ABSTRACT
In this paper, 15 batches of volatile oil from Schizonepeta tenuifolia herbs (STVO) originating from different areas of China were used to identify and quantify eight main components by GC-MS and to evaluate the antioxidant activity with 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity by the HPLC method. The results indicated that with respect to the antioxidant activity and the contents of the main compounds, there were notable differences among the STVOs. IC50 values of the STVOs ranged from 0.016 to 1.518 mg/mL. To determine the correlation of the chemical components and the antioxidant activities, multiple linear regression analysis (MLRA) and partial least square analysis (PLSA) were performed. As a result, (+)-pulegone and (-)-menthone showed much closer relationships than the other components in each analysis. Hence, it was concluded that (+)-pulegone and (-)-menthone were the characteristic components that may exhibit potent antioxidant activities in STVO.
Introduction
Schizonepeta tenuifolia (ST), belonging to the Lamiaceae family, is classified as a Traditional Chinese Medicine (TCM) to release the exterior, disperse wind, promote eruption, and relieve sores. As an aromatic medicinal plant, the herb is widely distributed in China, Korea, and Japan. The dried aerial part of ST, called Jingjie in China, is recorded in the Chinese Pharmacopoeia for the treatment of common cold, headache, measles, rubella, early onset of sore, and ulcer.[Citation1] Modern pharmacological studies have shown that ST herbs have various biological functions, such as anti-inflammation,[Citation2–Citation4] immunomodulation,[Citation2,Citation5] relieving itching,[Citation6] and antioxidation.[Citation3,Citation7] As we know, with anti-inflammatory,[Citation4,Citation8,Citation9] antitumor,[Citation10] and fumigant[Citation11] activities, the volatile oil of ST herbs (STVO) was used as a raw material in many TCM prescriptions recorded in Chinese Pharmacopoeia . Thus, STVO was considered responsible for the efficacies of ST herbs.
STVO contains many monoterpenoids, which mainly include (−)-limonene, (-)-menthone, (+)-menthofuran, and (+)-pulegone. These components have different bioactive or toxic activities. (+)-Pulegone is a natural monoterpene obtained from the volatile oils of a variety of plants. It was employed as flavouring agents and in perfumery. (+)-Pulegone decreased myocardial contractility and markedly reduced both the intracellular Ca2+ transient and L-type Ca2+ current. Additionally, (+)-pulegone caused changes in the action potential waveform and reductions of the outward and inward potassium current.[Citation12] As an important oxygenic terpenoid, (-)-menthone has been widely used for fragrance and flavour in the cosmetic, perfume, drug, and food industries. The component can also freely cross the blood–brain barrier and have been used as a cooling agent, a counterirritant for pain relief, and an antidepressant-like substance.[Citation13] (−)-Limonene is one of the most common monoterpenes in nature and has shown anxiolytic effect, regulatory effect on neurotransmitters, and antinociceptive effect.[Citation14] However, (+)-menthofuran is a toxic furan terpenoid that was oxidized by mammalian cytochrome P450 to hepatotoxic metabolites.[Citation15] In addition, 1-octen-3-ol, 3-octanone, β-myrcene, and β-caryophyllene are also the main constituents of STVO. Both 1-octen-3-ol and 3-octanone are typical “mouldy” odorants.[Citation16] β-myrcene has shown potent hepatoprotective, potential antimicrobial, antioxidant, and anti-inflammatory activities.[Citation17,Citation18] Thus, it is obvious that all these eight compounds are the key ones to STVO due to their bioactives.
However, to our knowledge, the antioxidant activity of STVO has not been documented while there have been several literatures about the bioactivity of other essential oils extracted from herbs.[Citation19,Citation20] At present, 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay,[Citation21,Citation22] 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assay,[Citation23,Citation24] and β-carotene bleaching assay[Citation25,Citation26] are the common in vitro methods of antioxidant activity evaluation. Among these methods, DPPH assay by HPLC was considered as the rapid, stable, and sensitive one.[Citation27–Citation29]
In this study, a DPPH assay method was designed and validated to evaluate the antioxidant activities of different STVOs. Furthermore, we also aimed to investigate the correlation of chemical components and antioxidation of STVO and identify the characteristic component(s) responsible for the bioactivity by some chemometric methods.
Materials and methods
Chemicals and samples
Chemicals and reagents we used in the experiment are listed in . Fifteen batches of the ST herbs were collected from different locations in China, and were identified by Prof. Qi-Nan Wu in Nanjing University of Chinese Medicine. The voucher specimen was stored at the herbarium of Nanjing University of Chinese Medicine, China. lists the sample’s information of different batches of ST herbs.
Table 1. Chemicals and reagents.
Table 2. Sample information of different bathes of ST herbs.
Herbal extraction and sample preparation
According to the Chinese Pharmacopoeia,[Citation1] STVOs were separately extracted with the hydro-distillation method using a set of standard instruments. Anhydrous sodium sulphate was added into the volatile oil at the ratio of 5 g:100 mL to remove the trace water. After standing overnight, all the STVOs were filtered and then stored in dark glass bottles at 4°C until use.
Gas chromatography–mass spectrometry analysis
Each STVO was dissolved and diluted with ethyl acetate to the concentration of about 80 μg/mL. Qualitative and quantitative analyses for 15 batches of the STVO were carried out using an Agilent 7890B-7000C GC-MS with an Agilent HP-5MS capillary column (30 m × 0.25 mm ID, 0.25 µm film thickness). Helium was the carrier gas at the flow rate of 1.0 mL/min. The oven temperature was set at 50°C for 2 min, increased to 90°C at a rate of 10°C/min and held for 3 min, and then increased to 150°C at a rate of 8°C/min and held for 3 min. The volume injected was 1 μL with a split ratio of 5:1. The inlet temperature was 220°C and the aux temperature was 200°C. Electron ionization (EI) worked as the ion source at 230°C with 70 ev and mass quadrupole was at 150°C. Scan mode was accepted from m/z 50 to 550.
Antioxidant activity assay
DPPH and samples preparation: Fresh DPPH stock solution was prepared by dissolving 80.32 mg DPPH in 100 mL methanol, which was stored at 4°C, and then diluted to the appropriate concentrations before use. Fifty milligrams of STVO were accurately weighed and transferred into a 10 mL volumetric flask, and then diluted to different concentrations for analysis (0.1, 0.25, 0.5, 1, 2, and 3 mg/mL).
HPLC analysis: The antioxidant activity was determined by the HPLC method as reported previously.[Citation27] Initially, an aliquot of 0.5 mL STVO solution was added to 0.5 mL DPPH solution (50 μg/mL). The mixture was vortexed for 30 s and reacted in the darkness for 60 min at room temperature. Then the mixture was filtered through a 0.45 μm microfiltration membrane and 10 μL of the sample was injected for HPLC analysis. A waters 2695 HPLC coupled with a photodiode array detector (Waters 2998) was used. The blank control was prepared by adding 0.5 mL methanol to 0.5 mL DPPH solution. The chromatographic separation was performed on a Hedera ODS-2 column (150 mm×4.6 mm×5 μm). Methanol-0.2% formic acid (80:20, v/v) was used as the mobile phase at the flow rate of 1.0 mL/min. The DPPH peak was monitored at 517 nm. The difference of DPPH peak areas (PAs) between the blank and the sample was used to calculate the radical scavenging activity according to the following equation: Radical scavenging rate (%) = (PAblank—PAsample)×100%/PAblank.
Optimization of reaction condition between DPPH and STVO: As is known, reaction time and DPPH concentration are the key factors to the final result. With radical scavenging rate as the evaluation index, the reaction time and DPPH concentration were optimized in the range of 0~120 min and 50~800 μg/mL, respectively.
Antioxidant capacity for samples using IC50: In this paper, IC50 value was named as the concentration of STVO scavenging 50% DPPH free radicals. STVO solutions of different concentrations (0.1, 0.25, 0.5, 1, 2, and 3 mg/mL) and their corresponding radical scavenging rates were used to establish the probit interpretation equation with IBM SPASS Statistics 19.0 software. IC50 value of each STVO could be calculated with the equation of this STVO.
Correlation analysis
Multiple linear regression analysis (MLRA)[Citation25,Citation30] was used to model the relationship between two or more variables with SPSS statistics software (IBM SPASS Statistics19.0). Partical least square analysis (PLSA)[Citation31,Citation32] was a wide class of methods for modelling relations between sets of observed variables by means of latent variables with SIMCA-P + 11.0 (Umetrics, Umea, Sweden). The general purposes of the two models were to learn about the relationship between the contents of the eight compounds and the antioxidant activity (IC50) of STVOs. p-Values less than 0.05 and 0.01 were considered statistically significant and very significant, respectively.
Results and discussion
Chemical composition analysis of STVO by GC-MS
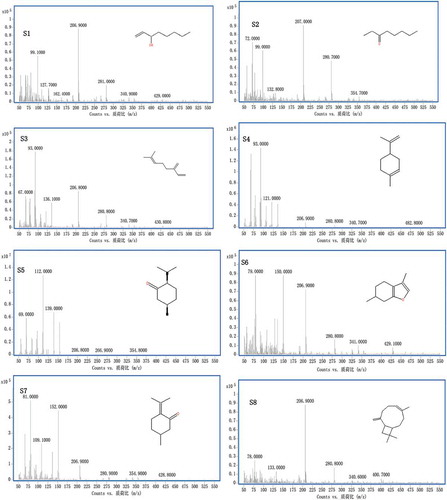
The GC-MS method was used to analyse the chemical compositions of 15 batches of hydro-distilled STVOs. Eight compounds (S1, 1-octen-3-ol; S2, 3-octanone; S3, β-myrcene; S4, (-)-limonene; S5, (-)-menthone; S6, (+)-menthofuran; S7, (+)-pulegone; and S8, β-caryophyllene) were identified with the data bank mass spectra (NIST libraries) and with the individual standard in each batch of STVO. The total ion chromatograms (TICs) and extract ion chromatograms (EICs) of STVO (sample 2) and the reference substances are shown in . The EI mass spectra of the eight compounds with their chemical structures are illustrated in . These figures showed good separation for the eight components and their accurate data spectra.
Figure 1. Total ion chromatograms of STVO (sample 2, A1) and reference substances (B1); extract ion chromatograms of STVO (sample 2, A1) and reference substances (B2).
1-octen-3-ol (S1), 3-octanone (S2), β-myrcene (S3), (-)-limonene (S4), (-)-menthone (S5).(+)-menthofuran (S6), (+)-pulegone (S7), β-caryophyllene (S8), and naphthalene (IS).
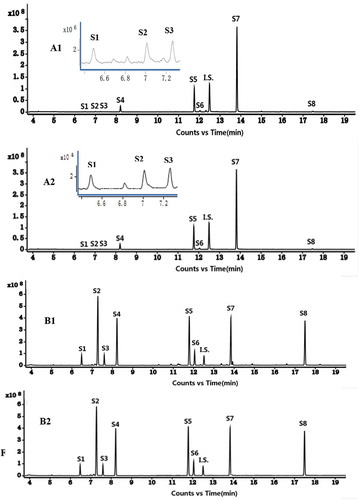
Figure 2. EI mass spectra and chemical structures of eight compounds in STVO.
1-octen-3-ol (S1), 3-octanone (S2), β-myrcene (S3), (-)-limonene (S4), (-)-menthone (S5), (+)-menthofuran (S6), (+)-pulegone (S7), β-caryophyllene (S8).
With naphthalene as the internal standard, the contents of the eight components were determined by our previously established method[Citation29,Citation33] (). As far as the components were concerned, each sample exhibited varying contents with other samples. The results showed that the intrinsic qualities of these samples were different to some extent. Some differences in the contents or even in the qualities of the STVOs might be due to some environmental factors and planting habits. (+)-Pulegone and (-)-menthone were determined as the predominant components of STVO, accounting for 85%~95%.
Table 3. Contents of eight components from 15 batches of STVO (means±SD‚n = 3).
Antioxidant activity assay
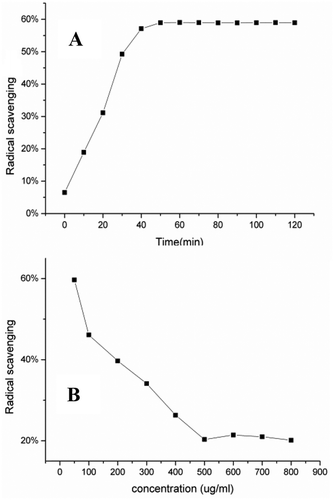
In this paper, the antioxidant activity of STVO in vitro was expressed as DPPH free radical scavenging activity. Before the antioxidant activity assay, the conditions of reaction between STVO (sample 2) and DPPH were optimized as follows: DPPH concentration for 50 μg/mL and the reaction time for 60 min (). According to , along with time increasing,the radical scavenging rate increased, and then reached the summit at 60 min, and would be a balanced condition. As seen in , the radical scavenging rate has been nearly 60% at 50 μg/mL. Under the optimum conditions, the scavenging percentage kept stable at a medium level. For each STVO sample, the interpretation equation of STVO concentration (X) and scavenging percentage (Y) was used to calculate the IC50 value, at which STVO could scavenge half of the DPPH free radicals. A lower IC50 value represented better DPPH radicals scavenging activity. The IC50 values of the STVOs ranged from 0.016 to 1.518 mg/mL, which are listed in with the interpretation equations. The different IC50 values of STVOs demonstrated that different antioxidant effects may be related to the different contents of eight constituents in STVOs and even the qualities of STVOs. The phenomenon might be caused by some environmental factors, planting habits, and harvesting time.
Table 4. Interpretation equation of scavenging DPPH free radical of STVOs (means ± SD).
Multi-linear regression analysis
The relationship between the independent variable X and the dependent variable Y was analysed with MLRA.[Citation25,Citation30] X1~X8 represented the contents of the eight components in one STVO while Y represented IC50 of this STVO. As a result, the regression equation was as follows:
Y = 22.887 + 0.971×X1 + 0.629×X2 + 1.373×X3 − 0.029×X4 − 0.079×X5 − 0.085×X6 − 0.315×X7-0.344×X8.
In this equation, r2 was 0.993 and F was 260.525, which showed the established MLRA model was available. The partial correlation coefficients and significant differences of the contents and IC50 are listed in .
Table 5. Partical correlations of content and antioxidant activity.
As shown in , (+)-pulegone and (-)-menthone had significant negative correlations with IC50 for −0.737 and −0.536, respectively, whose absolute values were much higher than those of the other components. Moreover, the absolute correlation value of (+)-pulegone was higher than that of (-)-menthone. The result indicated that these two compounds were most positively correlated with the antioxidant activity of STVO among the eight compounds and (+)-pulegone was the most prominent constituent to the antioxidant capacity.
Partial least square analysis
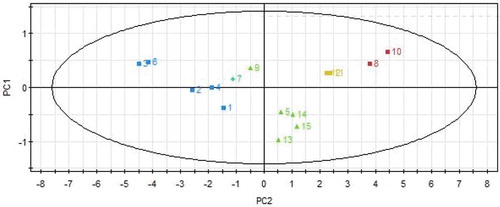
PLSA[Citation31,Citation32] is a latent-variables technique to express the relation between X and Y to derive the principal components (PCs). In this section, X and Y represent the same as in the MLRA section above. As shown in , PC1 relied heavily on the abundance of (+)-pulegone (coefficient = —0.298, VIP = 1.0814) whereas (-)-menthone (coefficient = —0.2169, VIP = 1.0496) had a greater impact than the other six chemicals on PC2. It was concluded that the two compounds were the characteristic ones and were responsible for the antioxidant function. In addition, according to coefficient and VIP value, (+)-pulegone was more prominent than (-)-menthone in antioxidant capacity. The result was consistent with that of MLRA above. The values of R2X and R2Y were 0.918 and 0.896, respectively, indicating that the model was stable and suitable for prediction. The PLSA scores plot of the 15 STVOs is shown in , which showed the good distribution from the 15 batches of samples on PLSA scores plot by PC1 and PC2.
Table 6. Coefficients of eight components and their antioxidant activity in PLEA.
Conclusion
In this study, our result () showed that STVO exhibited potential antioxidant activity and could be used as a promising source in food, cosmetic, and medicine due to the effect. Furthermore, it was also proved with two chemometric methods (MLRA and PLSA) that (+)-pulegone and (-)-menthone might be the two characteristic constituents responsible for the antioxidant effect of STVO. The study was reported for the first time to our knowledge and the findings were expected to be helpful in the researches of STVO and of antioxidant components from other phytomedicines.
Funding
This work was supported by Natural Science Foundation of China [81473313], University Science Research Project of Jiangsu Province [15KJB360010, 14KJD360001], Top-notch Academic Programs Project of Jiangsu Higher Education Institutions [TAPP-PPZY2015A070], Key Research Project of Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization [ZDXM-1-3], Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD-2014], and Postgraduate Innovation Project of Jiangsu Province [KYLX16-0001].
Additional information
Funding
References
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China 4; Chemical Industry Press: Beijing, China, 2015; 203–204.
- Kang, H.; et al. Immunomodulatory Effect of Schizonepeta tenuifolia Water Extract on Mouse Th1/Th2 Cytokine Production In-Vivo and In-Vitro. Journal of Pharmacy and Pharmacology 2008, 60, 901–907.
- Wang, B.S.; et al. Antioxidant and Anti-Inflammatory Activities of Aqueous Extracts of Schizonepeta tenuifolia Briq. Food and Chemical Toxicology 2012, 50, 526–531.
- Shan, M.;et al. Anti-Inflammatory Effect of Volatile Oil from Schizonepeta tenuifolia on Carrageenin-Induced Pleurisy in Rats and Its Application to Study of Appropriate Harvesting Time Coupled with Multi-Attribute Comprehensive Index Method. Journal of Ethnopharmacology 2016, 194, 580–586.
- Shin, T.Y., et al. Effect of Schizonepeta tenuifolia Extract on Mast Cell-Mediated Immediate-Type Hypersensitivity in Rats. Immunopharmacology and Immunotoxicology 1999, 21, 705–715.
- Tohda, C., et al. Inhibitory Effects of Methanol Extracts of Herbal Medicines on Substance P-Induced Itch-Scratch Response. Biological & Pharmaceutical Bulletin 2000, 23, 599–601.
- Yoon, M.Y., et al. Protective Effect of Schizonepeta tenuifolia Briquet Extracts on Oxidative DNA Damage in Human Leucocytes and on Hydrogen Peroxide-Induced Cytotoxicity in PC12 Cells. Food Science and Biotechnology 2007, 16, 858–862.
- Xie, Y.H., et al. Research on Effects of Pulegone to the Acute Inflammation Animal Models. Lishizhen Medicine and Materia Medica Research 2013, 24, 1344–1346.
- Zhao, L., et al. Effect of Herba schizonepetae Volatile Oil (STO) on Activity of 5-Lipoxygenase in Rat Thoracic Cavity Leukocytes. China Journal of Chinese Materia Medica 2008, 33, 2154–2157.
- Zang, L.Q.; Hu, F.; Wei, M. Research on Anti-Tumor Effect of Essential Oil in Schizonepeta tenuifolia Briq and Its Inducing Apoptosis. Guangxi Journal of Traditional Chinese Medicine 2006, 29, 246–248.
- Park, I.K., et al. Fumigant Activity of Plant Essential Oils and Components from Schizonepeta Tenuifolia against Lycoriella Ingenua (Diptera: Sciaridae). Pest Management Science 2006, 62, 723–728.
- De Cerqueira, S.V., et al. R(+)-Pulegone Impairs Ca2+ Homeostasis and Causes Negative Inotropism in Mammalian Myocardium. European Journal of Pharmacology 2011, 672, 135–142.
- Xue, J., et al. L-Menthone Confers Antidepressant-Like Effects in an Unpredictable Chronic Mild Stress Mouse Model via NLRP3 Inflammasome-Mediated Inflammatory Cytokines and Central Neurotransmitters. Pharmacology Biochemistry & Behavior 2015, 134, 42–48.
- Yun, J.;. Limonene Inhibits Methamphetamine-Induced Locomotor Activity via Regulation of 5-HT Neuronal Function and Dopamine Release. Phytomedicine International Journal of Phytotherapy & Phytopharmacology 2014, 21, 883.
- Khojasteh, S.C.; Oishi, S.; Nelson, S.D. Metabolism and Toxicity of Menthofuran in Rat Liver Slices and in Rats. Chemical Research in Toxicology 2010, 23, 1824.
- Tabata, J.; Moraes, C.M.D.; Mescher, M.C. Olfactory Cues from Plants Infected by Powdery Mildew Guide Foraging by a Mycophagous Ladybird Beetle. Plos One 2011, 6, e23799.
- Amin, K.A.; Nagy, M.A. Effect of Carnitine and Herbal Mixture Extract on Obesity Induced by High Fat Diet in Rats. Diabetology & Metabolic Syndrome 2009, 1, 1–14.
- Cho, J.Y., et al. Β-Caryophyllene Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice via Modulation of Gene Expression Associated Mainly with Colon Inflammation. Toxicology Reports 2015, 2, 1039–1045.
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic and Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chemistry 2016, 220, 153–161.
- Dawidowicz, A.L.; Olszowy, M. Does Antioxidant Properties of the Main Component of Essential Oil Reflect Its Antioxidant Properties? the Comparison of Antioxidant Properties of Essential Oils and Their Main Components. Natural Product Research 2014, 28, 1–12.
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Batooli, H. Chemical Composition of Essential Oil and in Vitro Antioxidant Activities of the Essential Oil and Methanol Extracts of Eucalyptus loxophleba. Natural Product Research 2012, 26, 669–674.
- Gong, J., et al. Antioxidant Capacities of Fractions of Bamboo Shaving Extract and Their Antioxidant Components. Molecules 2016, 21, 1–14.
- Vidic, D., et al. Essential Oil Composition and Antioxidant Activity of Four Asteraceae Species from Bosnia. Journal of Essential Oil Research 2016, 52, 1–13.
- Zhu, Y.Z., et al. Antioxidants in Chinese Herbal Medicines: A Biochemical Perspective. Natural Product Reports 2004, 21, 478–489.
- Han, N., et al. Optimization and Antioxidant Activity of Polysaccharides from Plantago Depressa. International Journal of Biological Macromolecules 2016, 93, 644–654.
- Locatelli, D., et al. Cooked Garlic and Antioxidant Activity. Correlation with Organosulphur Compound Compositions. Food Chemistry 2016, 220, 219–224.
- Chandrasekar, D., et al. Determination of DPPH Free Radical Scavenging Activity by Reversed-Phase HPLC: A Sensitive Screening Method for Polyherbal Formulations. Journal of Pharmaceutical and Biomedical Analysis 2006, 40, 460–464.
- Sun, L., et al. Antioxidant Anthocyanins Screening through Spectrum–Effect Relationships and DPPH-HPLC-DAD Analysis on Nine Cultivars of Introduced Rabbiteye Blueberry in China. Food Chemistry 2012, 132, 759–765.
- Shan, M., et al. Content Determination of 6 Monoterpenoids in Volatile Oil of Schizonepeta Tenuifolia by GC-MS. China Pharmacy 2013, 24, 1377–1379.
- Zhao, G., et al. HPLC Fingerprint-Antioxidant Properties Study of Buckwheat. Journal of Integrative Agriculture 2012, 11, 1111–1118.
- Luo, H., et al. Quality Evaluation of Salvia miltiorrhiza Bge. by Ultra High Performance Liquid Chromatography with Photodiode Array Detection and Chemical Fingerprinting Coupled with Chemometric Analysis. Journal of Separation Science 2015, 38, 1544–1551.
- Zheng, H.; Lu, H. Use of Kinetic, Weibull and PLSR Models to Predict the Retention of Ascorbic Acid, Total Phenols and Antioxidant Activity during Storage of Pasteurized Pineapple Juice. LWT - Food Science and Technology 2011, 44, 1273–1281.
- Yu, S., et al. Quantitative Comparative Analysis of the Bio-Active and Toxic Constituents of Leaves and Spikes of Schizonepeta tenuifolia at Different Harvesting Times. International Journal of Molecular Sciences 2011, 12, 6635–6644.