ABSTRACT
Soy protein isolate (SPI) was isolated from Indonesian soybean var. Grobogan and converted into protein nanofibrils. Their functionalities as a food thickener and building blocks for microcapsules were investigated and compared with those of commercial whey protein isolate (WPI). The isolation yield was about 58% with SPI’s protein content of about 90% on dry basis. Long, curved, and branched SPI fibrils with a few nanometers of diameter were obtained by heating SPI suspension at pH 2.0. The solution of SPI fibrils was shear thinning with much higher viscosity than the unheated SPI which was Newtonian. The fibrils showed a good potential as building blocks of microcapsules prepared by layer-by-layer adsorption method, which were similar to WPI fibrils.
Introduction
Soybean (Glycine max (L.) Merill) var. Grobogan is one of the superior varieties of non-GMO yellow soybeans. This variety is originally cultivated in Grobogan Regency, Central of Java, Indonesia. The soybean has yellowish white pod of seed with the weight of 16–20 g per 100 seeds, which are slightly larger than imported soybean seeds from USA that have weight of about 15 g per 100 seeds.[Citation1] The reported protein content of this soybean variety is 42.32%,[Citation2] which is higher than that of imported soybean from USA (31.06%).[Citation1] In some Eastern countries, soybean is an important protein source for daily diet in the form of tofu, tempeh, miso, natto, and so forth. In Western countries, soybean is mainly crushed to get soybean oil, and the defatted meal is used as animal feeds. These differences might be attributed to the different compositions between varieties of soybean.[Citation1,Citation2] Nevertheless, it is only a small portion of soybean that is further processed into food ingredients such as soy flour, soy protein concentrate (SPC), soy protein isolate (SPI), and texturised protein.[Citation3]
Soy protein has many advantages: for example, it contains all the essential amino acids, it has excellent processing abilities, and it contains functional compounds that lower the cholesterol and reduce the risk of hyperlipidemia and cardiovascular disease.[Citation4] Soy protein has been widely studied to develop meat analogue[Citation5,Citation6] and soy cheese.[Citation7,Citation8] The protein extracted from soybean also functions as an emulsifier,[Citation9] a gelling agent,[Citation10,Citation11] and a building block of capsules together with another type of biopolymer.[Citation12,Citation13] Further, SPI was also reported to form nanofibrils by prolonged heating at acidic condition.[Citation14] However, applications of SPI nanofibrils in food systems have not been explored extensively. The protein nanofibrils are defined as globular proteins, including whey proteins and egg white protein, that self-assemble into fibrillar structures with several nanometer thickness and several micrometer length during prolonged heating at very acidic conditions.[Citation14–Citation19]
This research specifically studied isolation of soy protein from soybean var. Grobogan and its potential applications in the form of nanofibrils. The choice of this variety was based on its high protein content as reported by Nurrahman,[Citation2] and therefore, high yield of SPI was expected. The SPI was then converted into protein nanofibrils as reported previously by Akkermans et al.[Citation14] and further investigated for their potentials as a food thickener and building blocks of microcapsules. The results were compared to the results obtained with nanofibrils of whey protein isolate (WPI) which partly have been established.[Citation20]
Materials and methods
Materials and chemicals
Soybeans var. Grobogan (non-GMO) was supplied by Grobogan Soybean House, Grobogan Regency, Central of Java, Indonesia. WPI (BiPro® JE 193-3-420) was kindly provided by Davisco Food International Inc. (Le Sueur, Minnesota, USA) with a reported protein content of 97.9% on dry basis. High methoxyl pectin (HMP) with degree of methoxylation of 70–75% was obtained from Sigma Aldrich, USA (CAS. No. 9000-69-5). The chemicals used were n-hexane (AR, Cat. No. A-1045, Smart-Lab, Indonesia), 2-Mercaptoethanol for synthesis (Cat. No. 805740, Merck, Germany), Tris(hydroxymethyl) aminomethane (Cat. No. 108382, Merck, Germany), n-hexadecane (Cat. No. 820633, Merck, Germany), and HCl 37% (Cat. No. 1000317, Merck, Germany). Double distilled water was used to dissolve protein.
Protein isolation
The protein was isolated according to modified methods of Kuipers et al.[Citation21] and Akkermans et al.[Citation14] Soybean meal (SBM) was prepared by milling the soybeans var. Grobogan using a hammer mill. The resulted meal was sieved twice using a 4 mm sieve and then a 0.5 mm sieve. The SBM was defatted five times with hexane at room temperature (SBM/hexane ratio of 1:5). Afterwards, the suspension was filtered by a filter paper to separate defatted SBM and hexane. The defatted SBM was left to dry at room temperature. The protein was isolated by suspending defatted SBM in a 30 mM Tris-HCl buffer pH 8 that contained 10 mM of 2-mercaptoethanol (145 g of meal in 1.5 L of buffer). The suspension was stirred at room temperature for 1.5 h followed by centrifugation at 19 000 × g, 20°C for 30 min (Beckman, Model J2-21 Ultracentrifuge, USA) and filtration (Whatman, Schleicher & Schuell, type 595, Cat. No. 1001125). The supernatant was set to pH 4.8 using 6 N HCl solution to induce protein precipitation and stirred for 1 h. The resulting suspension was centrifuged at 19 000 × g, 20°C for 30 min, and the precipitate was harvested. The precipitate was washed twice using double distilled water and centrifuged after each washing step. The precipitate was then re-suspended in double distilled water and set to pH 8 using 2 M Tris solution. This suspension was stirred overnight and re-adjusted to pH 8 prior to freeze drying for 24 h. The result was soy protein isolated from soybean var. Grobogan.
Proximate composition analysis
Proximate composition analysis of soybean, SBM, defatted SBM, and the resulted protein powder was conducted as follows. Crude protein was determined using Kjeldahl method with nitrogen conversion factor of 6.25.[Citation22] Fat, moisture, and ash contents were determined using standard AOAC methods 932.06, 925.09, and 923.03, respectively.[Citation23] The carbohydrate content was determined based on all other fractions obtained by proximate composition analysis according to James,[Citation24] as shown in Eq. (1).
Preparation of protein nanofibrils
Two types of protein were prepared to make protein nanofibrils, that is, SPI suspension and WPI solution. SPI suspension and WPI solution of 2% w/w were made by dissolving the proteins in double distilled water. The protein suspension and solution were stirred overnight to complete solubilisation. Afterwards, the pH was set to 2.0 using 6 N HCl solution. The SPI suspension and WPI solution at this pH were then heated in a water bath at 80°C (temperature of the solutions) while stirring for 16 h. The results were solutions containing protein fibrils made of SPI and WPI.
Flow behaviour of protein nanofibril solutions
Flow behaviour of SPI and WPI solutions in the form of nanofibrils and unheated SPI suspension and WPI solution was measured using a controlled-stress rheometer (Rheometer Anton Paar MCR301, Anton Paar GmbH, Austria). Protein concentration of each solution was 2% w/w, and the pH was 2.0. The solution was placed in a concentric cylinder measuring system (CC17) attached to the Peltier temperature device for concentric cylinder system (C-PTD 200, Anton Paar GmbH, Austria). Shear rate sweeps with up ramp from 1 s−1to 500 s−1 and down ramp from 500 s−1 to 1 s−1 were performed at 30°C. Thirty data points for each ramp were recorded in which each data point was measured for 5 s. The results were presented as viscosity (mPa·s) as a function of shear rate (s−1). The data were fit in Ostwald model as follows.
where is the viscosity (mPa·s),
is the shear rate (s−1), k (Pa·sn) is the consistency, and n is the power-law index, respectively.[Citation25] The power law index indicates flow behaviour of the solution. The solution that behaves as Newtonian fluid is shown by n = 1. If n < 1, then the solution behaves as pseudoplastic (shear thinning), and if n > 1, then the solution behaves as dilatant (shear thickening).
Microcapsules preparation using layer-by-layer adsorption method
Microcapsules made of protein nanofibrils as one of the building blocks were prepared following the method of Sagis et al.[Citation18] The method was specifically applied to prepare microcapsules made of WPI nanofibrils by LbL adsorption method. The method was adopted to prepare the microcapsules from SPI nanofibrils; meanwhile, those from WPI nanofibrils were also prepared as comparison. At the beginning, unheated WPI solution, SPI suspension, and HMP solution were prepared by dissolving 0.1% w/w WPI, SPI, or HMP in 25 mM sodium chloride solution at pH 3.5. The solution was centrifuged for 30 min at 925 ×g in a Kokusan centrifuge (H-103N Series, Japan) to precipitate undissolved materials and then filtered through 0.45 μm cellulose acetate syringe filter (25CS045AS, DISMIC-25CS, ADVATEC®, Japan). The capsule template was made by emulsifying 1% w/w hexadecane in unheated WPI solution or SPI suspension using a rotor-stator dispersion tool (T25 Ultra-Turrax®, Ika, Germany) equipped with S25N–25F dispersing element (IKA, Germany) at 9500 rpm for 1 min. The result was emulsion droplets that had positive charges because pH of the protein was below its isoelectric point. The droplets were separated from the remaining protein solution/suspension using centrifugation at 70 ×g to avoid any interactions between non-adsorbed proteins and the biopolymer in the next layer. The droplets were then dispersed in HMP solution that was set at pH 3.5. At this pH, the HMP solution had negative charges; therefore, it formed a layer on top of positively charged emulsion droplets. These bi-layered droplets were harvested by centrifugation and then dispersed in positively charge solution of protein fibrils. The protein nanofibrils deposited onto the droplets as the third layer. Subsequently, additional layers of HMP and protein nanofibrils can be deposited onto the droplets by repeating the same procedures until the desired number of layers is achieved. This research prepared microcapsules with seven layers. The final harvested droplets were then freeze-dried to evacuate hexadecane, and therefore, dried hollow microcapsules made of SPI and WPI nanofibrils were obtained.
Transmission electron microscopy
TEM was used to visualize the protein nanofibrils. TEM method was adapted from Bolder et al.[Citation16] The fibrils prepared as described previously were diluted to 0.05 wt% protein in HCl solution of pH 2.0. A drop of diluted sample was deposited onto a 5 nm thick carbon support film on a copper grid (400 mesh). The excess was removed after 15 s using a piece of filter paper. Electron micrographs were made using TEM (G2F20, FEI TechnaiTM, USA) operated at 120 kV.
Scanning electron microscopy
Morphology of the microcapsules was observed using SEM in which the method was adapted from Rossier-Miranda et al.[Citation20] The freeze-dried microcapsules were placed onto brass holders with double-sided sticky carbon tape. The microcapsules were attached to the sticky layer with pressurised air. The specimen holders with the microcapsules were sputter coated with gold in a dedicated preparation chamber. The analysis was performed with a field emission scanning electron microscope (EVO MA 10, Zeiss, Germany) at room temperature at a working distance of 15 mm, with electron high tension (EHT) detection at 17 kV.
Results and discussion
SPI from soybean var. Grobogan
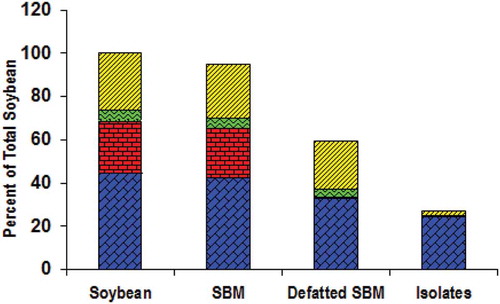
Soybean seeds var. Grobogan, SBM, and the resulted soy protein are depicted in , , and , respectively. The proximate compositions of the soybean, SBM, defatted SBM, and the protein on dry basis are shown in . Meanwhile, the compositions of SBM and SPI on wet basis and their comparison with Codex standard of SPI are presented in . shows that the SBM colour was yellowish white because the soybean var. Grobogan has yellowish white pod of seed as shown in . The yellowish colour was attributed by the fat contained in the beans, which was removed prior to protein isolation process. As the result, the protein powder has white colour as shown in . This is confirmed by the data in that shows much lower fat content in SPI than that in SBM. shows the changes of soy compositions in detail during the whole steps of isolation process. Assuming that the amount of matter in the form of soybean seeds was 100%, the yields during the isolation processes were 95% in the form of SBM, 59.28% in the form of defatted SBM, and 26.99% in the form of soy protein. The protein content (on dry basis) in soybean slightly decreased when the soybean was milled and sieved (from 44.63% to 42.40%). The protein content further decreased after defatting, that is, 32.68%, and finally, it was 24.32% in the form of soy protein. The fat content sharply decreased from 22.72% in SBM to 0.91% in defatted SBM. The carbohydrate content also sharply decreased, that is, 22.06% in defatted SBM to 1.81% in soy protein. The isolation of protein from soybean var. Grobogan yielded 26.99% soy protein consisting of 24.32% protein, 0.46% fat, 0.40% ash, and 1.81% carbohydrates (dry basis).
Table 1. Proximate analysis of SBM and SPI (wet basis).
Figure 2. Proximate compositions (dry basis) of the soybean var. Grobogan, the SBM, the defatted SBM, and the isolated protein. (![]()
Soy protein in general is classified according to its protein content, that is, SPC (about 70%) and SPI (90–95%) on dry basis; meanwhile, the protein content of SBM is about 40–50%.[Citation26] shows the proximate compositions of SBM and soy protein isolated from soybean var. Grobogan (wet basis). The final protein content in the soy protein was 85.83% w/w. This seems lower than the protein content defined for SPI, that is, ≥90% according to Codex Standard 175-1989.[Citation27] However, this standard value is on dry basis. The final protein content of soy protein in this research is about 90% on dry basis. Therefore, the protein can be classified as SPI. This could be an indication that the isolation processes conducted in this research were relatively good compared with some other studies. Using the same method, varied protein content between 82% and 88% were obtained (Akkermans et al.).[Citation14] The protein content variation might be attributed to the isolation methods. Yu et al.[Citation28] obtained a protein content of 92.46% from defatted soy hypocotyls by supercritical CO2. Another study obtained protein content of about 83% using conventional alkaline extraction.[Citation29] The protein content of soy protein in this research might be increased by further drying; therefore, the moisture content decreases or by repeating defatting process more than five times to further reduce the fat content.
Nanofibrils of SPI from soybean var. Grobogan and WPI
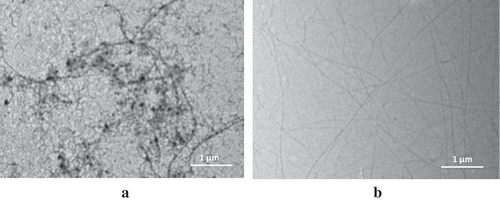
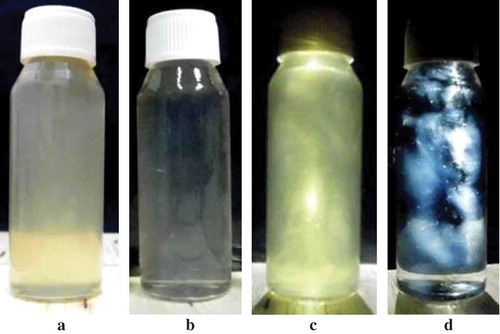
The solutions of SPI and WPI at pH 2 after heating were shown in and , respectively. Unheated SPI suspension became solution of SPI nanofibrils after heating; meanwhile, there was no visual difference between unheated WPI and heated WPI solution (figures are not shown). The existence of SPI and WPI nanofibrils in the solutions can be observed by placing the solutions in between a cross-polariser as shown in and , respectively. A cross-polariser is a pair of polarised optic that are arranged cross 90° and spaced between both where samples are placed and observed against natural light. The appearance of protein nanofibrils under a cross-polariser was also confirmed by Bolder et al.[Citation16] and Akkermans et al.[Citation30]
Figure 3. Solutions containing nanofibrils made of SPI from soybean var. Grobogan (a and c) and WPI (b and d). The pictures of c and d were taken by placing the solutions in between a cross-polariser.
Morphology of SPI and WPI nanofibrils is shown in and , respectively. WPI solution of 2% w/w at pH 2.0 under prolonged heating formed long and straight nanofibrils with a few nanometers of diameter, as previously reported by Sagis et al.[Citation18] and Rossier-Miranda et al.[Citation20] SPI suspension, at the same concentration and process conditions, formed long but curved and branched nanofibrils, as previously shown by Akkermans et al.[Citation14] Many stains are observed on the image of SPI nanofibrils that are not observed on the image of WPI nanofibrils. The quality sheet of WPI from Davisco reported that WPI contained 97.7% protein (dry basis), 0.3% fat, 1.8% ash, and 4.7% moisture. There was no carbohydrate content reported in the quality sheet of WPI. On the contrary, the SPI contained carbohydrate. It also had higher fat content than WPI. Therefore, the stains observed on the image of SPI nanofibrils might be attributed by relatively high fat and carbohydrate content of SPI.
Flow behaviour of SPI and WPI nanofibrils
Steady shear viscosity measurements were performed to study flow characteristic of SPI and WPI nanofibril solutions. Shear rate sweeps with increasing shear rate (up ramp) were performed to investigate the structural breakdown of the fibrils during shearing. Down ramp shear rate was performed to check structural recovery of the fibrils. shows the flow curve of 2% w/w SPI nanofibril solution at pH 2.0 and unheated SPI suspension at the same concentration and pH. The up ramp shear rates of the unheated SPI suspension show that the solution has Newtonian behaviour. It means that the viscosity of unheated SPI suspension does not change by increasing the shear rates. The flow curve of unheated SPI suspension during up ramp shear rates fits Ostwald model with flow behaviour index (n) of one that indicates Newtonian flow. This flow behaviour does not change when down ramp shear rates was applied. The apparent viscosity of SPI nanofibril solution at each shear rate is significantly higher than that of unheated SPI suspension. For an example, the viscosity of unheated SPI suspension at shear rate of 100 s−1 (30°C) is 1.65 mPa·s, which is about twice water viscosity at the same temperature. The apparent viscosity at this shear rate dramatically increases to 21.41 mPa·s when the SPI was in the form of nanofibrils. This might indicate that SPI nanofibrils are good candidate of food thickener or gelling agent.
Figure 5. Viscosity profiles of unheated protein suspension/solution (pH 2.0) and fibril solutions of (a) SPI of soybean var. Grobogan and (b) WPI, measured at 30°C. (![]()
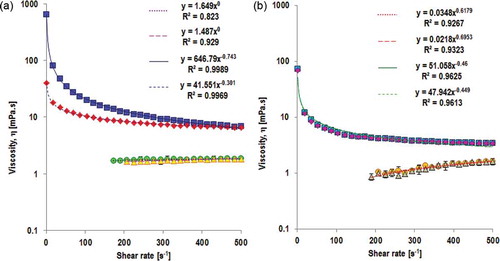
Converting globular structure of SPI into nanofibrils changed flow behaviour of the solution. The flow behaviour of the nanofibril solution became pseudoplastic/shear thinning. This behaviour might be attributed by alignment of the fibrils in the flow direction or by discharging of solvent (in this case water) from the fibrils that causes resistance to flow reduced. Shear thinning behaviour of the SPI nanofibril solution is confirmed by Ostwald model that shows flow behaviour index lower than 1 (n = 0.26). Down ramp shear rates of the solution of SPI nanofibrils show hysteresis. The hysteresis indicates that SPI nanofibrils might be thixotropic materials.[Citation31,Citation32,Citation33] Thixotropy is defined as ‘property of a body by virtue of which the ratio of shear stress to rate of deformation (viscosity) is temporarily reduced by previous deformation’ or ‘a comparatively slow recovery, on standing, of the consistency lost through shearing’ according to the Society of Rheology as quoted by Reiner and Scott-Blain.[Citation34] Other defined thixotropy as ‘the decrease (in time) of viscosity under constant shear stress or shear rate, followed by a gradual recovery the stress or shear rate is removed’ (Barnes, Hutton, and Walters, 1967).[Citation35] Flow curve of SPI nanofibril solution might indicate structural breakdown or rearrangement of the fibrils which does not completely recover to its initial state. Over longer time of period at rest, SPI nanofibril solution was observed to be at gel state.
Slightly shear thickening is observed in unheated WPI solution during up ramp shear rates (). This is confirmed by flow behaviour index of 1.62 in Ostwald model for up ramp shear rates. The protein structures at this condition recovered to their initial structures as indicated by similar value of flow behaviour index (n = 1.69) during down ramp shear rates. Converting globular structure of WPI into nanofibrils changes the flow behaviour of WPI solution. The solution with fibrillar structure of protein shows shear thinning behaviour () during up ramp shear rates. This is confirmed by flow behaviour index of 0.54. However, the viscosity changes were much lower than that in solution of SPI nanofibrils. For example, the viscosity of WPI nanofibril solution at shear rate of 1 s−1 (30°C) is 51.05 mPa·s, which is approximately 12 times lower than viscosity of SPI nanofibril at the same shear rate. The down ramp shear rates has similar flow behaviour index, that is, 0.56 which indicates that hysteresis does not exist in the solution of WPI nanofibrils. It means that WPI nanofibrils are not thixotropic. Rearrangement or reorientation of the fibrils with shearing might take place, but this structural reorientation might be completely recovered to the initial state when the shear rate is removed. The differences on flow behaviour between SPI and WPI nanofibrils might be attributed to the fibril structures.
Microcapsules SPI and WPI nanofibrils
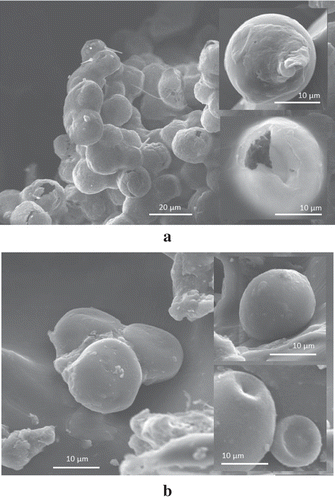
Other researches have reported that nanofibrils made of WPI were excellent building blocks for microcapsules prepared by LbL adsorption method.[Citation18,Citation20] Nevertheless, application of nanofibrils made of SPI for the same purposes has not been explored. and show SEM images of microcapsules prepared by LbL adsorption method using SPI and WPI nanofibrils, respectively. SEM images between both microcapsules show that the microcapsules are completely spherical microcapsules. Diameter of microcapsules from SPI nanofibrils was about 19 μm; meanwhile, the diameter of the microcapsules from WPI nanofibrils was around 17 μm. Rossier-Miranda et al.[Citation20] reported that a microcapsule with seven layers has a diameter larger than 10 μm and fibrous structure was observed on microcapsule’s surface. Based on our observation, the microcapsules made of WPI nanofibrils indicate smooth appearance of the surface; meanwhile, the surface of microcapsules made of SPI nanofibrils looks rough. The difference in microcapsule’s morphology might be attributed to the nature morphology of the fibrils, that is, SPI fibrils are branched and curved; meanwhile, WPI fibrils are straight and long. Several processes during microcapsules preparation using SPI nanofibrils need to be optimised, but these are still under study. Nevertheless, this research proved that SPI nanofibrils might act as building blocks of microcapsules prepared by LbL adsorption method, as performed by WPI nanofibrils.
Conclusion
The yellow soybean var. Grobogan yielded 24.32% of soy protein, which was only about 58% of the protein content reported for this soybean variety. Process optimisation is needed to increase the yield because half of the protein content of the soybean was wasted. Based on the protein content, the soy protein was classified as SPI. The SPI can be converted into branched and curved protein fibrils with a few nanometers of diameter. The nanofibril morphology was different from the nanofibrils made of WPI which was long and straight fibrils. The unheated SPI suspension and WPI solution showed Newtonian and slightly shear thickening flow behaviour, respectively, with complete recovery upon removing the shear rates. The flow characteristic changed into shear thinning, and the viscosity values increased when the protein structures were fibrils. The difference between SPI and WPI nanofibrils was marked upon down ramp shear rates. SPI nanofibrils indicate thixotropic materials; meanwhile, WPI nanofibrils recovered their initial structures completely. This flow behaviour indicates that SPI nanofibrils are good materials as a food thickener and a gelling agent. SPI nanofibrils are also good material as building block of microcapsules that were prepared by LbL adsorption method. The resulted microcapsules were similar to microcapsules made of WPI nanofibrils.
Acknowledgements
The authors thank PT Metrohm Indonesia for supporting Anton Paar Rheometer MCR301.
Funding
The research in this article was financially supported by Ministry of Research, Technology and Higher Education of the Republic of Indonesia in Beasiswa Pendidikan Pascasarjana Dalam Negeri (BPPDN), Penelitian Disertasi Doktor (PDD) Programme, and L’Oréal-UNESCO For Women in Science Fellowship Programme.
Additional information
Funding
References
- Yuwono, S.S.; Hayati, K.K.; Wulan, S.N. Karakteristik Fisik, Kimia Dan Fraksi Protein 7S dan 11S Sepuluh Varietas Kedelai Produksi Indonesian. Jurnal Teknik Pertanian 2003, 4, 84–90.
- Nurrahman. Evaluasi Komposisi Zat Gizi dan Senyawa Antioksidan Kedelai Hitam dan Kedelai Kuning. Jurnal Aplikasi Teknologi Pangan 2015, 4, 89–93.
- Renkema, J.M.S. Relations between Rheological Properties and Network Structure of Soy Protein Gels. Food Hydrocolloids 2004, 18, 39–47.
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review On Composition, Aggregation And Emulsification. Food Hydrocolloids 2014, 39, 301–318.
- Krintiras, G.A.; Göbel, J.; van der Goot, A.J.; Stefanidis, G.D. Production of Structured Soy-based Meat Analogues Using Simple Shear and Heat in a Couette Cell. Journal of Food Engineering 2015, 160, 34–41.
- Katayama, M.; Wilson, L.A. Utilization of Soybeans and Their Components Through the Development of Textured Soy Protein Foods. Journal of Food Science 2008, 73,158–164.
- Rinaldoni, A.N.; Palatnik, D.R., Zaritzky, N.; Campderrós, M.E. Soft Cheese-Like Product Development Enriched with Soy Protein Concentrates. LWT - Food Science and Technology 2014, 55, 139–147.
- Matias, N.S.; Bedani, R.; Castro, I.A.; Saad, S.M.I. A Probiotic Soy-based Innovative Product as an Alternative to Petit-suisse Cheese. LWT - Food Science and Technology 2014, 59, 411–417.
- Keerati-u-rai, M.; Corredig, M. Heat-induced Changes in Oil-in-water Emulsions Stabilized with Soy Protein Isolate. Food Hydrocolloids 2009, 23, 2141–2148.
- Cramp, G.L.; Kwanyuen, P.; Daubert, C.R. Molecular Interactions and Functionality of a Cold-gelling Soy Protein Isolate. Journal of Food Science 2008, 73, 16–24.
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and Gel Textural Properties of Soy Protein Isolate When Subjected to Extreme Acid pH-shifting and Mild Heating Processes. Journal of Agricultural and Food Chemistry 2015, 6, 4853–4861.
- Gómez-Mascaraque, L.G.; López-Rubio, A. Protein-based Emulsion Electrosprayed Micro- and Submicroparticles for the Encapsulation and Stabilization of Thermosensitive Hydrophobic Bioactives. Journal of Colloid and Interface Science 2016, 465, 259–270.
- Lee, H.; Yildiz, G.; dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy Protein Nano-aggregates with Improved Functional Properties Prepared by Sequential pH Treatment and Ultrasonication. Food Hydrocolloids 2016, 55, 200–209.
- Akkermans, C.; van der Goot, A.J.; Venema, P.; Gruppen, J.M.; Boom, R.M. Micrometer-sized Fibrillar Protein Aggregates from Soy Glycinin and Soy Protein Isolate. Journal of Agricultural and Food Chemistry 2007, 55, 9877–9882.
- Moayedzadeh, S.; Madadlou, S.; Khosrowshahi, A. Formation Mechanisms, Handling and Digestibility of Food Protein Nanofibrils. Trends in Food Science & Technology 2015, 45, 50–59.
- Bolder, S.G.; Hendrickx, H.; Sagis, L.M.C.; van der Linden, E. Fibril Assemblies in Aqueous Whey Protein Mixtures. Journal of Agricultural and Food Chemistry 2006, 54, 4229–4234.
- Akkermans, C.; van der Goot, A.J.; Venema, P.; der Linden, V.; Boom, R.M. Formation of Fibrillar Whey Protein Aggregates: Influence of Heat and Shear Treatment, and Resulting Rheology. Food Hydrocolloids 2008, 22, 1315–1325.
- Sagis, L.M.C.; de Ruiter, R.; Rossier-Miranda, F.J.; de Ruiter, J.; Schroën, K.; van Aelst, A.C.; Kieft, H.; Boom, R.; van der Linden, E. Polymer Microcapsules with a Fiber-reinforced Nanocomposite Shell. Langmuir 2008, 24, 1608–1612.
- Humblet-Hua, K.N.P.; Scheltens, G.; van der Linden, E.; Sagis, L.M.C. Encapsulation Systems Based on Ovalbumin Fibrils and High Methoxyl Pectin. Food Hydrocolloids 2010, 25, 307–314.
- Rossier-Miranda, F.J.; Schroën, K.; Boom, R. Mechanical Characterization and pH Response of Fibril-reinforced Microcapsules Prepared by Layer-by-layer Adsorption. Langmuir 2010, 26, 19106–19113.
- Kuipers, B.J.H.; van Koningsveld, G.A.; Alting, A.C.; Driehuis, F.; Gruppen, H.; Voragen, A.G.J. Enzymatic Hydrolysis as a Means of Expanding the Cold Gelation Conditions of Soy Proteins. Journal Agricultural Food Chemistry 2005, 53, 1031–1038.
- AOAC. Official Methods of Analysis of the AOAC; Association of Official Analytical Chemists: Maryland, USA, 2005.
- AOAC. Official Methods of Analysis of the AOAC; Association of Official Analytical Chemists: Arlington, USA, 1990.
- James, C.S. Analytical Chemistry of Foods; Springer Science and Business Media: New York, USA, 1995; 178 pp.
- Barnes, H.A. A handbook of Elementary Rheology; Cambrian Printers: Wales, UK, 2000; 201 pp.
- Shurtleff, W.; Aoyagi, A. History of Modern Soy Protein Ingredients: Isolates, Concentrates, and Textured Soy Protein Products (1911–2016); Soyinfo Center: Lafayette, 2016.
- Codex Standard 175–1989. Codex General Standard for Soy Protein Product; www.fao.org/input/download/standards/325/CXS_175e.pdf. 2016.
- Yu, J.; Liu, Y.; Qiu, A.; Wang, X. Preparation of Isoflavones Enriched Soy Protein Isolate from Defatted Soy Hypocotyls by Supercritical CO2. LWT- Food Science and Technology 2007, 40, 800–806.
- Lu, W.; Chen, X.; Wang, J.; Yang, X.; Qi, J. Enzyme-assisted Subcritical Water Extraction and Characterization of Soy Protein from Heat-denatured Meal. Journal of Food Engineering 2016, 169, 250–258.
- Akkermans, C.; van der Goot, A.J.; Venema, P.; der Linden, V.; Boom, R.M. Properties of Protein Fibrils in Whey Protein Isolate Solutions: Microstructure, Flow Behaviour and Gelation. International Dairy Journal 2008, 18, 1034–1042.
- Schramm, G. A Practical Approach to Rheology and Rheometry; Gebrueder HAAKE GmbH: Karisruhe, Germany, 2000; 291 pp.
- Derakhshandeh, B.; Vlassopolous, D.; Hatzikiriakos, S.G. Thixotropy, Yielding and Ultrasonic Doppler Velocimetry in Pulp Fibre Suspensions. Rheologica Acta 2012, 51, 201–214.
- Barnes, H.A. Thixotropy a Review. Journal of Non-Newtonian Fluid Mechanic 1997, 70, 1–33.
- Reiner, M.; Scott-Blair, G.W. Rheological Terminology. In Rheology, theory and applications; Eirich, F.R. Eds.; Academic Press: New York, 1967; 461–488.
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989; 199 pp.