ABSTRACT
Prebiotics are known as the ‘food’ for beneficial gut microbiota that are capable of promoting host health. Their effects depend on the product of gut fermentation or metabolites. This paper discusses the role of prebiotics on cytotoxicity, genotoxicity, and cell integrity. Metabolites produced from the fermentation of prebiotics can be used to understand gut diseases such as colorectal cancer. Fecal water from in vivo or in vitro studies can be used to understand the relationship between prebiotics and gut diseases because of its close contact with colon epithelium. Besides, fecal water has compounds that are capable of modifying colonocytes.
Introduction
The human gastrointestinal tract hosts diverse microbes such as beneficial and harmful microorganisms. Among the beneficial microbes are Bifidobacterium, Lactobacilli, Fecalibacterium, and Eubacterium,[Citation1] whereas the harmful or pathogenic microbes include Escherichia coli and clostridia.[Citation2] The growth of beneficial microbes is stimulated by prebiotics, such that these microbes suppress or decrease the population of the pathogens in the human gut. Prebiotics are selectively fermented ingredients that cause specific changes in the composition and/or activities of the gastrointestinal microbiota.[Citation3] Although prebiotics have been part of the human diet for many years, their benefits were only recently discovered.[Citation4] In recent times, several food ingredients and dietary fibres have been considered as prebiotics. However, the well-known prebiotics are inulin, fructo-oligosaccharides, and galacto-oligosaccharides. It is worth noting that for a food ingredient to be classified as prebiotic, it must fulfill the following criteria:[Citation4] (i) resistance to gastric acidity, hydrolysis by mammalian enzymes, and gastrointestinal absorption; (ii) fermentation by intestinal microbiota; (iii) selective stimulation of the growth and/or activity of intestinal bacteria associated with health and well-being.
Modification of gut microbiota through the utilization of prebiotic and/or probiotic affects the immune system and the gut epithelium of human beings.[Citation5] Studies on cell toxicity, gene, and membrane integrity on gut fermentation environment vis-à-vis prebiotics could clarify whether putative prebiotics are harmful to their hosts or not. In this review, we discuss the role of prebiotics on cytotoxicity, genotoxicity, and cell integrity.
Fecal water
In prebiotics research, focus on fecal water or aqueous extracts of gut fermentation is essential as this sample contains functional metabolites. Fecal water has compounds that are capable of modifying the growth of colonocytes better than in the solid phase.[Citation6] The toxicity of fecal water depends on the metabolites that are associated with it. Several studies have associated increase in colon cancer to the significant correlation between cytotoxicity and genotoxicity of fecal water. This could be one of the reasons for using fecal water toxicity as cancer biomarkers.[Citation7] Compounds in fecal water that are toxic to cells and DNA are bile acids, N-nitroso compounds, and heterocyclic amines.[Citation8] A number of processed foods have been reported to be high in carcinogenic metabolites such as bile acids (in fecal water),[Citation8] suggesting that people on processed foods could be prone to colon cancer. In addition, the fermentation of excessive protein in the gut produces isobutyrate, isovalerate, nitrogenous, and phenolic compounds that are toxic to the host.
The prebiotic oligofructose has been used to control the toxic effects of cycloalkanes, cycloalkenes, and esters on gut cells. These metabolites in fecal water samples indicate lower cytotoxicity compared with other metabolites. The effectiveness of the metabolites in reducing cytotoxicity is related to their antimicrobial and antioxidant properties.[Citation9] Another example is konjac glucomannan (KGM; soluble fibre), which is known to reduce the cytotoxicity of fecal water just as insoluble fibre does.[Citation10] In addition, KGM not only lowers the concentration of secondary bile acids but also increases short-chain fatty acids (SCFA) production.[Citation11]
Prebiotics should not react with the body since it is inert and harmless and must be safe when consumed.[Citation12] Besides, prebiotics are able to defy the acidic and enzymatic nature of the small intestine and because of this, prebiotics are able to move to the large intestine where they are utilized by the gut microbiota. This mechanism improves immunity and absorption, thus reducing the risk of acquiring gastrointestinal diseases.[Citation13] The prebiotic that enters the large intestine decreases the pH (acidic condition) of the large intestine due to the formation of SCFA.[Citation14] The main SCFAs are acetate, propionate, and butyrate with the molar ratio of 60:20:20. Generally, the health-promoting effects of prebiotics have been associated with the production of SCFA by colonic microbiota. Apart from being able to suppress the growth of enteric pathogens through the reduction of luminal pH, SCFA also affects intestinal motility because they are rapidly absorbed by the colonic mucosa, thus contributing to the energy requirements of the host.[Citation15] Acetate is mainly metabolized in human muscle, kidney, heart, and brain tissues. Propionate inhibits cholesterol synthesis in the liver and the regulation of adipose tissue deposition. Butyrate, which is mainly metabolized by the colonic epithelium, regulates cell differentiation. Butyrate also prevents colorectal cancer and colitis.[Citation1] A study has been conducted regarding the toxicity where galactooligosaccharides (GOS) are force-fed to Sprague-Dawley rats at 2500 or 5000 mg/kg BW/day for a length of 90 days. A few parameters were being carefully observed such as body weights, blood chemistries, and organ weight, and other examinations had no significant toxicology effect on the rat.[Citation16] Despite the innocuous nature of GOS in rats, utilization on human will still require further considerations. A sudden alteration in the intestinal microbiota could show adverse effects depending on the stimulated bacteria population. For example, the increase of butyrate formation by the intestinal flora metabolism with the butyrogenic substrate will lead to the growth of unwanted clostridia.[Citation17] However, overconsumption of prebiotic may cause stomach flatus, abdominal difficulties, and even diarrhoea. A normal dosage of oligofructose and inulin ranging between 10 and 20 grams is recommended to prevent the adverse effects. In addition, a study showed that the cytotoxicity of fecal water can be reduced after the uptake of oligofructose.[Citation18] Besides oligofructose, KGM are also known to reduce colonic toxicity in human and hence prevent colorectal cancer.[Citation10]
Cytotoxicity
Cytotoxicity is defined as the negative consequences resulting from disturbance with structures and/or processes required for cell survival, proliferation, and function.[Citation19] This causes cell necrosis (disintegration of membrane integrity) and lysis. The cells may decrease in viability by ceasing growth and dividing or even trigger apoptosis (death of program cell). Cytotoxic compounds in crude extracts kill either the target cells or the neighboring cells. Cytotoxicity screening is used to determine ‘hits’, i.e. the cutoff point of the minimum concentration of extracts used to stop the growth of the cells. The widely used cytotoxicity screening assay includes sulforhodamine B (SRB) and tetrazolium assay (MTT), and the comparison of both assays is summarized in .
Table 1. Comparison of sulforhodamine B (SRB) and tetrazolium assay (MTT).
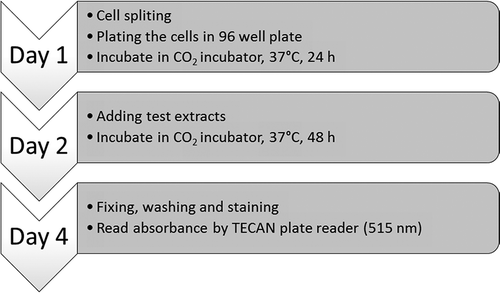
SRB assay was initially formulated as an endpoint mainly for the in vitro screening of anticancer agents.[Citation20] The SRB dye binds protein, normally amino acid residues in trichloroacetic acid (TCA) under acidic conditions to enable the sensitive scale of the cellular protein content, resulting in rapid and visible pink-red coloration.[Citation21] Once the proteins are stained with SRB, they are evaluated using enzyme-linked immunosorbent assay (ELISA) plate reader over a wide range of visible wavelengths. One of the advantages of the SRB assay is that samples that are fixed with TCA and stained with SRB can be stored indefinitely without degeneration. The SRB assay, which is closely related to in vivo toxicities, is a potential assay for in vitro toxicological studies because of its nature against operator bias, absorbance sensitivity, and long-term stability.[Citation22] shows the flow chart of cell passaging and the basic procedure for the SRB bioassay.
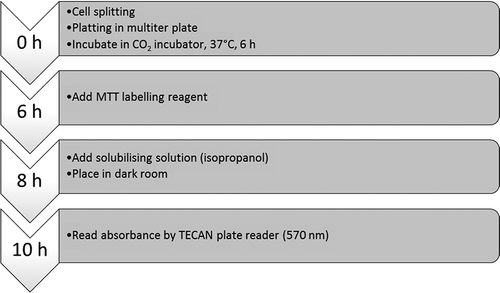
The MTT assay is suitable for high-throughput screening in a 96-well format.[Citation23] Formazan is produced through cells activity such as the reduction of tetrazolium salt.[Citation24] Cell activity refers to the cleavage of the tetrazolium ring of mitochondria in cells and this is why the MTT assay assesses only viable cells.[Citation25] The sites where mitochondria dehydrogenase are present convert the yellowish soluble salt to purplish insoluble formazan. Formazan, which is soluble in organic solvent such as dimethyl sulfoxide, is quantified using spectrophotometry.[Citation26] Thus, the ability to reduce MTT to formazan provides a denotation to the integrity and activity of mitochondria.[Citation21] shows the flow chart of cell passaging and the basic procedure for the MTT bioassay.
A study has revealed that prebiotics prevents cytotoxicity because they protect colorectal adenocarcinoma cells (HT-29 cell line against cytotoxicity).[Citation27] In that study, fecal water was analysed for bile acid content (lithocholic and deoxycholic acids) using Matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS). In an MTT assay with prebiotics (inulin and lactulose), in vitro cells survival increased by 100% for deoxycholic acid, by 30% for lithocholic acid, and by 40% for fecal water. Using wheat bran extract and oligofructose in a double-blind, crossover randomized controlled human trial, the presence of this prebiotic resulted in significant reduction in the cytotoxicity of fecal water.[Citation18]
In the intestinal barrier modulation of prebiotic and probiotic, intra-epithelial lymphocytes (CD8+) shut the epithelial barrier response to cause cytotoxicity.[Citation28] Commensal microbes and probiotics are assisted by prebiotics to compete for nutrients and binding sites on the cell surfaces of pathogenic organisms. Probiotics affect mucin expression and the secretion of mucus from goblet cells (GoC), whereas IgA neutralizes the pathogens in the mucus layer known to be controlled by polymeric immunoglobulin receptor-mediated (pIgR) transcytosis. Antimicrobial peptides are induced by probiotics against pathogens in two ways, directly as bacteriocins or indirectly by activating the epithelial cells to produce defensins. Cytotoxic foreign substances are killed due to the activation of the production of natural killer (NK) cell (IL-12 and IL-15) by antigen-presenting cells (APCs). The default setting of APCs is to present safe commensal peptides. In this case, it is the probiotics that activate the tolerogenic mechanism, suppressing the T-effector. Otherwise, if pathogenic peptides are detected, the tolerogenic mechanism is bypassed such that T-effector is initiated. For example, type-1 T-helper (Th1) responds to intracellular pathogens, type-17 T-helper (Th17) is for fungal protection whereas type-2 T-helper (Th2) is for extracellular pathogens. Appropriate modulation is essential for the prevention of allergy, bowel inflammation diseases, and tumour formation. For coeliac disease, because gluten (α-gliadin) from wheat is not tolerated by the gut, it causes discomfort in the digestive tract.[Citation29]
Genotoxicity
Genotoxicity is the property of chemical agents that damages the genetic information in cells such that mutations leading to cancer occur. The techniques that are used to examine genotoxicity include the Ames test, the SOS chromo test, and the Comet assay. The Ames test is used to examine the mutagenic potency of test chemicals using preexisting mutated Salmonella strains that inhibit amino acid histidine synthesis. This prevents bacterial colonies formation.[Citation30] However, if any other mutations take place in the genes, the preexisting mutated gene is recovered, thus enabling the synthesis of histidine. Initially, it has the mutated histidine– genotypic, but because of a secondary mutation, histidine– reverts to histidine+ genotypic to produce amino acid histidine. This leads to the growth of the bacterial strain in media, which are deficient in histidine.[Citation31] This is why the reversal of histidine is referred to as the reversion assay.
The SOS chromo test is a typical test to evaluate chemicals that cause damages to the gene. It is a short-term colorimetric assay that uses Escherichia coli PQ37 as a specific mutant.[Citation32] The SOS chromo test responds rapidly (few hours). Besides, the chromo test requires only a single tester strain.[Citation33] The SOS-inducing signal occurs when DNA stops replicating itself after exposure to genotoxic agents. Once the repressor is cleaved, genes sfiA and lacZ fuse together to promote the induction of β-galactosidase activities.[Citation34] The β-galactosidase activities are evaluated using 5-bromo-4chloro-3indolyl-β-D-galactosidase using the X-gal method.[Citation35]
In the Comet assay, the cells are placed in agarose, laid on a microscope slide, lysed with specific lysis solution, namely concentrated aqueous table salt and sarcosinate, to free the DNA. Afterward, the cells are treated under alkaline conditions followed by staining using either cyanine dye or ethidium bromide prior to electrophoresis. Finally, the comet scores are analysed using computer software.[Citation36] The results obtained will show various figures with head and tail that resemble a ‘comet’. The DNA strand breaks are rapidly detected based on the migrating denatured DNA fragments during electrophoresis.[Citation37] The DNA remaining in the comet head denotes undamaged DNA, whereas the comet tail denotes damaged DNA. The damaged DNA is exemplified visually or scored using a software.[Citation38]
Few studies have demonstrated how prebiotics and/or probiotics reduce cell genotoxicity. When probiotic microorganisms such as Bifidobacterium Bb12 and Lactobacillus plantarum were incubated with fecal water, genotoxicity was reduced.[Citation39] Fecal water-induced DNA damage was also reduced from supernatants incubated with fructo-oligosaccharides-based prebiotic.[Citation39] Both Lactobacillus and Bifidobacterium species have been used to reduce metabolites and enzymes that are linked to the production or activation of carcinogens, where the consumption of probiotic mix, i.e. Lactobacillus paracasei Lpc-37, Lactobacillus acidophilus 74–2, and Bifidobacterium animalis subsp. lactis DGCC 420, can lower the genotoxic potential of fecal water in atopic dermatitis patients.[Citation40] Furthermore, oral consumption of Lactobacillus acidophilus 145, Bifidobacterium longum 913, and Bifidobacterium animalis subsp. lactis DGCC 420 results in fecal water with lower genotoxic.[Citation40]
Cell membrane integrity
The cell membrane is composed of lipid bilayers that act as a barrier to enable substances to enter or exit the cell. When the membrane integrity is destroyed or weakened by test compounds, it loses its function, thus causing foreign substances to enter the cell. This results in necrosis and apoptosis. The cytoplasmic contents are released into the surrounding tissue due to the loss of cell membrane integrity. Moreover, the release of cytoplasmic contents leads to signal transmission (chemotactic signal) that has been implicated in cells inflammation.[Citation41] This process occurs either sequentially or simultaneously contingent upon the toxicity of the test compound. A cell with degraded membrane is unable to withstand external osmotic pressure and harmful substances.
In vitro measurement of cell membrane is performed through the assessment of cells tight junction integrity. Tight junction (zonula occludens) is a connector between adjacent epithelial cells that maintains the cell membrane integrity[Citation42] and regulates solutes movement across the epithelium.[Citation43] To determine the integrity of tight junctions physiological changes, trans-epithelial electrical resistance (TEER) is determined using Epithelial Voltohmmeter (EVOM).[Citation44] The TEER readings are taken after the cells had been seeded into Transwell® insert and are polarized after days of incubation. The Transwell® has inner and outer compartments that depict apical (lumen) and basolateral (extracellular fluids) layers in vivo. A decrease in TEER value (Ohm) indicates a weakening of the cell tight junction, thus suggesting a potential decrease in resistance that could cause death.[Citation45] This increases ion fluxes between the areas of medium and cells.[Citation46]
One of the significant biomarkers of colorectal cancer is the leaking and weakening of the tight junction.[Citation47] The missing or lack of tight junctions is due to the modification of the adhesive properties of tumour cells.[Citation48] Studies show that there is a significant difference in the number of tight junctions of normal and tumour cells. The number of tight junctions in normal cells is higher compared with those of tumour cells.[Citation48] Colon tumours are observed to have more ‘leakier’ tight junctions compared with those of normal colon cells. This enhances the permeability of epithelium and at the same time decreasing the barrier function.[Citation42]
Prebiotics and probiotics can be used to tighten junctions of tumour cells, e.g. TNF-α can induce Lactobacillus and Bifidobacterium species to prevent the disruption of epithelial barrier of the human intestine.[Citation49] Moreover, accession of TNF-α can induce Bifidobacterium to repair lesions in Caco-2 cell monolayers. SCFAs such as butyrate, which is produced during the fermentation of prebiotics, can improve the integrity of tight junctions. Studies have revealed that different relevant SCFAs are related to the paracellular permeability of the Caco-2 cell line model, and butyrate, in particular, not only reduces mannitol flux but also increases TEER in a concentration-dependent manner.[Citation50] Additionally, the paracellular permeability of cells is reduced with the possibility of more differentiated phenotypes of the cells. Studies on the mode of action of butyrate vis-à-vis tight junction cells integrity are currently limited to morphology, gene transcription, protein synthesis, β-oxidation, cell proliferation, and differentiation.[Citation50] In terms of morphology, there are no major differences between control cells and butyrate-treated cells such as swelling of cell and multilayer of Caco-2. For the gene transcription and protein synthesis aspect, the TEER is dependent on both, e.g. actinomysin D and cycloheximide as RNA synthesis inhibitors, where both completely inhibit butyrate effect on Caco-2 cells. For the cellular oxidation (β-oxidation) inhibition aspect of butyrate, the post-effect of sodium hydrosulphate (NaSH) is determined on the butyrate-mediated effect of cell stimulation. Significant increase in TEER values is shown when NaSH is present in butyrate. This observation has been reported to be similar when butyrate was supplemented without NaSH, suggesting that NaSH has no additional effect on butyrate activities. For cell proliferation and differentiation, a study showed that cell proliferation can be reduced with the treatment of butyrate after 48 h due to a lesser thymidine incorporation by Caco-2 cells. This is confirmed by the reduction in total cellular protein.[Citation50]
Conclusion
Prebiotics can potentially serve as functional food ingredients to improve or maintain gut health. This is possible through increasing the population of beneficial gut microbiota while suppressing the harmful ones. Carbohydrate-based prebiotic undergoes saccharolytic fermentation in the gut to produce beneficial metabolite, namely SCFAs. However, protein metabolism by pathogenic microbiota such as Escherichia coli, Clostridium, Streptococcus, and Staphylococcus can produce detrimental metabolites such as ammonia, amines, branched chain fatty acids, and phenolic compounds. The metabolites can damage the colonic epithelium and the associated gene. They can also compromise cell membrane integrity. Excessive fermentation of proteins, especially in the distal colon, has been linked to diseases such as colorectal cancer and inflammatory bowel diseases. Therefore, it is essential to asses these metabolites in the fecal phase in vitro and/or in vivo and clinically. Information on cytotoxicity, genotoxicity, and cell integrity of particular metabolites can be used as a biomarker, especially for colorectal cancer evaluation. This information could guide scientists to further understand how a prebiotic ingredient acts in the colonic environment. This will certainly boost confidence and acceptance among scientific community, regulators, and consumers of prebiotics.
Funding
This work was supported by the Malaysian Ministry of Science, Technology and Innovation through Science Fund Scheme, number: 02-01-04-SF16481.
Additional information
Funding
References
- Sarbini, S.R.; Rastall, R.A. Prebiotics: Metabolism, Structure, and Function. Functional Food Review 2011, 3(3), 93–106.
- Vernazza, C.L.; Rabiu, B.A.; Gibson, G.R. Human Colonic Microbiology and the Role of Dietary Intervention: Introduction to Prebiotics. In Prebiotics: Development & Application; John Wiley & Sons, 2006; 1–28.
- Roberfroid, M. et al. Prebiotic Effects: Metabolic and Health Benefits. British Journal of Nutrition 2010, 104(SupplementS2), S1–S63.
- Leach, J.D.; Gibson, G.R.; Loo, J.V. Human Evolution, Nutritional Ecology and Prebiotics in Ancient Diet. Bioscience and Microflora 2006, 25(1), 1–8.
- Vyas, U.; Ranganathan, N. Probiotics, Prebiotics, and Synbiotics: Gut and Beyond. Gastroenterology Research and Practice 2012, 2012, 16.
- Nordling, M.M. et al. Effects on Cell Proliferation, Activator Protein-1 and Genotoxicity by Fecal Water from Patients with Colorectal Adenomas. Scandinavian Journal of Gastroenterology 2003, 38(5), 549–555.
- Glinghammar, B. et al. Shift from a Dairy Product-Rich to a Dairy Product-Free Diet Influence on Cytotoxicity and Genotoxicity of Fecal Water–Potential Risk Factors for Colon Cancer. American Society for Clinical Nutrition 1997, 66, 1277–1282.
- Clark, M.J.; Robien, K.; Slavin, J.L. Effect of Prebiotics on Biomarkers of Colorectal Cancer in Humans. Nutrition Review 2012, 70(8), 436–443.
- Mohamed, A.A.; Ali, S.I.; El-Baz, F.K. Antioxidant and Antibacterial Activities of Crude Extracts and Essential Oils of Syzygium cumini Leaves. Plos ONE 2013, 8(4), e60269.
- Wu, W.-T.; Cheng, H.-C.; Chen, H.-L. Ameliorative Effects of Konjac glucomannan on Human Faecal Β-Glucuronidase Activity, Secondary Bile Acid Levels and Faecal Water Toxicity Towards Caco-2 Cells. British Journal of Nutrition 2011, 105(04), 593–600.
- Chen, H.-L. et al. Supplementation of Konjac glucomannan into A Low-Fiber Chinese Diet Promoted Bowel Movement and Improved Colonic Ecology in Constipated Adults: A Placebo-Controlled, Diet-Controlled Trial. Journal of the American College of Nutrition 2008, 27(1), 102–108.
- Anadón, A. et al. Chapter 54 - Prebiotics: Safety and Toxicity Considerations A2 - Gupta, Ramesh C, in Nutraceuticals; Academic Press: Boston, USA, 2016; 757–775.
- Thammarutwasik, P. et al. Prebiotics: A Review. Songklanakarin Journal Sciences Technological 2009, 31(4), 401–408.
- Den Besten, G. et al. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. Journal of Lipid Research 2013, 54(9), 2325–2340.
- Dass, N.B. et al. The Relationship between the Effects of Short-Chain Fatty Acids on Intestinal Motility in Vitro and GPR43 Receptor Activation. Neurogastroenterology and Motility 2007, 19(1), 66–74.
- Anthony, J.C.; Merriman, T.N.; Heimbach, J.T. 90-Day Oral (Gavage) Study in Rats with Galactooligosaccharides Syrup. Food and Chemical Toxicology 2006, 44(6), 819–826.
- Wang, Y. Prebiotics: Present and Future in Food Science and Technology. Food Research International 2009, 42(1), 8–12.
- Windey, K. et al. High Dose of Prebiotics Reduces Fecal Water Cytotoxicity in Healthy Subjects. Molecular Nutrition & Food Research 2014, 58(11), 2206–2218.
- Ekwall, B. Screening of Toxic Compounds in Mammalian Cell Cultures. Annals New York Academic Sciences 1983, 407(1), 64–77.
- Skehan, P. et al. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. Journal of National Cancer Institute 1990, 82(13), 1107–1112.
- Salati, N.A.; Ahmad, S.S.; Khwaja, K.J. Assessment of Cytotoxicity in Chemotherapeutic Drugs. Journal of Pharmacy and Biological Sciences 2013, 5(5), 73–76.
- Fricker, S.P. The Application of Sulforhodamine B as a Colorimetric Endpoint in a Cytotoxicity Assay. Toxic in Vitro 1994, 8(4), 2.
- Riss, T.L. et al. Cell Viability Assays. Eds. G.S. Sittampalam, N.P. Coussens, H. Nelson, M. Arkin, D. Auld, C. Austin, et al., Assay Guidance Manual [Internet], Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda (Retrieved August 15, 2016, http://europepmc.org/books/NBK144065/). 2013.
- Berridge, M.V.; Tan, A.S. Characterization of the Cellular Reduction of MTT. Journal of Archives of Biochemistry and Biophysics 1993, 303(2), 474–482.
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. Journal of Immunological Methods 1983, 65(1–2), 55–63.
- Van De Loosdrecht, A.A. et al. A Tetrazolium-Based Colorimetric MTT Assay to Quantitate Human Monocyte Mediated Cytotoxicity against Leukemic Cells from Cell Lines and Patients with Acute Myeloid Leukemia. Journal of Immunological Methods 1994, 174(1–2), 311–320.
- Adebola, O.; Corcoran, O.; Morgan, W.A. Protective Effects of Prebiotics Inulin and Lactulose from Cytotoxicity and Genotoxicity in Human Colon Adenocarcinoma Cells. Food Research International 2013, 52, 269–274.
- Hardy, H. et al. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5(6), 1869–1912.
- Meresse, B. et al. Coordinated Induction by IL15 of a TCR-Independent NKG2D Signaling Pathway Converts CTL into Lymphokine-Activated Killer Cells in Celiac Disease. Immunity 2004, 21(3), 357–366.
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2000, 455(1–2), 29–60.
- Goodson-Gregg, N.; De Stasio, E.A. Reinventing the Ames Test as a Quantitative Lab that Connects Classical and Molecular Genetics. Genetics 2009, 181(1), 23–31.
- Mersch-Sundermann, V.; Klopman, G.; Rosenkranz, H.S. Chemical Structure and Genotoxicity: Studies of the SOS Chromotest. Mutation Research/Reviews in Genetic Toxicology 1996, 340(2–3), 81–91.
- Quillardet, P. et al. SOS Chromotest, a Direct Assay of Induction of an SOS Function in Escherichia Coli K-12 to Measure Genotoxicity. Proceedings of the National Academy of Sciences of the United States of America 1982, 79(19), 5971–5975.
- Quillardet, P.; Hofnung, M. The SOS Chromotest, a Colorimetric Bacterial Assay for Genotoxins: Procedures. Mutation Research/Environmental Mutagenesis and Related Subjects 1985, 147(3), 65–78.
- Von Der Hude, W. et al. Evaluation of the SOS Chromotest. Mutation Research/Environmental Mutagenesis and Related Subjects 1988, 203(2), 81–94.
- Tice, R.R.;, et al. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environmental and Molecular Mutagenesis 2000, 35(3), 206–221.
- Liao, W.; McNutt, M.A.; Zhu, W.-G. The Comet Assay: A Sensitive Method for Detecting DNA Damage in Individual Cells. Methods 2009, 48(1), 46–53.
- Gratz, S.W.; Wallace, R.J.; El-Nezami, H.S. Recent Perspectives on the Relations between Fecal Mutagenicity, Genotoxicity, and Diet. Frontiers in Pharmacology 2011, 2, 4.
- Burns, A.J.; Rowland, I.R. Antigenotoxicity of Probiotics and Prebiotics on Faecal Water-Induced DNA Damage in Human Colon Adenocarcinoma Cells. Mutation Research 2004, 551, 233–243.
- Roessler, A. et al. The Effect of Probiotics on Faecal Microbiota and Genotoxic Activity of Faecal Water in Patients with Atopic Dermatitis. Clinical Nutrition 2012, 31, 22–29.
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology 2007, 35(4), 495–516.
- Martin, T.A.; Jiang, W.G. Loss of Tight Junction Barrier Function and Its Role in Cancer Metastasis. Biochimica Et Biophysica Acta (BBA): Biomembranes 2009, 1788(4), 872–891.
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harbor Perspectives in Biology 2009, 1(2), a002584.
- Bailey, C.A.; Bryla, P.; Malick, A.W. The Use of the Intestinal Epithelial Cell Culture Model, Caco-2, in Pharmaceutical Development. Advanced Drug Delivery Reviews 1996, 22(1–2), 85–103.
- Mathieu, F. et al. Influence of Different Calcic Antagonists on the Caco-2 Cell Monolayer Integrity or “TEER, a Measurement of Toxicity ?”. European Journal of Drug Metabolism and Pharmacokinetics 2005, 30(1–2), 85–90.
- Pasternak, A.S.; Miller, W.M. Measurement of Trans-Epithelial Electrical Resistance in Perfusion: Potential Application for in Vitro Ocular Toxicity Testing. Biotechnology and Bioengineering 1996, 50(5), 568–579.
- Commane, D.M. et al. Effects of Fermentation Products of Pro- and Prebiotics on Trans-Epithelial Electrical Resistance in an in Vitro Model of the Colon. Nutrition and Cancer 2005, 51(1), 102–109.
- Easty, G.C.; Mercer, E.H. An Electron Microscope Study of the Surfaces of Normal and Malignant Cells in Culture. Cancer Research 1960, 20(11), 1608–1613.
- Hsieh, C.Y. et al. Strengthening of the Intestinal Epithelial Tight Junction by Bifidobacterium Bifidum. Physiological Reports 2015, 3(3), e12327.
- Mariadason, J.M.; Barkla, D.H.; Gibson, P.R. Effect of Short-Chain Fatty Acids on Paracellular Permeability in Caco-2 Intestinal Epithelium Model. American Journal of Physiology: Gastrointestinal and Liver Physiology 1997, 272(4), G705–G712.