the GraphPad Prism 5.0 Software using one-way ANOVA analysis.
Results and discussion
Polyphenol, flavonoid, condensed tannin, and protein contents in the total exudates of green gram obtained at two different temperatures
Green gram seeds soaked in water at room temperature (27±2°C) had a lower yield of total exudate (2.08%) compared with that obtained from warm condition (45°C) (2.75%). As can be seen from , protein content as well as polyphenol, flavonoid, and tannin contents in warm seed exudates was higher compared to that of room-temperature exudate. For example, the polyphenol content was 1.8-fold higher and the protein content was 1.5-fold higher in warm seed exudate compared with that of room-temperature exudate. Palavalli et al.[Citation5] reported the release of several proteins from soybean seeds when they were soaked in warm water (50°C). Hirano et al.[Citation29] also reported the release of a large amount of proteins from soybean seeds when they were immersed in warm water between 50 and 60°C. Ndakidemi and Dakora[Citation30] reported the release of flavonoids, alkaloids, terpenoids, peptides, and amino acids as chemical signals during imbibition of legume seeds.
Table 1. Contents of protein and phenolics in exudates obtained from green gram seeds at two different temperatures.
Antioxidant properties of total seed exudates obtained at two different temperatures
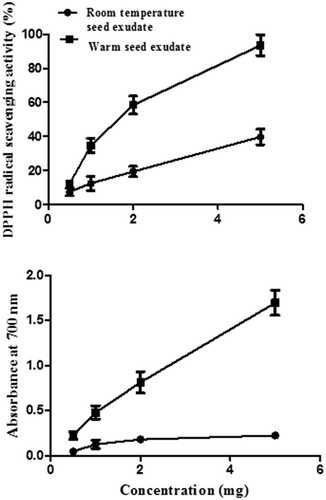
Antioxidative properties like DPPH radical scavenging activity and reducing power capacity of total seed exudates obtained at room temperature and warm temperature were determined. Exudates obtained at room temperature showed 7.56%, 12.45%, 19.48%, and 39.8% while warm seed exudates showed 12.26%, 34.65%, 58.48, and 99.49% DPPH radical scavenging activities, respectively, at 0.5, 1, 2, and 5 mg/ml concentrations. The IC50 value for warm exudate was 1.8 mg/ml, which is lower than that of room-temperature exudates (IC50, 7.2 mg mg/ml), indicating that warm exudate had a higher DPPH radical scavenging activity. The reducing power capacity of total seed exudates obtained from room temperature and warm condition at 0.5, 1, 2, and 5 mg/ml concentrations showed absorbance of 0.045, 0.126, 0.179, and 0.225, and 0.223. 0.478, 0.812, and 1.694 at 700 nm, respectively (). Absorbance at 700 nm is proportional to the reducing capacity of a sample. As warm seed exudate showed higher OD at 700 nm, the reducing power capacity was higher for warm seed exudate compared with that of room-temperature seed exudate. Thus, the warm seed exudate showed better radical scavenging activity (lower IC50 value) and higher reducing power capacity, indicating its better antioxidant potential compared with that of the total exudate obtained at room temperature. Hence, further experiments were carried out on total warm seed exudate.
Identification of polyphenols, and the beneficial biological properties of ethanol extract and proteins of warm exudates
As total warm seed exudate showed better antioxidant properties, the total exudate was fractionated into polyphenol fraction (80% ethanol extract) and protein fraction as described in the methods section. These two fractions were separately studied for their beneficial biological properties.
Identification and characterization of polyphenols in the ethanol extract of warm seed exudate and its beneficial biological properties
Identification of phenolic acids and flavonoids in ethanol extract
The phenolic acids identified in the extract were gallic acid, ferulic acid, and sinapic acid, whereas the flavonoids identified were vitexin and isovitexin (). Among these, vitexin content was the highest, followed by isovitexin, gallic acid, sinapic acid, and ferulic acid. Li et al.[Citation1] reported the presence of phenolic acids and flavonoids in green gram seed. It is possible that phenolic acids may be released from seed into the exudates during the imbibition of seeds.
Table 2. Phenolic acids and flavonoids identified in the ethanol extract of warm seed exudates using RP-HPLC.
Antioxidant properties of ethanol extract
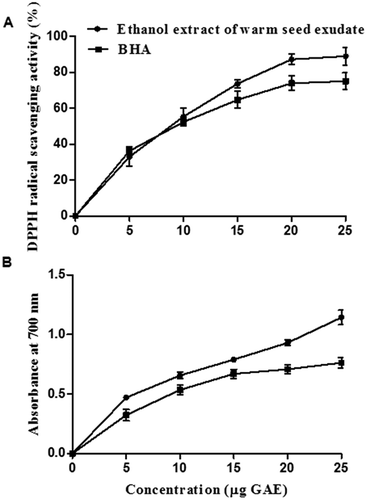
Antioxidant properties like DPPH radical scavenging activity and reducing power capacity of ethanol extract are shown in . DPPH radical scavenging activity of ethanol extract of warm seed exudate showed IC50 value of 9 μg GAE, which was comparable to the value obtained with BHA, while the reducing power capacity of warm exudate extract was better than that of BHA. Warm seed exudate ethanol extract showed 0.7 absorbance at 700 nm, while BHA showed an absorbance of 0.6 at 15 μg concentration. Thus, the antioxidant properties of warm seed exudate were comparable to that of BHA (). These antioxidant properties could be attributed to the phenolic acids and flavonoids present in the extract.
Inhibition of AChE activity
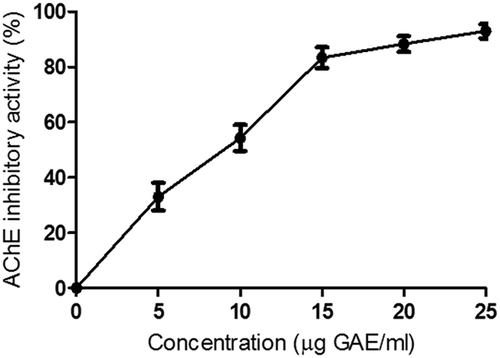
Ethanol extract of warm seed exudate inhibited AChE activity in a concentration-dependent manner (). The warm seed exudate extract at 9.6 µgGAE/ml concentration showed 50% AChE inhibition (). Murray et al.[Citation31] also reported that phenolic acids and flavonoids showed either moderate or strong AChE inhibitory properties. A potent inhibitor like galanthamine for AChE was identified from bulbs of the common snowdrop and several amaryllidaceae plants by Rizzi et al.[Citation32] Conforti et al.[Citation33] reported that flavonoids like isovitexin and Isoorientin from the flowers and rhizomes of Iris pseudopumila were responsible for AChE inhibition. It is to be noted that warm seed exudate is rich in vitexin and isovitexin as well as phenolic acids and these compounds may be responsible for the inhibition of AChE.
Platelet aggregation inhibition
Ethanol extract of warm seed exudate at 27.2, 54.4, and 108.4 μg GAE/ml concentrations showed 16, 39, and 72% inhibition of ADP-induced platelet aggregation, respectively, in a dose-dependent manner. The inhibition of platelet aggregation by ethanol extract may be due to the presence of phenolic acids like gallic acid and ferulic acid, which inhibited the platelet activity. Zia-Ul-Haq et al.[Citation34] reported that methanol extracts of green gram, lentil, mash bean, and soya bean dose-dependently inhibited platelet aggregation. Earlier Zia-Ul-Haq et al.[Citation34] showed the inhibition of platelet aggregation of seed extract, while in the present study, we showed the inhibition of platelet aggregation by its exudate. The exudate extract may regulate platelet aggregation by acting as an ADP receptor antagonist.
Identification and characterization of proteins, and the beneficial biological properties of proteins
Protein fractionation
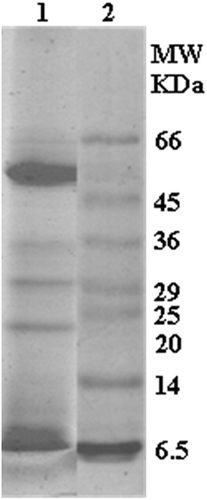
Initially, we intend to characterize the protein fraction, which was left out after ethanol (80%) extraction. Although proteins were precipitated with 80% ethanol, it was gummy in nature. Therefore, the dialysis method was used to retain the protein in the dialysis bag after removing the low molecular weight compounds like phenolics. Total warm seed exudate proteins were fractionated by SDS-PAGE as well as by gel filtration chromatography to know its heterogeneity. The total warm seed exudate had protein subunits with the molecular weights of 8, 16, 24, 32, 36, and 50 kDa (). When the total protein was separated on gel filtration chromatography, four protein peaks with molecular weights of 150 kDa (Peak 1), 66 kDa (Peak 2), 29 kDa (peak 3), and 12.4 kDa (Peak 4) were obtained (). Earlier, Hirano et al.[Citation29] reported the release of 7S globulin fraction that consisted of 27 kDa and 16 kDa subunits from green gram into warm water.
Inhibition of AChE activity
Seed exudate protein and its fractions were evaluated for their AChE inhibitory properties. Total protein of seed exudate at 20.28 µg/ml concentration showed 50% AChE inhibition whereas high molecular weight protein fractions, i.e. peak 1 and peak 2, did not show AChE inhibition. However, low molecular weight proteins, i.e. peaks 3 and 4, showed 50% inhibition at the concentrations of 8 μg/ml and 6.8 μg/ml, respectively (). Thus, the results indicate that phenolic acids, flavonoids, and low molecular weight proteins of total exudate from green gram may contribute to the inhibition of AChE activity.
Table 3. Acetylcholinesterase inhibition by total proteins of warm seed exudate and its protein fractions.
Inhibition of platelet aggregation
Protein and its fractions of seed exudates were evaluated for platelet aggregation inhibitory property. Total proteins of seed exudate at a concentration of 1 mg/ml showed 66.7% inhibition of platelet aggregation. Protein fractions, peak 1, peak 3, and peak 4 showed platelet aggregation inhibition. Among these protein fractions, peak 1 showed the highest inhibition of platelet aggregation (83%), and peak 3 and peak 4 showed 16 and 36% inhibition of platelet aggregation, respectively, at 1 mg/ml concentration ().
Table 4. Inhibition of platelet aggregation by total proteins warm seed exudate and its protein fractions.
Although earlier studies by Hirano et al.[Citation29] showed the presence of proteins in green gram seed warm exudates, they did not study ACh esterase inhibition or prevention of platelet aggregation by seed exudates. However, they identified low molecular weight proteins such as 16 and 27 kDa proteins, which were associated with the 7S globulin fraction. The 29 kDa (peak 3) and 12.4 kDa (peak 4) may also belong to the 7S fraction as they are comparable to the molecular weights reported by Hirano et al.[Citation29]
Conclusions
This study was conducted to investigate the ability of exudates from green gram to act as an antioxidant, an inhibitor of AChE, and an inhibitor for platelet aggregation. The crude seed exudate obtained at warm condition had higher contents of polyphenols and it showed an increase in the antioxidant activity in a dose-dependent manner compared with the exudate obtained at room temperature. Both DPPH radical scavenging and reducing power capacity were comparable to that of BHA. The antioxidant, AChE inhibition, and platelet aggregation inhibitory property by the ethanol extract of warm seed exudate may be due to the presence of gallic acid, ferulic acid, sinapic acid, vitexin, and isovitexin, which were reported to be potent antioxidants. The total proteins and their high MW protein fraction showed inhibition of platelet aggregation, while low molecular weight fractions showed AChE inhibition. These results suggest that higher temperature increases the release of bioactive compounds and proteins from green gram seeds into the exudate and, therefore, warm seed exudate can be used as a source of natural compounds to inhibit both platelet aggregation and AChE activity, which are beneficial properties for maintaining cardiovascular health.
Acknowledgements
Shaik Akbar Basha gratefully acknowledges the award of Senior Research Fellow by Indian Council of Medical Research Institute, New Delhi, India.
References
- Li, H.; Cao, D.; Yi, J.; Cao, J.; Jiang, W. Identification of the Flavonoids in Mungbean (Phaseolus radiatus L.) Soup and Their Antioxidant Activities. Food Chemistry 2012, 135, 2942–2946.
- Zhu, S.; Li, W.; Li, J.; Jundoria, A.; Sama, A.E.; Wang, H. It is not Just Folklore: The Aqueous Extract of Mung Bean Coat Is Protective against Sepsis. Evidence-Based Complementary and Alternative Medicine 2012, e498467.
- Scarafoni, A.; Ronchi, A.; Prinsi, B.; Espen, L.; Assante, G.; Venturini, G.; Duranti, M. The Proteome of Exudates from Germinating Lupinus albus Seeds is Secreted through a Selective Dual-Step Process and Contains Proteins Involved in Plant Defence. FEBS Journal 2013, 280, 1443–1459.
- Mel’nikova, N.N.; Omel’chuk, S.V. Effect of Legume Seed Exudates on the Formation of Rhizobium-Legume Symbiosis. Applied Biochemistry and Microbiology 2009, 45, 297–302.
- Palavalli, M.H.; Natarajan, S.S.; Wang, T.T.; Krishnan, H.B. Imbibition of Soybean Seeds in Warm Water Results in the Release of Copious Amounts of Bowman–Birk Protease Inhibitor, a Putative Anticarcinogenic Agent. Journal of Agricultural and Food Chemistry 2012, 60, 3135–3143.
- Hartwig, U.A.; Phillips, D.A. Release and Modification of nod-Gene-Inducing Flavonoids from Alfalfa Seeds. Plant Physiology 1991, 95, 804–807.
- Anusuya, S.; Sathiyabama, M. Identification of Defence Proteins from the Seed Exudates of Cicer arietinum L. and its Effect on the Growth of Fusarium oxysporum F.Sp. Ciceri. Archives of Phytopathology and Plant Protection 2014, 47, 1611–1620.
- Rocha, R.O.; Morais, J.K.; Oliveira, J.T.; Oliveira, H.D.; Sousa, D.O.; Souza, C.E.A.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Antonino de Souza Júnior, J.D.; Grossi de Sá, M.F. Proteome of Soybean Seed Exudates Contains Plant Defense-Related Proteins Active against the Root-Knot Nematode Meloidogyne incognita. Journal of Agricultural and Food Chemistry, 2015, 63, 5335–5343.
- Nóbrega, F.M.; Santos, I.S.; Cunha, M.D.; Carvalho, A.O.; Gomes, V.M. Antimicrobial Proteins from Cowpea Root Exudates: Inhibitory Activity Against Fusarium oxysporum and Purification of a Chitinase-Like Protein. Plant and Soil 2005, 272, 223–232.
- Abbasi, E.; Nassiri-Asl, M.; Shafeei, M.; Sheikhi, M. Neuroprotective Effects of Vitexin, a Flavonoid, on Pentylenetetrazole-Induced Seizure in Rats. Chemical Biology and drug design 2012, 80, 274–278.
- Choi, J.S.; Nurul Islam, M.; Yousof Ali, M.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food and Chemical Toxicology 2014, 64, 27–33.
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant Properties of the Mung Bean Flavonoids on Alleviating Heat Stress. PLOS ONE, 2011, 6, e21071.
- Schrör, K. Aspirin and Platelets: the Antiplatelet Action of Aspirin and its Role in Thrombosis Treatment and Prophylaxis. Seminars in Thrombosis and Hemostasis 1997, 23, 349–356.
- Smart, S.; Aragola, S.; Hutton, P. Antiplatelet Agents and Anaesthesia. Continuing Education in Anaesthesia, Critical Care & Pain 2007, 7, 157–161.
- El Haouari, M.; Rosado, J.A. Medicinal Plants with Antiplatelet Activity. Phytotherapy Research 2016, 30, 1059–1071.
- Chang, C.P.; Bruneau, B.G. Epigenetics and Cardiovascular Development. Annual Review of Physiology 2012, 17, 41–68.
- Kim, K.; Bae, O.-N.; Lim, K.-M.; Noh, J.-Y.; Kang, S.; Chung, K.Y.; Chung, J.-H. Novel Antiplatelet Activity of Protocatechuic Acid through the Inhibition of High Shear Stress-Induced Platelet Aggregation. Journal of Pharmacology and Experimental Therapeutics, 2012, 343, 704–711.
- Maheswaraiah, A.; Jaganmohan Rao, L.; Naidu, K.A. Anti-Platelet Activity of Water Dispersible Curcuminoids in Rat Platelets. Phytotherapy Research 2015, 29, 450–458.
- Rose, T.L.; Conceição, A.da S. ; Xavier-Filho, J.; Okorokov, L.A.; Fernandes, K.V.S.; Marty, F.; Marty-Mazars, D.; Carvalho, A.O.; Gomes, V.M. Defense Proteins from Vigna unguiculata Seed Exudates: Characterization and Inhibitory Activity Against Fusarium oxysporum. Plant and Soil 2006, 286, 181–191.
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 1976, 72, 248–254.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture, 1965, 16, 144–158.
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity of Phaseolus vulgaris L.Dry Beans.Journal of Agricultural and Food Chemistry 2005, 53, 3053–3056.
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified Vanillin. Journal of the Science of Food and Agriculture 1978, 29, 788–794.
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chemistry 2006, 95, 466–473.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30.
- Yen, G-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. Journal of Agricultural and Food Chemistry 1995, 43, 27–32.
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for Acetylcholinesterase Inhibitory Activity in Plants Used in Thai Traditional Rejuvenating and Neurotonic Remedies. Journal of Ethnopharmacology 2003, 89, 261–264.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Hirano, H.; Kagawa, H.; Okubo, K. Characterization of Proteins Released from Legume Seeds in Hot Water. Phytochemistry, 1992, 31, 731–735.
- Ndakidemi, P.A.; Dakora, F.D. Legume Seed Flavonoids and Nitrogenous Metabolites as Signals and Protectants in Early Seedling Development. Functional Plant Biology 2003, 30, 729–745.
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Current Neuropharmacology 2013, 11, 388–413.
- Rizzi, A.; Schuh, R.; Brückner, A.; Cvitkovich, B.; Kremser, L.; Jordis, U.; Fröhlich, J.; Küenburg, B.; Czollner, L. Enantiomeric Resolution of Galanthamine and Related Drugs Used in Anti-Alzheimer Therapy by Means of Capillary Zone Electrophoresis Employing Derivatized Cyclodextrin Selectors. Journal of Chromatography B: Biomedical Sciences and Applications 1999, 730, 167–175.
- Conforti, F.; Rigano, D.; Menichini, F.; Loizzo, M.R.; Senatore, F. Protection Against Neurodegenerative Diseases of Iris Pseudopumila Extracts and Their Constituents. Fitoterapia 2009, 80, 62–67.
- Zia-Ul-Haq, M.; Ahmed, S.; Rizwani, G.H.; Qayum, M.; Ahmad, S.; Hanif, M. Report: Platelet Aggregation Inhibition Activity of Selected Legumes of Pakistan. Pakistan Journal of Pharmaceutical Sciences 2012, 25, 863–5.