ABSTRACT
Responding to the ever-growing concern about safe foods and security, the food industries are forced to seek an emerging technology capable of detecting and quantifying contaminations, especially those of biological origin. Among the different emerging technologies, hyperspectral imaging is considered a good alternative as it can be easily applied at all steps of the food production process and is a non-destructive technique. This paper reviews targeted analytical applications of hyperspectral imaging in monitoring biological contaminants in food. First, traditional techniques for detection of biological contaminants in foods are presented, where disadvantages for practical applications are highlighted and explained in detail. Second, prominent applications of hyperspectral imaging from the last decade to food safety and quality assessment are reviewed, specifically focusing on both deteriorative and pathogenic microorganisms, microbial toxins, and parasites; whether acting individually or collectively in spoiling food products and/or represent a health risk to the consumers. Finally, relevant current and future challenges, advantages and disadvantages of hyperspectral imaging applications are briefly examined.
Introduction
Food quality and safety problems are receiving increasing attention in both developed and developing countries,[Citation1] and these issues are frequently confronted in our daily life because in today’s markets there is an increasing demand for safe and quality products and also because food safety legislations have become more and more stringent.[Citation2]
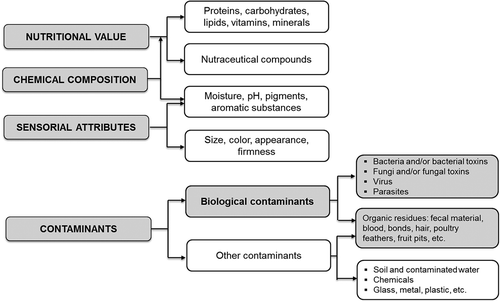
The food industry is actually focused on developing innocuous products and requires a constant commitment to the design and implementation of procedures and systems to control various parameters in food products (). Currently available analytical techniques are mostly slow methods and destructive too. Therefore, it is urgent to develop non-invasive, efficient, and quick testing method for monitoring food quality and safety.[Citation3] Of all the available options, hyperspectral imaging (HSI) technology is shown as one of the most promising alternatives, being a non-destructive analysis technology that can easily engage in productive processes.
Several recent review articles have been published on applications of HSI which is primarily concerned with overall food quality and safety evaluation,[Citation3–Citation13] and others have been mainly focused on determination of chemical and microbiological contamination on food products.[Citation1,Citation2,Citation14,Citation15] However, there is not any paper which specifically addresses on the application of HSI to evaluate different contaminants of biological origin in food products, such as spoilage microorganisms that can attack agricultural crops and cause losses in production output and quality; microorganisms causing deterioration and quality loss in ready-to-eat foods, especially pathogens and/or their toxins; different organic residues that can be a vehicle for different microbial contaminants; and parasites.
It is estimated that between 10% and 50% of agricultural production, especially grains and vegetables, are lost each year as a result of microbial contamination.[Citation16] Besides, it is estimated that 42% of foodborne illnesses are mainly caused by contaminating microorganisms, especially pathogenic bacteria.[Citation17] For instance, in the United States, it is estimated that each year roughly 1 in 6 Americans (about 48 million people) gets sick, where around 128,000 were hospitalized, and 3000 died of foodborne diseases.[Citation18] Therefore, this review focuses on the most notable application of HSI for detecting such contaminations at any level of food production chain, which includes the production of agricultural raw materials, and the industrial processing, packaging, and the storage conditions, that is, from farm to table.
Traditional techniques used to detect biological contaminants in foods
summarizes some of the most common techniques used for detecting the presence of contaminants such as bacteria and/or their toxins, fungi and/or their toxins, viruses, and parasites in different food products.
Table 1. Some of most common analytical techniques for detecting biological contaminants in foods.
Evaluation of viable microorganisms
Many of the employed techniques have some disadvantages for practical applications, for example, the culture and colony counting methods, commonly used as standard approaches for detection of microorganisms, have major drawbacks being labor-intensive and time-consuming, and often take 2–3 days for initial results, and up to 7–10 days for confirmation.[Citation19] On the other hand, early detection of pathogens can be useful to prevent potential negative effects on consumers’ health and degradation of the food products, which is not always possible by conventional plate count, especially with pathogens such as Escherichia coli.[Citation20]
For its part, the conventional direct microscopic techniques are time-consuming, particularly when a large number of samples are analysed or they do not provide complete quality and safety assurance responses,[Citation21,Citation22] and do not adequately differentiate between viable and non-viable cells. As alternative, methods based on cell staining have been designed,[Citation23,Citation24] which in turn can be tedious and expensive.
Meanwhile, the immunology-based methods such as enzyme-linked immunosorbent assay (ELISA) have been successfully employed for the detection of microbial contamination (). However, with excessive total microbial count, these methods show low sensitivity, low affinity of the antibody to the microorganism or other analysed objects, and potential interference from contaminants.[Citation25] Furthermore, the polymerase chain reaction (PCR) method is also a broadly used method for the detection of pathogens in foods (). In spite of its advantages, PCR is considered to be excessively expensive and complicated, and skilled workers are needed to carry out the tests.[Citation19] Besides, PCR and ELISA tests are destructive and take longer to get results, as well as have limited application at laboratory level,[Citation26] and there could be possible interference of inhibitors present in foods,[Citation27] which could produce erroneous results with these techniques.
Other alternatives include the use of biofunctional magnetic nanoparticles (BMNPs) combined with ATP bioluminescence, for example, for rapid detection of E. coli in pasteurized milk;[Citation28] as well as a quartz crystal microbalance (QCM)–based DNA biosensor to detect the main viral RNA encoding G protein in viral haemorrhagic septicemia infection (VHS),[Citation29] one of the most serious viral diseases damaging both fresh and marine fish species. According to the obtained results, these detection techniques are rapid, specific, and sensitive. However, most of them are technically complicated and costly, and require well-trained specialists.[Citation20]
Ultrasound technology is also used in food-quality characterization, which is based on the variation of flight time of the ultrasonic waves as a result of chemical changes induced by microbial contaminants.[Citation30] However, among its limitations the difficulty to be applied for a wide variety of microorganisms with different replication time is highlighted. This technology usually has been applied for detecting seal defects in food packages, for predicting chemical composition, and for detecting physicochemical changes and foreign objects, such as bone, glass, or metal fragments.[Citation4]
Regarding visual inspection, it is difficult for the workers to detect contaminated products, especially when they work on an inspection table, examining foods moving at a relatively high speed.[Citation31] Also, the identification of microbial contamination by imaging appears to be a good alternative; nevertheless, conventional imaging lacks the ability to measure material composition through spectral analysis.[Citation32] Besides, operating at visible wavelengths (i.e., monochromatic or RGB images), it is incapable in differentiating samples with similar colour, classifying complex objectives, predicting chemical components, and in detecting invisible defects.[Citation11] Likewise, regarding detection of fecal residue and/or digested materials, the problem associated with the classification accuracy is that only the thick feces could be observed and easily identified, while the thin layers normally become problematic for detection when only one band is used.[Citation33]
For quantitative analysis, visible (Vis) and near-infrared (NIR) spectroscopies[Citation34,Citation35] are not independent of the disadvantages arising from the reference method used for calibration, which requires a series of samples with known analyte (standard) concentrations.[Citation14] For instance, information about “where” and “how” microorganisms are physically distributed in food samples cannot be acquired, which is essential for visualizing the distribution of the microbial spoilage, because measurements only involve a limited portion of the sample.[Citation7,Citation36] Moreover, the spectroscopy is applicable to characterize homogenous samples by providing mean values of analysed samples, so a single measurement may be misleading to represent the bulk value of the entire sample when heterogeneous samples are evaluated.[Citation7]
As a solution to this situation, HSI combines the complementary approaches of spectral analysis and image processing, thus offering the advantage to evaluate simultaneously the composition (spectral information) and the external characteristics (traditional image analysis) in different foodstuffs.[Citation3,Citation6]
Evaluation of microbial toxins
Different techniques have been explored to determine the presence of microbial toxins, such as ELISA,[Citation37–Citation40] high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), gas chromatography (GC),[Citation41–Citation43] molecular identification techniques,[Citation44] and other techniques based on chemical analysis. Although these techniques have many merits such as specificity, accuracy, selectivity, and very low limit of detection, most of them are generally expensive, labor-intensive, destructive, and time-consuming to achieve appropriate results,[Citation45] some of them are unable to handle a large number of samples,[Citation11] require unfriendly organic reagents,[Citation46] and their application is also limited at a laboratory level. In addition, there is risk of discarding an entire lot or accepting a contaminated lot as safe due to the uneven distribution of contaminated products in storage[Citation47] with the economic losses that it implies.
Detection of microbial toxins by fluorescence (induced reaction between peroxidase enzyme with kojic acid and toxins) is also used because certain photosensitive compounds fluoresce when they are exposed to shortwave radiation such as UV radiation,[Citation43,Citation48–Citation50] for example, toxins such as aflatoxins (produced by Aspergillus flavus and Aspergillus parasiticus), while others, such as fumonisin, cannot be detected by fluorescence. Besides, in some cases A. flavus has also been noted to produce kojic acid, which can convert to fluorescent compounds, but does not produce aflatoxin,[Citation51] likely to give false positive or false negative results, and therefore discarding an entire lot or accepting a contaminated lot as safe.
Likewise, transient infrared spectroscopy (TIRS) is a technique for online, non-contact detection of A. flavus, which is potentially effective in screening bulk quantities of grain,[Citation52] for example, early tests based on TIRS spectral differences to distinguish healthy from infected grains. However, the limitation of TIRS is the special requirements on heating the media surface for producing proper thermal emission,[Citation53] which is inviable in large batches of food products.
Techniques for detection of insect infestation
There is an increasing need to detect insect infestation during postharvest handling practices and before the food products are shipped to the market. There is not extensive information available in the bibliography regarding studies performed on the possible methods for identifying the insect infestation in agricultural products,[Citation54] although several technologies such as acoustic devices and signal processing methods, conductivity, or NIR spectroscopy have been studied. However, these technologies are complex and destructive, and detecting dead and larva insects is difficult through these.[Citation55]
Likewise, X-ray technique has also been researched for insect detection,[Citation56,Citation57] but this technology is not currently in use because of its cost and difficulty in effectively discriminating normal and infested tissues.[Citation58] On the other hand, since the insect larvae usually produce small holes on the surface of food products, the common machine vision technology in the visible range can be a possible means of recognition. Nevertheless, penetrating the inner part of the samples using machine vision technology with a visible light source is hardly possible.[Citation55] Besides, this technology is sensitive to surface features and may provide information regarding other types of surface damage with similar symptoms to holes as in the case of insect larvae. Therefore, machine vision has been considered to be unreliable for inspecting the insect infestation in agricultural products.[Citation57,Citation59]
Evaluation of contamination by parasites
Fish fillets are inspected by transillumination on candling tables and parasites are removed manually by trained personnel,[Citation60] achieving to identify up to 70% of contaminated pieces.[Citation61] However, this procedure is expensive; the results depend on the training and skills of inspectors and are prone to both human error and inspector-to-inspector variation.[Citation60,Citation61] Besides that, it is performed at room temperature, which increases the risk of degradation by endogenous enzymes and contaminant microorganisms.[Citation61,Citation62]
Other techniques explored include the use of fluorescence to spot parasites.[Citation63] However, this method was limited to the detection of surface nematodes, because the UV light does not penetrate deeply into the fish muscle, therefore is no longer commercially available.[Citation64] Hafsteinsson et al.[Citation65] reported detection of parasites deeply embedded into the fish muscle by use of ultrasonic waves, on the basis of the difference in acoustic properties between the fish tissue and parasite. The drawback of this method was the requirement of direct coupling between detector and measurement medium, therefore it is one of the reasons why it has not been industrialized.
Also, PCR and ELISA have been successfully applied for the detection of parasites in meat,[Citation66–Citation71] although with the same disadvantages as previously mentioned. For its part, liquid chromatography–mass spectrometry (LC-MS) has been shown to be a powerful tool for isolating proteins from Anisakis.[Citation72] Although chromatographic techniques have many merits such as specificity, accuracy, selectivity, and very low limit of detection, most of them are generally expensive, labor-intensive, destructive, require long time to get the results and introduce unfriendly chemicals,[Citation45,Citation46] and their application is also limited to a laboratory level.
Because of these disadvantages, the traditional analytical techniques are normally not suitable for online and real-time detection, and for large-scale operations in a rapid and non-destructive/non-invasive way.[Citation5,Citation14] So that, of all the available options, HSI technology is shown as one of the most promising alternatives, which overcomes all of the aforementioned disadvantages of conventional technologies used to evaluate foodstuffs, because these methods are not practical when fast analysis and early detection of biological contaminants in industrial and commercial processing are required with the minimum human intervention.[Citation6]
Hyperspectral imaging technology
Also known as “imaging spectroscopy” or “imaging spectrometry,” HSI is an emergent technology that integrates the advantages of spectroscopy and imaging, allowing to evaluate simultaneously the composition (spectroscopic component) and external characteristics (traditional image analysis) of a sample.[Citation3,Citation6,Citation8,Citation11] That is, the spectroscopy responds to the questions “what” and “how much” and the image analysis provides an answer to the question “where.” Therefore, HSI systems have had a significant influence on the outcome to the combined questions “where and how much of what,” providing a comprehensive characterization of a sample.[Citation73]
. shows a typical laboratory experimental setup of the HSI system. The hyperspectral images obtained are three-dimensional data cubes (hypercubes) made up of hundreds of images of the same object at different spectral wavelengths (), where spectra of each pixel can be used to characterize the composition of that specific position, and surface-feature information can be obtained according to the spatial images.[Citation4,Citation11]
Figure 2. Schematic of the hyperspectral imaging (HSI) system: (a) acquisition of hyperspectral image; (b) hypercube: spectral and special information in a hyperspectral image at different wavelengths; (c) spectral signature for each constituent of the sample plotted against different wavelengths.
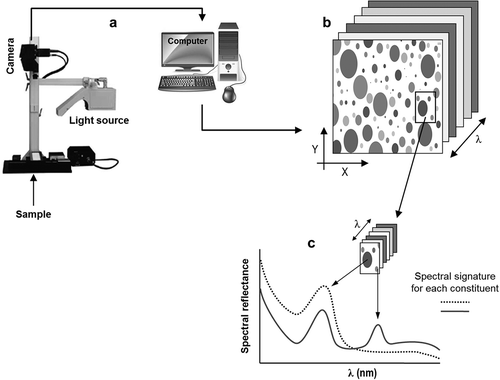
The basic principle of HSI is based on the fact that all materials reflect, scatter, or absorb energy differently when they are subjected to an electromagnetic radiation source at different wavelength ranges due to the difference in their chemical composition and physical structure.[Citation7] Light scattering is related with physical characteristics of the samples such as particle size, cellular structure, and tissue density; whereas light absorption is related with chemical composition of the objective material.[Citation74,Citation75]
Each food constituent has a typical “spectral signature” or “spectral fingerprint” when it interacts with the incident light, which can be used to characterize, identify, and discriminate between different samples.[Citation76] This “spectral signature” tells about its chemical composition and can be plotted against different wavelengths, resulting in the feature curve of reflectance, absorbance, or transmittance for each material ().
Although the constituents of a sample present certain reflectance (or absorbance) values at specific wavelengths, as in the case of conventional spectrophotometers, it is possible to obtain complete information of such constituents in different spectral bands, from ultraviolet–visible (UV–vis) to NIR,[Citation45] which allows to measure a wide range of quality parameters, as well as the presence of contaminants and their spatial distribution in a given food sample.[Citation77]
There are several studies regarding food analysis by HSI, which offers advantages like speed, accuracy, reliability, and furthermore being a non-destructive analysis technology that can be applied along the different production phases, simultaneous assessment, and real-time data processing of numerous sample chemical and physical properties,[Citation7,Citation53] in addition to the simplicity in sample preparation, and being a chemical-free analysis technology,[Citation15] aspects that with other technologies are not always possible.
Since 2011 literature on the use of HSI in food analysis has increased considerably,[Citation78] indicating that the acceptance of this technology is relevant because of the advantages mentioned previously. These studies deal with various parameters ranging from chemical composition, nutritional value, sensory attributes, and detecting defects and contaminants in different foodstuffs (details are given in ). Among an increasing number of food contaminants, those of biological origin may cause a marked accelerated chemical and structural degradation within a short period of time. These changes could be detected depending on the magnitude of scattering or absorption intensity of the incident radiation by the sample during the analysis.[Citation74,Citation75,Citation79]
In this sense, this review is focused on the most relevant studies conducted using HSI as an alternative tool and summarizes the outstanding results in the detection and evaluation of contaminants of biological origin such as pathogenic microorganisms, undesirable and/or harmful substances as a result of microbial metabolism (such as toxins), as well as the presence of viruses, parasites, and organic residues that can act as carriers of contaminating organisms; which acting individually or collectively can deteriorate food products and/or represent a health risk to consumers.
Applications to evaluate biological contaminants in plant-based foods
Microbial contamination in agricultural crops
Early detection of crop diseases benefit farmers as they can remove infected plants quickly before diseases spread and inflict further damage.[Citation80] In this sense, adequate monitoring of cultivars can be a useful and cost-effective approach for mapping of infected crops, for instance, by the using hyperspectral airborne imaging. In , a summary of studies related to spectral imaging for detecting microbial contamination in different cultivars is presented.
Table 2. Spectral imaging applications for detecting microbial contamination in agricultural crops.
A pioneering study was done by Ausmus and Hilty,[Citation81] where spectral reflectance (400–2700 nm) was used to evaluate the presence of fungi Helminthosporium maydis and of the maize dwarf mosaic virus (MDMV), by taking into account the injuries and colour changes in the maize leaves. Their results revealed that healthy, MDMV infected, and H. maydis–infected leaves could be spectrally differentiated in the NIR range (800–2600 nm), especially at early infection stages when symptoms are not yet visible by direct observation (reflectivity: healthy leaves > MDMV-infected leaves > H. maydis–infected leaves).
Later, Hamid Muhammed and Larsolle[Citation82] used HSI (360–900 nm in reflectance mode) to detect the fungal disease known as “tan spot” in wheat, caused by the pathogenic fungi Drechslera tritici-repenti. However, according to the authors, the possibilities for fungal disease control using HSI has to be further investigated.
On the other hand, contamination by Fusarium ssp. is a serious problem for the cereal industry due to its potential capacity of producing mycotoxins. Detection of contamination at the early stages can be an effective strategy to prevent substantial loss in cereal production as well as prevent that toxins reach the consumer in later stages of the production chain. In this regard, Delwiche and Kim[Citation83] used HSI (425–860 nm) to identify wheat grains affected by “Fusarium head blight”, disease that causes wheat grain to be shriveled, underweight, difficult to mill, and is a health concern because of the possible production of the mycotoxin deoxynivalenol. According to the authors, this disease is more suitable to detect in the NIR region than in the visible spectrum.
Also, Bauriegel et al.[Citation84] evaluated wheat plants using HSI (400–1000 nm) in order to detect “Fusarium head blight” disease before harvest. In Fusarium-infected ear tissues, absorption in the range of the chlorophyll bands (560–675 and 682–733 nm) rapidly decreased with development of infection as a result of destruction of chloroplasts. Likewise, they applied spectral angle mapper (SAM), achieving 91% of correct classification for the degree of disease. Nevertheless, due to the time-consuming and complex classification algorithm (SAM), this method is not recommended for practical applications. So a fastest classification was achieved with a new head blight index (HBI), based on principal component analysis (PCA) to identify distinct wavelength ranges with pronounced difference between healthy and diseased tissues, obtaining an accuracy rate of 67%, which could be improved with this index.
Regarding to fruit crops, Qin et al.[Citation85] applied HSI (reflectance mode at 450–930 nm) for early detection of “citrus canker”, a bacterial disease caused by Xanthomonas axonopodis, where symptoms are visible just after 7–10 days of infection. Correlation analysis (CA) and PCA were used for hyperspectral band selection. Based on a two-band ratio selected by CA (R834/R729), algorithms for multispectral image were developed to differentiate canker from other peel conditions, obtaining an overall classification accuracy of 95.7% highlighting that the two-band ratio images have great potential to be adopted by a multispectral imaging (MI) system for early and real-time “citrus canker” detection.
The same two-band ratio (R834/R729) selected by CA gave a correlation value of 0.811 and classification accuracies between 93.3% and 96.7% in a previous study carried out by Zhao et al.[Citation86] Later, on the basis of these key wavelengths, Qin et al.[Citation87] developed an online commercial fruit sorting machine that achieved an overall classification accuracy of 95.3%, operating at speed of 5 fruits/second.
Similarly, early detection of infected vine by grapevine leafroll-associated virus 3 (GLRaV-3), an important tool to overcome a decrease in grape production yield,[Citation88] is an aspect that with conventional techniques such as ELISA and PCR[Citation89,Citation90] would not be possible. This virus reduces the total level of chlorophyll in vine leaves, which can be detected by measuring the differences in reflectance between infected leaves and healthy ones.[Citation91] Besides, although there is no specific treatment for the disease, early detection can limit its spread.[Citation92]
According to Naidu et al.,[Citation93] the presence of this virus in vineyards of Cabernet Sauvignon and Merlot tends to decrease the absorption of chlorophyll at 550 nm in infected leaves (due to changes in the pigment), but without any visible symptoms. They applied a stepwise discrimination analysis (SDA) to select key wavelengths that discriminate infected from healthy vine leaves in the spectral range between 350 and 2500 nm, obtaining an overall accuracy of 0.81. More recently, MacDonald et al.[Citation92] have showed that remote monitoring (hyperspectral airborne imaging at 400–1000 nm) of GLRaV-3 infected Cabernet Sauvignon vineyards can be a useful and cost-effective approach for mapping infected crops, with a success rate of leafroll detection of 94.1% using a classification and regression tree (CART) algorithm. However, according to the authors, future studies should focus on the use of this technology for detecting GLRaV-3 in other grape varieties, as well as other grapevine pathogens.
Similarly, early detection of “anthracnose” would avoid a decrease in the strawberry yield because not only the healthy and symptomatic stages can be recognized but also at the incubation stage before any “anthracnose” symptoms become visible. A pioneering study has been developed by Yeh et al.,[Citation80] who have demonstrated that the different infection stages can be identified through HSI (400–1000 nm) in artificially inoculated strawberry leaves with Colletotrichum gloeosporioides to simulate foliar “anthracnose”. Different statistical analysis methods were evaluated, with the results indicating SDA as the most appropriate method, with an average accuracy between 80% and 93%.
Finally, the cucurbit diseases caused by cucumber green mottle mosaic virus (CGMMV) have led to a serious problem to cucurbit producers because pathogen-infected seeds transmission phenomenon is difficult to combat. Recently Lee et al.[Citation94] have applied an NIR–HSI system (reflectance mode at 946–2016 nm) to discriminate virus-infected watermelon seeds from healthy ones using key wavelengths: 1411, 1456, 1792, and 1867 nm, selected by partial least square discriminant analysis (PLS-DA), obtaining a classification accuracy of 83.3%. Besides that, the authors propose phenolic compounds as a prominent key discriminating factor between virus-infected and healthy seeds where the most common feature of major peaks were consistent with the absorption peaks of these compounds, which plants produce to defend themselves not only against herbivores, but also against competing plants and microorganisms.
These aforementioned results clearly demonstrate that the use of spectral methods provide useful information to avoid significant agricultural productivity losses, because it would be possible to detect infected cultivars before the damage becomes visible.[Citation26,Citation80] Additionally, compared with traditional detection methods, HSI offers a potentially valuable alternative for monitoring crop diseases, since this technology is cost-effective, reliable, and automatable.[Citation92]
Contamination by microbial toxins in plant-based foods
Microbial toxins are among the most controlled food contaminants and its consumption can lead to health problems such as jaundice, liver carcinomas, esophageal cancer, neural tube defects, immunosuppression, etc.[Citation95,Citation96] These substances are metabolites produced by fungi (mycotoxins) and bacteria (bacterial toxins), and the most common in food products are aflatoxins produced by A. flavus and A. parasiticus,[Citation97] ochratoxin produced by Aspergillus,[Citation96,Citation98] fumonisins and trichothecenes produced by Fusarium,[Citation96] and patulin produced by Penicillium, Aspergillus, or Byssochlamys.[Citation99]
Analytical techniques such as ELISA, HPLC, TLC, GC, and visual analytical techniques such as fluorescence-based detection methods[Citation43,Citation45,Citation48–Citation50] are applied for detecting toxins in food products. However, these techniques are susceptible of the disadvantages mentioned earlier. There is thus a growing need for rapid, non-destructive, non-invasive, and accurate techniques that do not require a major resource investment. The HSI technology is the best option to fulfill these requirements, allowing not only the detection of microbial toxins in foods[Citation45,Citation100] but also the detection of the presence of viable microorganisms, and either toxigenic or non-toxigenic strains from both bacteria[Citation20,Citation101] and fungi.[Citation22,Citation43] summarizes some of the research works carried out using HSI technology to evaluate contamination caused by viable microorganisms and/or their toxins.
Table 3. Hyperspectral imaging (HSI) for detecting microbial contamination and damages caused by insects and/or their organic residues in plant-based foods.
Pearson et al.[Citation102] evaluated the presence of aflatoxin in maize grains by using of HSI (500–950 nm) and by an affinity chromatography commercial procedure. Results were similar when using either discriminant analysis (DA) or partial least squares regression (PLSR) for classification, and more than 95% of the grains were correctly classified as containing either high (>100 parts per billion, ppb) or low (<10 ppb) levels of aflatoxin. Besides that, they determined that the wavelength of 735 nm is related to the presence of this toxin. Also, Yao et al.[Citation103] estimated aflatoxin concentration in maize grains inoculated with A. flavus spores, obtaining a R2 of 0.72 using a multiple linear regression (MLR) model. The multivariate analysis of variance (α = 0.01) showed significant differences between four aflatoxin groups tested: <1, 1–20, 20–100, and ≥100 ng/g ppb, and the classification accuracy ranging from 0.84 to 0.91 when a threshold of either 20 or 100 ng/g was used.
Later, Yao et al.[Citation43] inoculated maize ears with two strains of A. flavus (one toxigenic strain and other non-toxigenic strain), and production of aflatoxin in both germ and endosperm fractions of grains was detected by an affinity chromatography commercial procedure and by fluorescence HSI (400–700 nm), thereafter the range between 450 and 500 nm was figured out as the best spectral region to detect fungal contamination. Results from the DA classification indicated overall higher classification accuracy for a 100 ppb threshold on the germ side (94.4%).
More recently, Wang et al.[Citation104] assessed the potential of NIR-HSI (1000–2500 nm) to detect aflatoxin on maize grains, and a minimum classification accuracy of 88% was achieved and as low as 10 ppb of aflatoxin was detected by applying PCA and factorial discriminant analysis (FDA) methods. Likewise, the same group[Citation105] evaluated the feasibility of detecting aflatoxin in artificially inoculated maize ears, and two wavelengths (1729 and 2344 nm) were identified to characterize aflatoxin exclusively, achieving a higher detection accuracy (92.3%).
Moreover, applying Vis/NIR-HSI (600–1000 nm) and HPLC analysis, Wang et al.[Citation106] analysed maize grains contaminated with aflatoxin, and an overall classification accuracy of 98% was achieved. A combination of PCA and FDA (PCA-FDA) was performed to detect and differentiate different levels of aflatoxin, pointing out the detection of this toxin at 735.2 nm as it was obtained by Pearson et al.[Citation102] Similar results were obtained by Kandpal et al.,[Citation48] who applied a SWIR-HSI system (reflectance mode at 1100–1700 nm) to detect aflatoxin contamination on maize grains inoculated with four different aflatoxin concentrations: 10, 100, 500, and 1000 mg/kg. They developed a PLS-DA model to categorize infected grains, obtaining an overall classification accuracy of 96.9%.
In the case of other food products, Kalkan et al.[Citation47] detected the presence of aflatoxins by the using of multispectral images and liquid chromatography (LC) in artificially contaminated hazelnut samples with A. parasiticus. A two-dimensional local discriminant bases (LDB) algorithm was developed and a classification accuracy of 92.3% was achieved for aflatoxin-contaminated and uncontaminated hazelnuts. The algorithm was also used to classify fungal contaminated and uncontaminated samples, and an accuracy of 95.6% was achieved.
On the other hand, unlike aflatoxins that can be detected by fluorescence,[Citation43,Citation50] fumonisin is known for its difficulty to be detected directly by optical methods. In this respect, reflectance analyses in the NIR region can be useful to detect this toxin in cereals. Firrao et al.[Citation42] evaluated the presence of fumonisin in milled maize using HPLC and multispectral images (720–940 nm), with a coefficient of determination R2P of 0.68 for fumonisin concentration (linear regression model fitting). Furthermore, on the basis of the predictions provided by a neural network (NN), the samples were assigned to one of three classes named high (>2.8 ppm), medium (0.5–2.8 ppm), and low (≤0.5 ppm), for their predicted fumonisin content.
For its part, the presence of ochratoxin A in stored barley has been recently evaluated by Senthilkumar et al.[Citation98] using NIR-HSI (reflectance mode at 1000–1600 nm). They have applied PCA to select key wavelengths: 1260, 1310, and 1360 nm (to evaluate Aspergillus glaucus, Penicillium spp., and non-ochratoxin A producing Penicillium verrucosum–infected grains), and 1310, 1360, and 1480 nm (to differentiate ochratoxin A contaminated grains). Selected wavelengths were used as input for statistical classifiers, which differentiated fungal infected grains with more than 80% (initial periods of infection) and 100% classification accuracy (four weeks after infection). These authors also highlight the fact that the time taken for image acquisition, processing, and data analysis for five grains is less than 2 min, which would be reduced to less than 1 min when it is performed later with selected key wavelengths.
Contamination by viable microorganisms in plant-based foods
Safety problems as a consequence of contaminants of biological origin of most plant-based foods might be severe because a small portion of infected products may contaminate the whole batches, and ingestion of contaminated products might incur health problems to consumers.[Citation9] Therefore, it is crucial and necessary to apply rapid, accurate, and non-invasive technique in food analysis procedures to overcome previously mentioned drawbacks. In a summarized list of approaches to evaluate the presence of viable microorganisms and organic residues (vehicle for transmitting microorganisms to foods) of insects and other animals is shown.
Viable microorganisms in cereals
One of the biggest problems for the cereal industry is the significant loss caused by so-called storage fungi. If it is not eliminated by proper drying processes after harvest, it may flourish during storage under favourable conditions,[Citation107,Citation108] affecting important features such as appearance, chemical composition, or germination capacity. Apart from that, this fungal growth promotes some undesirable side-effects such as production of toxins and off-odours originating from the synthesis of volatile compounds such as 3-methyl-1-butanol, 1-octanol, and 3-octanone.[Citation109]
The possibility of spectral information for detecting characteristic compounds at certain wavelengths is reported by Fernández-Ibáñez et al.,[Citation41] who related the spectral range of 870–1200 nm with compounds that include the functional group –NH, like amino acids and aromatic rings of furans and phenols, formed as a consequence of fungal degradation of cereals.
HSI is one of the best alternatives for the identification of different fungal species, because it shows a specific “spectral signature” for each species.[Citation110] The “spectral signature” can be useful for detecting fungal contamination in foods and identify unknown fungal species on the basis of their spectral profile in comparison to the referential spectra of known fungal species.[Citation45] The studies mentioned below demonstrate the advantage of the HSI detection on microbial contamination when symptoms are not yet visible by direct observation.
Shahin and Symons[Citation32] designed a low-cost MI system from an HSI system (400–1000 nm), and reported that 917 nm is a relevant wavelength to detect mildew damage on wheat grains. A partial least square (PLS) model analysis based on four wavelengths (450, 561, 861, and 917 nm) achieved values of R2 = 0.89 and RMSE = 0.68 (calibration model), and R2 = 0.87 and RMSE = 0.74 (validation model). According to this study, compared with the full-wavelength models (HSI systems), the simplified models (MI systems) present small differences in R2 and RMSE values, and the proposed MI system could be more suitable for online applications, due to its relatively low instrument cost and high analytical speed.[Citation4–Citation6,Citation9]
Meanwhile, Singh et al.[Citation111] used SW-NIR-HSI (700–1100 nm) for fungal detection in wheat grains infected by Penicillium spp., A. glaucus, and Aspergillus niger. Linear discriminant analysis (LDA) classifier was applied and classification accuracy between 97.3% and 100.0% was achieved for healthy and fungal-infected samples.
On the other hand, it is very well known that toxigenic fungi grown in grain are toxic for humans and animals. Jin et al.[Citation53] used HSI (400–1000 nm) to discriminate toxigenic and non-toxigenic A. flavus strains. The prominent dimensionality reduction technique PCA and a support vector machine (SVM) model were successfully applied for the classification. Under halogen light source, 83% of toxigenic strains and 74% of non-toxigenic strains were classified correctly; whereas using UV light, the classification was 67% and 85%, respectively. Similarly, Zhang et al.[Citation112] applied NIR-HSI (1000–1600 nm) to evaluate wheat grain infection by different fungi. The dimensionality was reduced by PCA and a multiclass SVM with kernel of radial basis function was used for classification, achieving differentiation rates of 92.9% for those contaminated by A. niger, 87.2% by A. glaucus, and 99.3% by Penicillium strains.
Similarly another study realized by Del Fiore et al.[Citation45] evaluated the presence of A. flavus, A. niger, A. parasiticus, and Fusarium verticillioides in maize grains by HSI (400–1000 nm). PCA was performed on the whole set of spectral data, and the principal components obtained were used to create a model for fungal identification by DA. Results revealed that between 500 and 700 nm, the absorbance increased while fungal and mycotoxin contamination increased, however, between 850 and 950 nm the absorbance values decreased, especially with an A. niger strain.
The variation of absorbance observed in the visible spectrum (500–700 nm) could be caused by fungi, which alters the spectral properties of maize grains related with colour changes,[Citation41] while the variations in the NIR region (850–950 nm) have been possibly due to scattering of light caused by the increased porosity in the endosperm, as a result of fungal growth.[Citation113]
Regarding the chemical composition changes, Farag et al.[Citation114] observed an increase of 0.5% in the lipid content and between 3% and 9% in the protein content, as well as an increase in the reducing sugars content up to six fold in wheat grains, especially those infected by A. niger. These chemical composition changes would be the reason for the altered spectral properties in contaminated grains[Citation45] as consequence of metabolism of these constituents by fungus. Similar results were obtained by Williams et al.[Citation100] in maize grains inoculated with spores of F. verticillioides and analysed using HSI (1000–2498 nm). They observed changes in the composition of the grain components, especially with prominent peaks at 1405 and 2136 nm, which are related to the absorbance for starch (O–H stretch) and protein (N–H stretch and a C=O stretch), respectively, substances used by the fungus for growth.[Citation115]
For its part, Shahin and Symons[Citation116] used Vis/NIR-HSI (400–1000 nm) to detect Fusarium-damaged grains of wheat. LDA was applied and an overall classification accuracy of ≥92% was obtained. Later, the same authors[Citation117] applied PLS-DA for detecting Fusarium, however a lower overall accuracy was achieved (90%).
More recently, Siripatrawan and Makino[Citation118] used HSI (400–1000 nm) to evaluate rice grains inoculated with a non-toxigenic strain of Aspergillus oryzae. Partial least squares regression (PLSR) was used to predict fungal growth from the HSI reflectance spectra, obtaining a high R2 (0.97) between formed colonies and spectral data. Besides this, they also observed that spectral reflectance is inversely proportional to the infection level, which would be related to the formation of cellular structures of the fungus (as spores and mycelium), and synthesis of metabolites and changes in the chemical composition and colour of the grains.[Citation41,Citation45,Citation113] Also, higher fungal growth was observed in the portion of germ, which is the fraction rich in proteins, lipids, and minerals, while the endosperm is mainly constituted by starch.[Citation119]
Viable microorganisms in fruits and vegetables
Fruit and vegetables are exposed to different sources of microbial contamination. At the early growing stage, the most common sources are organic residues from soil and water used for irrigation. Other vehicles are mainly insects that can spread pathogens through their bite or by the means of their organic residual deposits on and around plants, while in postharvest stages the main source is the cross-contamination.
Bacterial contamination
In recent years, diseases caused by foodborne pathogens such as E. coli, Salmonella, Listeria monocytogenes, or Shigella flexneri have become an important public health problem in the world.[Citation120–Citation123] Irrigation-induced contamination is one of the principal routes of vegetable spoilage, especially in those crops where the edible portion is in contact with the soil, such as lettuce, spinach, etc., which may be easily contaminated by pathogenic bacteria. Therefore, early detection plays a crucial role in protecting the consumer against infectious microorganisms, so being that the HSI technology one of the best options in this regard.
E. coli is among the most common foodborne pathogens, naturally present in the intestinal flora and whose presence is used as an indicator of food fecal contamination. Siripatrawan et al.[Citation20] evaluated the presence of E. coli after inoculating it into spinach leaves and analysed by HSI (400–1000 nm), obtaining a coefficient of determination R2 of 0.97, between plate count method and the spectral reflectance computed using PCA (to remove redundant information of the hyperspectral data). An artificial neural network (ANN) algorithm was used to construct a prediction map of all pixel spectra of an image by colouring each pixel with respect to the calculated number of E. coli, expressed as log (CFU/g). These results suggested that tandem HSI-chemometrics can be useful for a rapid and innovative approach, where an early detection of pathogenic bacteria in packaged fresh foods is required.
Furthermore, these pathogenic bacteria are closely associated with fecal matter as in the case of proximity of food crops and processing operations to livestock or wildlife intrusion.[Citation124] Comparisons between HSI reflectance and fluorescence modes for detecting fecal contamination was performed by Kim et al.[Citation125] They used a commercial machine to sorting artificially contaminated apples at speeds of three and even more samples per second, achieving an accuracy of 100% by using HSI in fluorescence mode. For reflectance mode, the classification accuracy was 99.5%.
Yang et al.[Citation126] employed an HSI system (fluorescence mode at 320–400 nm) for the detection of bovine fecal contaminants on lettuce and spinach leaves, and a two-band ratio, 666 nm/680 nm, to differentiate the contaminated spots from uncontaminated leaf area was used. Later, the same research team[Citation127] developed and evaluated three multispectral algorithms derived from hyperspectral line-scan fluorescence imaging (481–780 nm) for the detection of fecal contamination on apples at four wavebands: 680, 684, 720, and 780 nm, and using linear regression models they detected 99% of fecal contamination spots on apple surfaces. The study highlighted that this fast and non-destructive method for detection of fecal contamination can be implemented in the food production chain to help the farmer and manufacturer to prevent or minimize the potential foodborne illness.
Fungal contamination
This is a serious problem since a small set of infected fruit or vegetables can contaminate the whole batch, especially during the storage and transportation processes. Diseases such as “green mold” (caused by Penicillium digitatum) and “blue mold” (caused by Penicillium italicum) are two examples, which affect several cultivars over the whole world.[Citation128] Fungal infection at early stages cannot be detected with the naked eye because in the most of cases the appearance of the damaged fruit is very similar to the uncontaminated ones,[Citation31] and therefore cannot be detected in postharvest by manual inspection.
Mehl et al.[Citation129] used HSI (430–900 nm) to analyse apples contaminated by fungi. An asymmetric second difference method using a chlorophyll absorption band (685 nm) and two bands in the NIR region (722 and 869 nm) provided an excellent detection of the defective/contaminated portions of the apples examined. Also, the carotenoid absorption band (450 nm) offered good contrast between wholesome apples with respect to samples affected by fungal contamination, either soil contamination or bruises. Besides that, this study showed that the asymmetric second difference and PCA methods give very similar results. However, PCA is complex to use, because it requires longer data processing time, while the asymmetric second difference method requires only three wavelengths and much less computation time to process the images. So, it can be easily implemented in a three-band MI system.
Citrus fruit also are susceptible to fungal contamination. These fruits are manually selected by trained personnel exposed to a dangerous UV light source;[Citation12,Citation130] furthermore, it is not always possible to detect the presence of fungi in the early stages of infection. Among fungal species that cause spoilage in citrus fruit are included P. digitatum and P. italicum, which are also analysed by HSI.[Citation131]
In this regard, an HSI system (320–1100 nm) was employed for early detection of rottenness caused by P. digitatum in mandarins.[Citation132] The optimal number of bands required to optimize the classification was estimated to be 20, selected by genetic algorithms based on LDA (GA-LDA). The classification rate of contaminated samples, obtained by CART, was above 91%, highlighting the capability of HSI for early detection of this kind of contamination, which is extremely relevant from the economical point of view.
More recently, Folch-Fortuny et al.[Citation31] have developed a N-way partial least squares regression discriminant analysis (NPLS-DA) method to detect symptoms of P. digitatum in citrus fruits using Vis/NIR-HSI (650–1080 nm). They selected five key wavelengths: 650, 660, 700, 750, and 760 nm to discriminate between control and contaminated oranges, where 96.8% of control samples and 95.7% of fungus-contaminated fruits were correctly classified. In accordance with the results obtained and from a practical point of view, the NPLS-DA model was an effective means to reduce the losses in fruit industry during the storage process caused by infected fruits.
Better results have been obtained by Li et al.[Citation130] using only four key wavelengths: 575, 698, 810, and 969 nm selected by PCA in order to develop a faster classification method and making it easier to establish an online MI system. They used a Vis/NIR-HSI system (reflectance mode at 325–1100 nm) to discriminate between uncontaminated and decayed oranges (affected by P. digitatum), obtaining an overall classification accuracy for training set and test set of 100% and 98.6%, respectively, with no false negatives.
These results significantly improve those obtained by Folch-Fortuny et al.,[Citation31] providing an additional alternative for the citrus fruit decay detection problem. Nevertheless, despite these good results, according to the authors, further research is still needed to establish online MI system for industrial applications, as well as verify performance of the proposed algorithm to detect early decay caused by other fungal species.
Furthermore, the most frequent signs of microbial deterioration in fruits are the reduction of sugar contents, elevated acidity, and superficial growth of aerobic bacteria, yeasts, and molds.[Citation133] To that respect, Teena et al.[Citation22] evaluated the growth of A. flavus in date fruits and their results revealed that the symptoms of contamination were visible only from the sixth day of infection. At the same time (for 10 days) they evaluated the date fruits by HSI (960–1700 nm), observing changes in the spectral properties caused by the chemical modification of constituents, especially a reduction of the sugar content. PCA was applied to select the most significant wavelengths, and LDA and quadratic discriminant analysis (QDA) were applied in the statistical classification. Classification accuracies higher than 91% were achieved, and in most of the cases QDA yielded better accuracy in all the models tested. However, the authors highlighted that further works are required to test this technique for other fungal species and its effect on the chemical composition of different fruits.
Previous results suggest that a simple microbial detection system and non-destructive imaging inspection method may have significant potential to help and ensure food quality and safety in the fruit and vegetable industries, an aspect of vital importance since in many cases the symptoms of contamination are visible by direct observation just several days after the infection.
Contamination by insects in plant-based foods
Detection of insect damages in plant-based foods is another challenge as they can cause serious economic loss.[Citation12,Citation134] Non-invasive measurement is a complicated task due to the fact that larvae generally hide themselves deep inside the fruits and vegetables and they cannot be easily detected from outside.[Citation9] In some studies carried out using HSI technology to detect insect infestation in different plant based foods are shown.
Xing et al.[Citation135] developed an MI system to evaluate internal insect infestation in cherries by using Vis/NIR-HSI in reflectance (590–1550 nm) and transmittance (580–980 nm) modes. A GA was applied to select optimal wavebands in both modes, and the best results were obtained in reflectance mode in the NIR region. The PLS-DA results indicated that models built with three or four GA selected wavebands (MI system) gave similar classification accuracy to the HSI model. Nevertheless, the authors highlighted that due to the stochastic nature of the GA, the efficiency of this MI system needs to be verified in future works.
Also, Lu and Ariana[Citation58] used HSI in transmittance (740–1000 nm) and reflectance (450–740 nm) modes to detect fruit fly infestation in pickling cucumbers. PLS-DA was performed to discriminate wholesome and infested samples, and results showed a better accuracy in transmittance mode (88%–93%). The finding pointed out that the HSI system offers a better detection than manual inspection (75%), and whose performance decreased significantly for smaller-size cucumbers.
On the other hand, Singh et al.[Citation136] used NIR-HSI (1000–1600 nm) for detecting insect damage caused by Sitophilus oryzae, Rhyzopertha dominica, Cryptolestes ferrugineus, and Tribolium castaneum in wheat grains. The acquired spectral data were reduced by PCA, and by using LDA and QDA, they correctly classified 85%–100% healthy and insect-damaged wheat grains.
Later, discrimination between insect-damaged (Etiella zinckenella Treitschke) and undamaged vegetable soybeans was performed by Huang et al.[Citation55] applying hyperspectral transmittance images technique at wavelengths of 400–1000 nm. Support vector data description (SVDD) algorithm was used to develop the classification models, achieving a discriminate overall accuracy of 95.6% for the validation set of samples.
More recently, Mireei et al.[Citation54] have developed a new approach to detect insect infestation in tomatoes based on an MI system (400–1100 nm). Correlation-based feature selection (CFS) algorithm was used to find the best wavelengths (733, 746, 821, 911, 944, and 957 nm). To classify tomatoes, three different machine learning techniques: ANN, SVM, and Bayesian networks (BN) were implemented, obtaining the best performance by ANN, based on spectral difference features with a classification accuracy of 95.0%.
Furthermore, insects are an important source of microbial contamination in plants (at larval and adult stages), for example, the “tomato hornworm,” a common pest affecting tomatoes and other fresh products, whose excrements can be a vehicle of pathogenic bacteria.[Citation137] Yang et al.[Citation138] used a hyperspectral fluorescence line-scan imaging system (400–800 nm) in order to develop a simple MI algorithm to detect frass contamination at different concentrations on surfaces of mature red tomatoes. Five wavelengths of 515, 640, 664, 690, and 724 nm were selected to be used in three ratio functions: R515/640, R724/690, and R664/690, for a multispectral frass-detection algorithm. Results show that this procedure is capable of detecting over 99% of contaminated samples with 0.2 and 0.1 kg/L frass contamination spots, which is very difficult to achieve with a simple visual inspection. However, differentiation of 0.05 and 0.02 kg/L frass contamination spots was more difficult. Therefore, the efficiency of this MI system needs to be improved in future works.
Applications to evaluate biological contaminants in animal-based foods
Microbial contamination in meats and fish
Meats are an excellent source of high-quality nutrients, ensuring a good balance of energy, protein, vitamins, and minerals. However, they are also an ideal substrate for the growth of both spoilage and pathogenic microorganisms.[Citation75,Citation139] Spoilage in meats is caused by the growth and enzymatic activity of microorganisms.[Citation140] As a consequence of deterioration, undesirable metabolites are formed which could result in off flavours, nutrient loss, changes in appearance, colour, etc. In many cases, consumer-purchasing decisions depend on meat colour more than any other quality parameters because consumers use discoloration as an indication of freshness and wholesomeness.[Citation6]
Beyond this, a high microbial population may represent a serious danger to consumer health, especially in the case of pathogens like Salmonella, Pseudomona, E. coli, Campylobacter, among others.[Citation74,Citation101,Citation141,Citation142] Furthermore, there is a growing trend to use HSI to measure total microbial counts, Enterobacteriaceae, Pseudomonas, E. coli, and lactic acid bacteria loads,[Citation12,Citation14] due to the sufficient spectral information obtained from the samples and their correlation with traditional microbial count methods.[Citation141,Citation143,Citation144] Enterobacteriaceae is a group of pathogens including E. coli, Shigella, Salmonella, or Yersinia, whose presence is used as an indicative of hygienic practices. shows some applications of HSI for detecting microbial contamination in different types of meat and marine products.
Table 4. Hyperspectral imaging (HSI) for detecting viable microorganisms and parasites in meat and marine products.
Microbial contamination in beef and pork meat
Peng et al.[Citation74,Citation145] evaluated microbial contamination in beef steaks applying HSI (400–1100 nm) and total viable count (TVC) methods. A multiple linear regression (MLR) model was established to predict log10 TVC, obtaining an R2p = 0.95. Besides that, they observed a relation between meat spoilage and oxidation of haemoglobin and myoglobin, which was localized in the absorption bands of 596 and 760 nm, corresponding to the spectral signature of oxymyoglobin and oxyhaemoglobin, respectively.
Similar findings were reported by Panagou et al.[Citation146] where beef samples were analysed by multispectral reflectance (405–970 nm) and microbial counts (TVC, Pseudomonas, and Brochothrix thermosphacta) methods, Pseudomonas being the dominant microbial group due to its fast growth. PLS-DA analysis was employed for the discrimination in three microbiological quality classes: class 1 (TVC < 5.5 log10 CFU/g), class 2 (5.5–7.0 log10 CFU/g), and class 3 (TVC > 7.0 log10 CFU/g), obtaining an overall classification rate for the three quality classes of 91.8% and 80.0% for calibration and validation, respectively. Furthermore, PLS regression models were developed to provide quantitative estimations of microbial counts during meat storage, obtaining values of R of 0.90–0.93 and 0.78–0.86 for model development and validation, respectively.
Additionally, this study revealed that reflectance decreased as the microbial population increased, especially between 600 and 700 nm, phenomenon related to synthesis of oxymyoglobin, deoxymyoglobin, and metmyoglobin, in concordance with Peng et al.[Citation74] Also, a decrease in reflectance values at 850 and 970 nm was observed, which could be related with the modification of fat content, whose absorption band is around 940 nm.[Citation147] However, the authors suggest the need of new models for the determination of specific microbial groups, as it was performed in other studies,[Citation101,Citation141,Citation142] because the counts of TVC does not fully reflect the spoilage dynamics in meat.
More recently, Tsakanikas et al.[Citation79] have employed a multispectral system in reflectance mode (405–970 nm) for the discrimination of beef samples in two quality classes: TVC < 2 log10 CFU/g and TVC > 2 log10 CFU/g (mix of mesophilic and psychrophilic bacteria), obtaining classification rates over 80% at different storage temperatures using Gaussian mixture models (GMM). Moreover, the calculated R2 to predict counts of TVC from the spectral information was 0.98, as it was estimated using a support vector machine regression (SVMR) model. The authors addressed the need for validation of developed models in different storage conditions, since different temperatures favour the growth of specific microorganisms.
Regarding pork, Peng and Wang[Citation148] applied HSI (400–1000 nm) to detect bacterial contamination, determining five optimal wavelengths by stepwise discrimination method: 480, 525, 650, 720, and 765 nm. Least square support vector machine (LS-SVM) was adopted as the modelling method to predict the TVC, and best TVC prediction results were observed taking into account the data for the five optimal wavelengths with an R = 0.87. The proposed method demonstrated better results than ANN or MLR methods.
Likewise, Wang et al.[Citation149] by using an LS-SVM method obtained an R2P of 0.9426 in a study performed to predict the TVC of fresh pork meat based on HSI data. Also based on LS-SVM, Wang et al.[Citation150] developed a model from eight selected wavelengths (477, 509, 540, 552, 560, 609, 720, and 772 nm), obtaining a similar result (R2P = 0.9236).
Later, Dissing et al.[Citation151] used a MI system in 18 different wavelengths (405–970 nm) for spoilage degree detection of pork. In addition, a sensory evaluation panel composed of five members was recommended to judge the spoilage degree into one of three classes (fresh, semi-fresh, and spoiled). The classification of images in these three categories, and prediction of TVC, was done using a logistic regression model. Results indicated that the MI system was capable of differentiating the meat samples with an overall classification rate of 76.13% according to the defined sensory scale. For the microbial counts, an overall classification performance of 80.0% was achieved. Besides, the TVC value was also successfully predicted with an RMSEP of 0.551 and a SEP of 7.47%.
For their part, Barbin et al.[Citation152] evaluated the effectiveness of NIR-HSI (900–1700 nm) in predicting microbial contamination in pork. They applied PLSR, obtaining values of R2P = 0.82 for TVC, and R2P = 0.85 for psychrotrophic plate counts (PPC), as well as for the determination of contamination maps (chemical images) of pork samples stored at different temperatures. Alongside, they were able to discriminate between both fresh and contaminated meat with 95% and 93% of accuracy, respectively, using LDA model. They also observed an increase in absorbance values in the contaminated samples between 1300 and 1600 nm, as result of protein degradation by contaminating microorganisms.
More recently, Ma et al.Citation[153] applied an MI system utilizing 19 different wavelengths (400–1000 nm) to determine aerobic plate counts (APC) in cooked pork sausages stored at 4°C. PLSR was applied to establish prediction models for APC, obtaining an R2 of 0.89. The prediction model was then transferred to each pixel in the image for visualizing the distribution of APC of the samples. According to the authors, future studies with more samples under different technological or storage conditions and types of packages should be studied to establish more accurate and robust detection models which can be applied in the meat industry.
The versatile application of HSI would seem to offer numerous potential advantages, including reduced labour costs, the elimination of human error and/or subjective judgment, and allowing a real-time product data creation and document generation reliable for traceability and labelling in the meat industry.Citation[152] However, in spite of good results, the presence of different micro flora would lead to variations in model precision,Citation[154] which indicates the need of new models for the determination of specific microbial groups, because some of these analytical methods, mainly TVC, does not fully reflect the spoilage dynamics. Therefore, HSI is an interesting technological alternative for identification of specific bacterial species. For instance, Tao et al.Citation[142] used an HSI system (scattering mode at 400–1100 nm) to determine E. coli concentration in pork meat by using an MLR model, obtaining a reasonable performance with an RCV of 0.88.
Microbial contamination in chicken meat
Current contaminated pieces detection procedures are mainly conducted by human visual perceptions, which is both labor-intensive and prone to both human error and inspector-to-inspector variation.[Citation7,Citation155] HSI has been used to detect chicken meat contamination with pathogenic microorganisms based on spectral differences between contaminated meat and meat from healthy chickens.[Citation156,Citation157]
It is known that most research works related to the HSI detection of meat contamination by organic residuals were carried out on chicken meat, where contamination by pathogenic bacteria can potentially occur as a result of exposure of the pieces to fecal and/or digested materials during or after slaughter.[Citation6,Citation7] However, many of these studies have not been evaluated in real-time applications.[Citation158] It is estimated that only 5 mg of fecal material can pose a danger to humans due to its high pathogenic bacteria load such as Campylobacter, Shigella, Salmonella, Yersinia, or E. coli,[Citation141,Citation159] so that early detection can prevent adverse effects on consumer health and quality. HSI is the tool that provided the best results in this regard.
Windham et al.[Citation160] extracted four optimal wavelengths: 434, 517, 565, and 628 nm using principal component (PC) loading weights, and developed effective hyperspectral image processing algorithms, specifically band ratio of 565/517 for the identification of contamination in poultry carcasses, being able to detect 99% of the fecal contaminants using single-term linear regression (STLR). The same ratio was used later in a similar study, obtaining a prediction accuracy of 96.4% at wavelengths between 430 and 900 nm.[Citation161]
Likewise, Chao et al.[Citation156] developed a system to classify fecal contaminated from uncontaminated chicken meat at a high-speed processing (140 pieces per minute), applying HSI at 580 and 620 nm (since these wavelengths showed the greatest difference between the average wholesome and average unwholesome chicken spectra). Similar results were obtained in a later study of in-plant real-time detection, in which they identified fecal spots on the carcasses at 140 pieces per minute, and the detectable feces could be as little as 10 mg.[Citation162]
A similar study was developed by Yoon et al.[Citation158] in an online real-time HSI system (400–1000 nm). The fecal detection algorithms using 517, 565, and 802 nm allowed to differentiate fecal and digested material contamination on fresh cuts of chicken meat at a speed of 140 and 180 pieces per minute, with a prediction accuracy of 98% and 89%, respectively, with minimum false-positive errors (less than 1% in some cases). Besides that, they provided a commercially viable imaging platform for fecal detection.
More recently, a new two-band freshness index (TBFI) was used by Ye et al.[Citation163] to develop a bacterial prediction model (HSI vs. TVC) with an R2P of 0.6833, based on the wavelengths 650 and 700 nm [TBFI = (Rλ1 – Rλ2)/(Rλ1 + Rλ2)]. Additionally, HSI has been also implemented to detect specific microbial groups such as Enterobacteriaceae and Pseudomonas on chicken fillets, correlating with the traditional microbiological plate count techniques.
On the other hand, Feng and Sun[Citation143] evaluated the effectiveness of NIR-HSI (910-1700 nm) to predict contamination in chicken meat. Ten wavelengths were selected for the simplified PLSR model based on absorbance spectra (AS-PLSR): 961, 1054, 1081, 1084, 1191, 1198, 1201, 1208, 1218, and 1328 nm. The values of R and RMSEs for this model were 0.96 and 0.40 log10 CFU/g (calibration) as well as 0.94 and 0.50 log10 CFU/g (cross-validation), respectively. Meanwhile the residual predictive deviation (RPD) value was 2.75 (). When the best PLSR models (both full wavelength or simplified models) were finally confirmed, they were applied for predicting bacterial loads in each pixel of the sample images (chemical image).
Table 5. Multispectral imaging (MI) models developed from hyperspectral imaging (HSI) models by selecting key wavelengths.
Feng et al.[Citation141] applied NIR-HIS (900–1700 nm) for determination of Enterobacteriaceae loads on chicken fillets. They developed simplified models (MI system) by applying second derivative spectra and weighted PLS regression coefficients to select three key wavelengths: 930, 1121, and 1345 nm, obtaining values of R2 of 0.89, 0.86, and 0.87 and RMSEs of 0.33, 0.40, and 0.45 log10 CFU/g for calibration, cross-validation, and prediction models, respectively. The prediction map (chemical image) provided a good approach for visualizing the distribution of Enterobacteriaceae. Moreover, an increase in spectral reflectance as the microbial load increased was observed, due to the changes caused by microbial metabolism.[Citation164]
Also, Feng and Sun[Citation101] correlated the spectral information obtained by HSI (900–1700 nm) with traditional counts of Pseudomonas in chicken fillets by using PLSR. To enhance the model, they selected 14 wavebands in five spectral segments (1138–1155, 1195–1198, 1392–1395, 1452–1455, and 1525–1529 nm) based on genetic algorithm (GA), producing R and RMSEs of 0.91 and 0.55 log10 CFU/g, 0.87 and 0.65 log10 CFU/g, and 0.88 and 0.64 log10 CFU/g for calibration, cross-validation, and prediction models, respectively.
Furthermore, the rapid growth of Pseudomonas is responsible for the spoilage, exerted in substrates such as glucose to synthesize 2-oxo-gluconate and gluconate,[Citation165,Citation166] as well as an increase of ammonia production and pH, as a result of amino acid metabolism,[Citation167] hence the formation and increased concentrations of these substances causing undesirable odours and tastes.[Citation166]
According to these studies,[Citation101,Citation141,Citation143] the full-wavelength models (HSI systems) compared with the simplified models (MI systems) showed better statistical indicators (R/R2 and RMSE, ). Therefore, already established MI system could be more suitable for online applications in agreement with its higher robustness, relatively low instrumental cost, and high analytical speed.[Citation4–Citation6,Citation9] Nevertheless, according to the authors, more studies should be carried out to further verify the effectiveness of HSI and to figure out the feasibility of MI systems for real-time prediction of Pseudomonas and Enterobacteriaceae loads in chicken or other meat.
On the other hand, organic residues including fat, blood, and feces on equipment surfaces in poultry processing plants can generate cross-contamination and increase the risk of unsafe food for consumers. Qin et al.[Citation168] applied a line-scanning HSI system (500–700 nm), and two soft independent modeling by class analogy (SIMCA) models were developed to differentiate organic residues and stainless steel samples: two-class (“stainless steel” and “organic residue”) and four-class (“stainless steel,” “fat,” “blood,” and “feces”), whose classification accuracies were 100% and 97.5%, respectively, identifying an optimal single band (666 nm, false-negative errors for chicken blood) and a band-pair ratio (503/666, for detecting various chicken residues on stainless steel surfaces) by correlation analysis.
Similarly, Jun et al.[Citation169] carried out a study for detecting microbial biofilms of pathogenic E. coli and Salmonella on food-contact surfaces such as stainless steel, high-density polyethylene (HDPE), plastic laminate (formica), and two variations of polished granite, by using hyperspectral fluorescence imaging (421–700 nm). PCA was used to reduce the dimensionality of the data, and the result showed an overall rate of 95% for detecting biofilm spots on stainless steel, HDPE, and granite. However, too many false positives were present to accurately determine an effective biofilm detection rate on formica. This could be due to the lower microbial density observed on formica (approximately 4.3–6.4 log CFU/cm2). Besides, a single band image at 559 nm was able to detect the biofilm spots on stainless steel surfaces.
Microbial contamination in fish
Fish and other marine products are highly perishable due to its nutritional richness, which makes them suitable for the growth of pathogenic and spoilage microorganisms. The growing demand for new conservation techniques to maintain quality and safety of these products as well as the accomplishment of vigorous controls at different stages of the distribution chain entails the use of potent analysis techniques to detect possible contamination.
Deterioration of fish can occur either as the result of autolytic endogenous enzymatic degradation or by the microbial spoilage,[Citation170] producing undesirable modifications such as textural changes, discoloration, development of off-odours, production of slime, increase of acidity, and formation of toxic metabolites.[Citation171–Citation174] The presence of aerobic microorganisms can promote unstable oxygen derivatives that can accelerate lipid oxidation and pigment oxidation such as haemoglobin and myoglobin,[Citation175–Citation177] and thereby generating changes in the colour and other characteristics. In recent years, HSI has been extensively studied () and implemented as a technological alternative to traditional analytical methods and has the proven potential to detect the presence of biological contaminants in fish.[Citation5]
Sone et al.[Citation170] used Vis/NIR-HSI (400–1000 nm) and total bacterial counts (TBC) methods to assess bacterial contamination degree on salmon fillets. K nearest-neighbour (Knn) classifier was used for the classification of fillets. The best classification was achieved using five single wavelengths: 606, 636, 665, 705, and 764 nm, with an accuracy rate of 88%. In addition, the authors reported an increment of absorbance at 636 nm, phenomenon related to the oxidation of haemoglobin and myoglobin to methemoglobin and metmyoglobin, respectively.[Citation146]
In the same way, Wu and Sun[Citation144] used time series-hyperspectral imaging (TS-HSI) between 400 and 1700 nm to predict contamination in salmon fillets. Competitive adaptive reweighted sampling (CARS) was conducted to identify the most important wavelengths that had the greatest influence on the TVC prediction, and eight wavelengths: 495, 535, 550, 585, 625, 660, 785, and 915 nm were selected. On the basis of these wavelengths, a CARS-PLSR model to predict TVC was developed, with an R2 = 0.985 and RPD = 5.127. Similar work was carried out by Cheng and Sun[Citation178] to predict TVC in carp fillets by using HSI (400–1000 nm), observing an increase in reflectance values. Seven optimal wavelengths: 410, 488, 553, 665, 750, 825, and 960 nm were selected by successive projections algorithm (SPA), and a simplified SPA-PLSR model gave values of R2 = 0.90, RMSE = 0.57 log10CFU/g, and RPD = 3.13.
In both studies,[Citation144,Citation178] the best model was used to predict the TVC values of each pixel within the region of interest (ROI) of fish pieces (chemical image), and the multispectral (MI) systems were also recommended to be developed for online applications.
Regarding specific microbial species, among the most important fish-contaminating bacteria are some species of Enterobacteriaceae or Pseudomonas and lactic acid bacteria (LAB).[Citation179–Citation181] He and Sun[Citation181] applied HSI (900–1700 nm) for evaluating Enterobacteriaceae contamination of salmon flesh by correlating the spectral information with plate counts (CFU/g). By applying SPA, eight key wavelengths: 924, 931, 964, 1068, 1262, 1373, 1628, and 1668 nm were selected to develop a simplified SPA-PLS model, obtaining values of RP, RMSEP, and RPD of 0.95, 0.47, and 3.23, respectively. The same research team[Citation180] applied HSI to predict Enterobacteriae and Pseudomonas counts (EPC) in salmon fillets. The better performance was found with a simplified PLS model, established with nine key wavelengths: 931, 1138, 1175, 1242, 1359, 1628, 1641, 1652, and 1655 nm, obtaining values of R, RMSE, and RPD of 0.964, 0.429, and 3.715, respectively.
Similar results were obtained in their previous study[Citation172] aimed to predict LAB loads in salmon fillets by NIR-HIS. Eight important wavelengths: 1155, 1255, 1373, 1376, 1436, 1641, 1665, and 1689 nm were selected using a CARS algorithm, and an optimized CARS-LS-SVM model was established, leading to RP of 0.925 with RMSEP of 0.531 log10 CFU/g. Besides, the authors concluded that an increase in spectral reflectance is directly proportional to the bacterial counts. Also, Cheng and Sun[Citation182] evaluated E. coli contamination in carp flesh using HSI (400–1000 nm), and values of R2P and RDP over 0.87 and 5.22, respectively, were obtained by application of PLSR and MLR models by mining either full-wavelength spectra or six selected key wavelengths: 424, 451, 545, 567, 585, and 610 nm.
In all cases, the best model was transferred to each pixel of images, and colourful distribution maps (chemical image) with different colours and representing different numbers of Enterobacteriaceae, EPC, or LAB counts were produced. Given the promising results, the simplified models (MI systems) showed better statistical indicators (R/R2, RMSE, and RPD, ). Therefore, the developed MI system could be more suitable for online applications, due to its higher robustness, relatively low instrument cost, and high analytical speed.[Citation4–Citation6,Citation9] However, more studies are still required to refine its applicability at an industrial level.
Microbial contamination in eggs
The most studies aimed to evaluate this type of food by using HSI have been applied to inspect quality attributes such as polyunsaturated fatty acids content (omega-3),[Citation183] freshness, bubble formation, or scattered yolk.[Citation184] There is no comprehensive information available regarding the application of HSI to evaluate microbial contamination in these food products.
It is known that organic residues on eggshells such as blood, feathers, feces, etc., can be a vehicle for transmitting pathogens such as Salmonella, Enterobacter, or Staphylococcus,[Citation185] hence their early detection is the most important way to identify contaminated products and prevent future risk. To that respect, Lunadei et al.[Citation186] used MI (440–940 nm) to discriminate clean and contaminated eggs with organic residues. They employed an image algorithm based on a combination of red (700 nm) and blue (450 nm) images, achieving a classification rate near 98% based on the geometric characteristics of the detected stains, with a very short processing time (0.05 s). Afterwards, the system was validated using a second set of samples, obtaining a classification rate of 97%.
Given a simple algorithm based on only two wavelengths, the proposed method is a cheap, easy, accurate, and fast alternative that could be implemented in an online process. However, in spite of the obtained results, further research is necessary in order to optimize the accuracy, for example, employing samples with different kinds of defects and monitoring whether changes in the illumination system affect the results of the imaging process.
Evaluation of parasitic contamination
shows some of the studies carried out by HSI application for detecting parasites in different types of meat and marine products. The presence of parasites, such as Anisakis simplex in fish or Trichinella spp. and Taenia solium in pork and beef, is related to the incidence of many diseases.[Citation187–Citation189] Parasitic infection is a severe food safety challenge, and it is important for the food industry to rely on compatible preventive action and online detecting methods to avoid the presence of parasites.[Citation64] For example, fish fillets are inspected by transillumination on candling tables and parasites are removed manually by trained personnel,[Citation60] achieving to identify up to 70% of contaminated pieces.[Citation61] However, this procedure and other options have disadvantages as previously mentioned.
Another alternative to assess the presence of parasites in fish is the application of image analysis techniques. Although it is sometimes invisible to human eyes, parasites could be easily detected by HSI due to the fact that their presence in fish flesh present distinctive “spectral fingerprints” compared with normal fish muscles.[Citation6] In this regard, a pioneering study was developed by Wold et al.,[Citation77] who investigated how MI (transmittance mode at 400–1000 nm) in combination with SIMCA classification can be used for automatic detection of parasites in cod fillets, detecting nematodes (Gadus morhua) to a depth of 6 mm. However, despite promising results, the authors recommended further studies to verify feasibility for the fish industry.
Heia et al.[Citation64] achieved a greater depth detection of nematode G. morhua in cod fillets using HSI (350–950 nm). The spectral images were analysed by discriminant partial least square (DPLS) regression in order to identify pixels representing fish sample with embedded nematodes. This method demonstrated the great potential of HSI to detect parasites to a greater depth (8 mm), which is 2–3 mm deeper compared with the classic visual inspection,[Citation60,Citation61] besides the method is non-intrusive and should thus be feasible for industrial purposes.
For their part, Sivertsen et al.[Citation190] applied HSI (400–1000 nm) to evaluate the presence of parasites in cod fillets using a mobile system at an operating speed of 25 mm/s, obtaining in some cases better results than the visual inspection. The Gaussian maximum likelihood (GML) classifier achieved a detection rate of 50.7% for Anisakis simplex and 65.3% for Pseudoterranova decipiens. However, the system could not process the fillets with skin and the speed was slow. Later on, the same authors[Citation62] optimized the application of HSI in order to detect the presence of the same nematodes at a required industrial speed of 400 mm/s in cod fillets, and the GML classifier improved the detection rates to 60.3% (A. simplex) and to 70.8% (P. decipiens). In addition, these parasites were classified based on the depth or the section of fish piece where they are localized. However, the authors suggest the need to improve the depth of detection of parasites and the correct detection rate in future applications.
It is also reported the possible use of HSI to evaluate the presence of non-pathogenic parasites such as Edotea magellanica, which is not risky to human health but its presence affects food quality standards and generates rejection from consumers.[Citation191] In this regard, Coelho et al.[Citation192] working with a HSI system (400–1000 nm), and a summary of the spectral information contained at the wavelengths 624.1, 760.4, and 888.6 nm, achieved 85% of detection accuracy to predict the presence of this parasite in cooked clam. Besides, they observed a decrease in transmittance between 600 and 950 nm, which was attributed to the spectral properties of the parasite, especially at 720 nm.
As seen so far, the different research works regarding HSI application for detecting the presence of parasites are related to the assessment of fish and other marine products, while there is not comprehensive information available in the bibliography regarding the application of HSI in other type of meats. One similar work in this regard was performed by Gómez-De-Anda et al.,[Citation193] using spectroscopy to evaluate Trichinella spiralis in pork. They were able to discriminate between both infected and non-infected meat by using SIMCA. In all cases, the recognition and rejection rates were 100% and a higher concentration of trichinosis in the masseter muscle was detected, suggesting that it could be considered as the best muscle for parasite identification purposes in contaminated pork.
Finally, it should be noted that there is no information available in the bibliography regarding the application of HSI to detect Taenia solium (tapeworm), one of the parasites most prevalent in Latin America, whose infections can lead to “cysticercosis” disease as a result of raw or undercooked pork consumption.[Citation187,Citation194]
Current and future challenges
Advantages and disadvantages of hyperspectral imaging
This review gives special prominence to HSI as a promising non-destructive technology for the evaluation of contamination of biological origin in foods, as it offers several improvements, such as speed, accuracy, and reliability over other methods. Besides, no sample preparation is required; it is a chemical-free analytical method that does not bring environmental pollution,[Citation5] and is capable to determinate several features simultaneously in the same sample.[Citation7,Citation15,Citation53] In addition, this non-destructive and non-invasive technology has potential to realize quantitative and qualitative analysis as well as rapid real-time and online detection,[Citation11] aspects that with other technologies are not always possible.
However, HSI technology still has some disadvantages that need to be resolved for further application at the industrial level. For instance, HSI systems need accurate reference calibration and robust model transfer algorithms, and do not have good detection limits compared with chemical-based analytical methods.[Citation11] Moreover, hyperspectral images contain much unnecessary information than a single-color image and therefore require more processing time to obtain valuable information. Besides that, the hardware speed of an HSI system needs to be enhanced to satisfy the fast acquisition and analysis of the enormous hyperspectral data. So, the HSI technology would not be yet advisable for direct achievement of the online purpose.[Citation5]
To overcome these disadvantages, current studies are aiming to develop models that: (a) allow a rapid data discrimination and retrieve interesting information, given the large amount of data from the wide range of spectral bands in which the HSI works, and (b) identify optimal wavelengths for each food or food constituents, affected by biological contaminants. This is possible due to the potential of HSI to identify and discriminate different constituents,[Citation76] biological contaminants,[Citation110] or metabolites produced by contaminant microorganisms, such as toxins[Citation42,Citation45,Citation100,Citation106] or other substances causing undesirable attributes in food products.[Citation109,Citation172–Citation174]
Future challenges
On the basis of aforementioned disadvantages, scientific and technological challenges that must be overcome for further application of HSI at the industrial level are
Further research for detecting biological contaminants in liquid foods is needed,[Citation15] since most applications have been focused on solid samples (–).
Quantification of low concentrations of microorganisms, since most studies have been focused on assessing microbial densities higher than 102 CFU/g.[Citation2]
Current studies will need to be extended to other microbial species,[Citation2,Citation92] since most of these studies are limited in detecting and quantifying metabolites produced by microbial contaminants.
Advanced methodologies that allow discrimination between biological contaminants based on its specific “spectral signature.”
Optimize the existing models or create more robust models that allow to predict biological contamination from the spectral data obtained, without running the risk of losing valuable information.
Validation of developed models on several storage conditions, simulating real life, for example, at different storage temperatures.[Citation79]
Design of techniques able to fuse the spatial and spectral data, since most of available hyperspectral data processing techniques focus on analysing the spectral data without incorporating information on the spatial data.[Citation3]
Besides, the implementation of online applications, due to the big size of hyperspectral images, requires efficient technologic tools to analyse such images in a real-time mode. The most hyperspectral raw data are currently used in off-line systems at laboratory level to select key wavelengths for building MI systems suitable for online applications.[Citation6,Citation10,Citation14] Therefore, it is needed to develop a fast and efficient technological support for processing the spectral information, which is considerably disadvantageous due to the high cost to introduce HSI at an industrial level. An interesting alternative could be the integration of image-processing algorithms into specialized hardware, then reducing significantly the test time, as well as development of MI systems for industrial applications due to its relatively low instrument cost and high analytical speed.[Citation8,Citation9]
Compared with the full-wavelength models (HSI systems), the simplified models (MI systems) turned out to be more robust. Some works carried out in this respect are shown in , in which the improvement of models based on MI can probably be attributed to the elimination of the uninformative or even misleading wavelengths, and small differences in R/R2, RMSE, and RPD for models were observed. In addition, the reduction of spectral multicollinearity could also be part of the reasons for model enhancement.[Citation14]
Likewise, some disadvantages in analysis of microorganisms concerning food quality could be overcome with the technical integration of HSI with fluorescence microscopic imaging (FMI) and Raman microscopic imaging (RMI), improving the capability for inspection of bacterial contamination. In this sense, Chen et al.[Citation4] anticipate that future technological development in the HSI system for food-safety analysis will promote some novel imaging techniques, such as hyperspectral FMI or hyperspectral RMI.
On the other hand, HSI systems cannot acquire the deeper constituent spectral information inside certain samples; penetration depth of the incident radiation will generally be a function of employed wavelength,[Citation15] for example, in meat samples. This disadvantage could be overcome by identifying specific spectral bands. Heia et al.[Citation64] detected parasites at a depth of 8 mm at wavelengths between 350 and 950 nm, using the spectral difference between the parasite and the contaminated food. It could also overcome the disadvantages of similar technologies, such as infrared spectroscopy Fourier transform with attenuated total reflectance (ATR-FTIR), whose penetration depth of incident radiation is approximately 1 μm, limiting its application to surface analysis.[Citation195] Additionally, according to previous results obtained by Sivertsen et al.,[Citation62,Citation190] transmittance mode allowed to obtain better results in detecting parasites, as well as the interactance mode, which has less surface effects and reduces the influence of thickness.[Citation12]
Moreover, in the case of plant-based foods, most of the research is being conducted to evaluate specific cultivars. Obviously such results are variety dependent and cannot be used to evaluate different varieties. So, future studies should focus on the use of HSI technology for detecting microbial contamination in different varieties.[Citation92] From a practical point of view, this would imply to build different systems incorporating different wavelengths depending on the variety.[Citation31] Finally, regarding high cost of the HSI systems compared with other technologies, this is expected to be a minor barrier in the next years because the increase of commercial suppliers could reduce the cost and improve its availability.[Citation6] In the last years, commercial HSI/MI systems for food applications such as Hyperspec Inspector (Headwall Photonics, Fitchburg, USA) or SisuCHEMA (Specim, Oulu, Finland) are appearing in the market,[Citation10] and recently, Goel et al.[Citation196] have developed a prototype capable of capturing hyperspectral images with a low-cost camera (called HyperCam), which uses 17 wavelengths that are spread between 450 and 990 nm, with a cost of about US$ 800, but could reach US$ 50 to fit on mobile phones.
Critical appreciation and conclusions
Of the numerous applications of HSI, there are different studies aiming to determine their suitability for detecting contaminants of biological origin, which can cause deterioration of the product at different stages in the food production chain that will pose a danger to quality products and/or to consumer health. Contamination is very important at any level, which includes the production of agricultural raw materials of both animal and plant origin, the industrial processing, packaging and the storage conditions.
A number of review papers about application of HSI published in the last years are primarily associated with quality evaluation of food products, however, our work is focused to review the most notable application of HSI for detecting: (a) viable microorganisms that cause damage to different levels, such as fungi, bacteria, or viruses that can attack agricultural crops and cause losses in production and quality, and once harvested may retain residual contaminants that pose a further risk; (b) microorganisms causing deterioration and quality loss in ready-to-eat foods, reaching certain levels of microbial population, specially pathogens and/or their toxins, which can be a danger to the health of consumers; (c) different organic residues such as feces, digestive material, etc., which can be a vehicle for different microbial contaminants; and (d) parasites, applied mainly to marine products. All of them, individually or in combination, pose a danger to the quality of the product and/or a risk to consumer health and safety.
On the other hand, some disadvantages are also presented and discussed. Further research on the application of HSI to a larger number of microbial species, liquid foods, presence of parasites in other types of meat (such as pork), and development of models on several storage conditions is needed, which together with the technical integration of HSI with other technologies such as FMI and RMI, the increase of commercial suppliers that can reduce the cost and improve the availability of HSI systems, technological improvements for processing spectral information and search for robust and optimized models, without running the risk of losing valuable information, would give this technology a better chance of industrial application as an alternative to traditional techniques such as the liquid chromatography, the MID-FTIR spectroscopy, or the ELISA and PCR tests, which besides being tedious, are expensive and their application is limited on the laboratory level. Finally, although many researches for evaluating biological contamination in foods have been performed, much studies are still needed to standardize the procedure of HSI in terms of data mining, MI development, and further real-time and online applications along the various stages in the food production chain.
Funding
This study was funded by the following institutions: Programa Nacional de Innovación para la Competitividad y Productividad Innóvate Perú – Ex-FINCyT (Contract 407-PNICP-PIAP-2014) and Universidad Nacional de Trujillo – UNT (PIC2-2013/UNT).
Additional information
Funding
References
- Feng, Y.Z.; Sun, D.W. Application of Hyperspectral Imaging in Food Safety Inspection and Control: A Review. Critical Reviews in Food Science and Nutrition 2012, 52(11), 1039–1058.
- Gowen, A.A.; Feng, Y.; Gaston, E.; Valdramidis, V. Recent Applications of Hyperspectral Imaging in Microbiology. Talanta 2015, 137, 43–54.
- Liu, D.; Zeng, X.A.; Sun, D.W. Recent Developments and Applications of Hyperspectral Imaging for Quality Evaluation of Agricultural Products: A Review. Critical Reviews in Food Science and Nutrition 2015, 55(12), 1744–1757.
- Chen, Q.; Zhang, C.; Zhao, J.; Ouyang, Q. Recent Advances in Emerging Imaging Techniques for Non-Destructive Detection of Food Quality and Safety. Trends in Analytical Chemistry 2013, 52, 261–274.
- Cheng, J.H.; Sun, D.W. Hyperspectal Imaging as an Effective Tool for Quality Analysis and Control of Fish and Other Seafoods: Current Research and Potential Applications. Trends in Food Science and Technology 2014, 37(2), 78–91.
- ElMasry, G.; Barbin, D.F.; Sun, D.W.; Allen, P. Meat Quality Evaluation by Hyperspectral Imaging Technique: An Overview. Critical Reviews in Food Science and Nutrition 2012a, 52(8), 689–711.
- ElMasry, G.; Kamruzzaman, M.; Sun, D.W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Critical Reviews in Food Science and Nutrition 2012b, 52(11), 999–1023.
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral Imaging: An Emerging Process Analytical Tool for Food Quality and Safety Control. Trends in Food Science and Technology 2007, 18(12), 590–598.
- Pu, Y.Y.; Feng, Y.Z.; Sun, D.W. Recent Progress of Hyperspectral Imaging on Quality and Safety Inspection of Fruits and Vegetables: A Review. Comprehensive Reviews in Food Science and Food Safety 2015, 14(2), 176–188.
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T. Hyperspectral and Multispectral Imaging for Evaluating Food Safety and Quality. Journal of Food Engineering 2013, 118(2), 157–171.
- Wu, D.; Sun, D.W. Advanced Applications of Hyperspectral Imaging Technology for Food Quality and Safety Analysis and Assessment: A Review: Part I: Fundamentals. Innovative Food Science and Emerging Technologies 2013a, 19, 1–14.
- Wu, D.; Sun, D.W. Advanced Applications of Hyperspectral Imaging Technology for Food Quality and Safety Analysis and Assessment: A Review: Part II: Applications. Innovative Food Science and Emerging Technologies 2013b, 19, 15–28.
- Wang, N.N.; Sun, D.W.; Yang, Y.C.; Pu, H.; Zhu, Z. Recent Advances in the Application of Hyperspectral Imaging for Evaluating Fruit Quality. Food Analytical Methods 2015a, 9(1), 178–191.
- Cheng, J.H.; Sun, D.W. Recent Applications of Spectroscopic and Hyperspectral Imaging Techniques with Chemometric Analysis for Rapid Inspection of Microbial Spoilage in Muscle Foods. Comprehensive Reviews in Food Science and Food Safety 2015b, 14(4), 478–490.
- He, H.J.; Sun, D.W. Hyperspectral Imaging Technology for Rapid Detection of Various Microbial Contaminants in Agricultural and Food Products. Review. Trends in Food Science and Technology 2015a, 46(1), 99–109.
- Baird-Parker, T.C. The Production of Microbiologically Safe and Stable Foods. In Microbiological Safety and Quality of Food; Lund, B.M.; Baird-Parker, T.C.; Gould, G.W.; Eds.; Springer Science & Business Media: New York, 2000; 3–18.
- Yeni, F.; Acar, S.; Polat, Ö.G.; Soyer, Y.; Alpas, H. Rapid and Standardized Methods for Detection of Foodborne Pathogens and Mycotoxins on Fresh Produce. Food Control 2014, 40, 359–367.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States: Major Pathogens. Emerging Infectious Diseases 2011, 17(1), 7–15.
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An Overview of Foodborne Pathogen Detection: In the Perspective of Biosensors. Biotechnology Advances 2010, 28(2), 232–254.
- Siripatrawan, U.; Makino, Y.; Kawagoe, Y.; Oshita, S. Rapid Detection of Escherichia coli Contamination in Packaged Fresh Spinach using Hyperspectral Imaging. Talanta 2011, 85(1), 276–281.
- Takeuchi, K.; Frank, J.F. Confocal Microscopy and Microbial Viability Detection for Food Research. Journal of Food Protection 2001, 64(12), 2088–2102.
- Teena, M.A.; Manickavasagan, A.; Ravikanth, L.; Jayas, D.S. Near Infrared (NIR) Hyperspectral Imaging to Classify Fungal Infected Date Fruits. Journal of Stored Products Research 2014, 59, 306–313.
- Betts, R.P.; Bankes, P.; Banks, J.G. Rapid Enumeration of Viable Micro-Organisms by Staining and Direct Microscopy. Letters in Applied Microbiology 1989, 9(5), 199–202.
- Koch, H.A.; Bandler, R.; Gibson, R.R. Fluorescence Microscopy Procedure for Quantitation of Yeasts in Beverages. Applied and Environmental Microbiology 1986, 52(3), 599–601.
- Meng, J.; Doyle, M.P. Introduction. Microbiological Food Safety. Microbes and Infection 2002, 4(4), 395–397.
- Zhang, M.; Meng, Q. Automatic Citrus Canker Detection from Leaf Images Captured in Field. Pattern Recognition Letters 2011, 32(15), 2036–2046.
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors: Occurrence, Properties and Removal. Journal of Applied Microbiology 2012, 113(5), 1014–1026.
- Cheng, Y.; Liu, Y.; Huang, J.; Li, K.; Zhang, W.; Xian, Y.; Jin, L. Combining Biofunctional Magnetic Nanoparticles and ATP Bioluminescence for Rapid Detection of Escherichia coli. Talanta 2009, 77(4), 1332–1336.
- Hong, S.R.; Jeong, H.D.; Hong, S. QCM DNA Biosensor for the Diagnosis of a Fish Pathogenic Virus VHSV. Talanta 2010, 82(3), 899–903.
- Elvira, L.; Durán, C.M.; Urréjola, J.; Montero de Espinosa, F.R. Detection of Microbial Contamination in Fruit Juices using Non-Invasive Ultrasound. Food Control 2014, 40, 145–150.
- Folch-Fortuny, A.; Prats-Montalbán, J.M.; Cubero, S.; Blasco, J.; Ferrer, A. VIS/NIR Hyperspectral Imaging and N-way PLS-DA Models for Detection of Decay Lesions in Citrus Fruits. Chemometrics and Intelligent Laboratory Systems 2016, 156, 241–248.
- Shahin, M.A.; Symons, S.J. Design of a Multispectral Imaging System for Detecting Mildew Damage on Wheat Kernels. The Canadian Society for Engineering in Agricultural, Food, Environmental and Biological Systems, 2009, Paper CSBE09-701.
- Kim, M.S.; Lefcourt, A.; Chao, K.; Chen, Y.; Kim, I.; Chan, D. Multispectral Detection of Fecal Contamination on Apples based on Hyperspectral Imagery: Part I. Application of Visible and Near-Infrared Reflectance Imaging. Transactions of the ASAE 2002, 45, 2027–2037.
- He, H.J.; Sun, D.W. Microbial Evaluation of raw and Processed Food Products by Visible/Infrared, Raman and Fluorescence Spectroscopy. Review. Trends in Food Science and Technology 2015c, 46(2), 199–210.
- Kim, S.; Park, J.; Chun, J.H.; Lee, S.M. Determination of Ergosterol used as a Fungal Marker of Red Pepper (Capsicum annuum L.) Powders by Near-Infrared Spectroscopy. Food Science and Biotechnology 2003, 12(3), 257–261.
- Cozzolino, D. Recent Trends on the use of Infrared Spectroscopy to Trace and Authenticate Natural and Agricultural Food Products. Applied Spectroscopy Reviews 2012, 47(7), 518–530.
- Muthomi, J.W.; Ndung’u, J.K.; Gathumbi, J.K.; Mutitu, E.W.; Wagacha, J.M. The Occurrence of Fusarium Species and Mycotoxins in Kenyan Wheat. Crop Protection 2008, 27(8), 1215–1219.
- Notermans, S.; Dufrenne, J.; Van Schothorst, M. Enzyme-linked Immunosorbent Assay for Detection of Clostridium botulinum Toxin Type A. Japanese Journal of Medical Science and Biology 1978, 31(1), 81–85.
- Rammanee, K.; Hongpattarakere, T. Effects of Tropical Citrus Essential Oils on Growth, Aflatoxin Production, and Ultrastructure Alterations of Aspergillus flavus and Aspergillus parasiticus. Food and Bioprocess Technology 2011, 4(6), 1050–1059.
- Saunders, G.C.; Bartlett, M.L. Double-Antibody Solid-phase Enzyme Immunoassay for the Detection of Staphylococcal Enterotoxin A. Applied and Environmental Microbiology 1977, 34(5), 518–22.
- Fernández-Ibáñez, V.; Soldado, A.; Martínez-Fernández, A.; de la Roza-Delgado, B. Application of Near Infrared Spectroscopy for Rapid Detection of Aflatoxin B1 in Maize and Barley as Analytical Quality Assessment. Food Chemistry 2009, 113(2), 629–634.
- Firrao, G.; Torelli, E.; Gobbi, E.; Raranciuc, S.; Bianchi, G.; Locci, R. Prediction of Milled Maize Fumonisin Contamination by Multispectral Image Analysis. Journal of Cereal Science 2010, 52(2), 327–330.
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Bhatnagar, D.; Cleveland, T. Detecting Maize Inoculated with Toxigenic and Atoxigenic Fungal Strains with Fluorescence Hyperspectral Imagery. Biosystems Engineering 2013, 115(2), 125–135.
- Borman, A.M.; Linton, C.J.; Miles, S.J.; Johnson, E.M. Molecular Identification of Pathogenic Fungi. Journal of Antimicrobial Chemotherapy 2008, 61(1), 7–12.
- Del Fiore, A.; Reverberi, M.; Ricelli, A.; Pinzari, F.; Serranti, S.; Fabbri, A.A.; Bonifazi, G.; Fanelli, C. Early Detection of Toxigenic Fungi on Maize by Hyperspectral Imaging Analysis. International Journal of Food Microbiology 2010, 144(1), 64–71.
- Christensen, D.; Allesø, M.; Rosenkrands, I.; Rantanen, J.; Foged, C.; Agger, E.M.; Andersen, P.; Nielsen, H.M. NIR Transmission Spectroscopy for Rapid Determination of Lipid and Lyoprotector Content in Liposomal Vaccine Adjuvant System CAF01. European Journal of Pharmaceutics and Biopharmaceutics 2008, 70(3), 914–920.
- Kalkan, K.; Beriat, P.; Yardimci, Y.; Pearson, T.C. Detection of Contaminated Hazelnuts and Ground Red Chili Pepper Flakes by Multispectral Imaging. Computers and Electronics in Agriculture 2011, 77(1), 28–34.
- Kandpal, L.M.; Lee, S.; Kim, M.S.; Bae, H.; Cho, B.K. Short Wave Infrared (SWIR) Hyperspectral Imaging Technique for Examination of Aflatoxin B1 (AFB1) on Corn Kernels. Food Control 2015, 51, 171–176.
- Klich, M.A. Aspergillus Flavus: The Major Producer of Aflatoxin. Molecular Plant Pathology 2007, 8(6), 713–722.
- Yao, H.; Hruska, Z.; Brown, R.; Cleveland, T. Hyperspectral Bright Greenish-Yellow Fluorescence (BGYF) Imaging of Aflatoxin Contaminated Corn Kernels Proceeding of SPIE.2006, 6381, 63810B-1-63810B-8.
- Basappa, S.C.; Sreenivasamurthy, V.; Parpia, H.A.B. Aflatoxin and Kojic Acid Production by Resting Cells of Aspergillus flavus Link. Journal of General Microbiology 1970, 61(1), 81–86.
- Gordon, S.H.; Jones, R.W.; McClelland, J.F.; Wicklow, D.T.; Greene, R.V. Transient Infrared Spectroscopy for Detection of Toxigenic Fungi in Corn: Potential for On-Line Evaluation. Journal of Agricultural and Food Chemistry 1999, 47(12), 5267–5272.
- Jin, J.; Tang, L.; Hruska, Z.; Yao, H. Classification of Toxigenic and Atoxigenic Strains of Aspergillus flavus with Hyperspectral Imaging. Computers and Electronics in Agriculture 2009, 69(2), 158–164.
- Mireei, S.A.; Amini-Pozveh, S.; Nazeri, M. Selecting Optimal Wavelengths for Detection of Insect Infested Tomatoes based on SIMCA-aided CFS Algorithm. Postharvest Biology and Technology 2017, 123, 22–32.
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of Insect-Damaged Vegetable Soybeans using Hyperspectral Transmittance Image. Journal of Food Enginnering 2013, 116(1), 45–49.
- Hansen, J.; Schlaman, D.W.; Haff, R.P.; Yee, W.L. Potential Postharvest Use of Radiograph to Detect Internal Pests in Deciduous Tree Fruits. Journal of Entomological Science 2005, 40(3), 255–262.
- Jackson, E.S.; Haff, R.P. X-ray Detection and Sorting of Olives Damaged by Fruit Fly. ASAE, St. Joseph, MI, United Sates, 2006, Paper number 066062.
- Lu, R.; Ariana, D.P. Detection of Fruit Fly Infestation in Pickling Cucumbers using a Hyperspectral Reflectance/Transmittance Imaging System. Postharvest Biology and Technology 2013, 81, 44–50.
- Xing, J.; Guyer, D. Detecting Internal Insect Infestation in Tart Cherry using Transmittance Spectroscopy. Postharvest Biology and Technology 2008, 49(3), 411–416.
- Hafsteinsson, H.; Rizvi, S. A Review of the Sealworm Problem. Journal of Food Protection 1987, 50(1), 70–84.
- Bublitz, C.G.; Choudhury, G.S. Effect of Light Intensity and Color on Worker Productivity and Parasite Detection Efficiency during Candling of Cod Fillets. Journal of Aquatic Food Product Technology 1992, 1(2), 75–89.
- Sivertsen, A.H.; Heia, K.; Hindberg, K.; Godtliebsen, F. Automatic Nematode Detection in Cod Fillets (Gadus morhua L.) by Hyperspectral Imaging. Journal of Food Engineering 2012, 111(4), 675–681.
- Pippy, J.H.C. Use of Ultraviolet Light to Find Parasitic Nematodes in situ. Journal of the Fisheries Research Board of Canada 1970, 27(5), 963–965.
- Heia, K.; Sivertsen, A.H.; Stormo, S.K.; Elvevoll, E.; Wold, J.P.; Nilsen, H. Detection of Nematodes in Cod (Gadus morhua) Fillets by Imaging Spectroscopy. Journal of Food Science 2007, 72(1), E011–E015.
- Hafsteinsson, H.; Parker, K.; Chivers, R.; Rizvi, S. Application of Ultrasonic Waves to Detect Sealworms in Fish Tissue. Journal of Food Science 1989, 54(2), 244–247.
- Cuttell, L.; Owen, H.; Lew-Tabor, A.E.; Traub, R.J. Bovine Cysticercosis-development of a Real-time PCR to Enhance Classification of Suspect Cysts Identified at Meat Inspection. Veterinary Parasitology 2013, 194(1), 65–69.
- Fæste, C.K.; Plassen, C.; Løvberg, K.E.; Moen, A.; Egaas, E. Detection of Proteins from the Fish Parasite Anisakis simplex in Norwegian Farmed Salmon and Processed Fish Products. Food Analytical Methods 2015, 8(6), 1390–1402.
- Nöckler, K.; Reckinger, S.; Broglia, A.; Mayer-Scholl, A.; Bahn, P. Evaluation of a Western Blot and ELISA for the Detection of anti-Trichinella-IgG in Pig Sera. Veterinary Parasitology 2009, 163(4), 341–347.
- Ogunremi, O.; Benjamin, J. Development and Field Evaluation of a New Serological Test for Taenia saginata Cysticercosis. Veterinary Parasitology 2010, 169(1–2), 93–101.
- Ramahefarisoa, R.M.; Rakotondrazaka, M.; Jambou, R.; Carod, J.F. Comparison of ELISA and PCR Assays for the Diagnosis of Porcine Cysticercosis. Veterinary Parasitology 2010, 173(3–4), 336–339.
- Werner, M.T.; Fæste, C.K.; Levsen, A.; Egaas, E. A Quantitative Sandwich ELISA for the Detection of Anisakis simplex Protein in Seafood. European Food Research and Technology 2011, 232(1), 157–166.
- Fæste, C.K.; Moen, A.; Schniedewind, B.; Anonsen, J.H.; Klawitter, J.; Christians, U. Development of Liquid Chromatography-Tandem Mass Spectrometry Methods for the Quantitation of Anisakis simplex Proteins in Fish. Journal of Chromatography A 2016, 1432, 58–72.
- Foca, G.; Ferrari, C.; Ulrici, A.; Sciutto, G.; Prati, S.; Morandi, S.; Brasca, M.; Lavermicocca, P.; Lanteri, S.; Oliveri, P. The Potential of Spectral and Hyperspectral-Imaging Techniques for Bacterial Detection in Food: A Case Study on Lactic Acid Bacteria. Talanta 2016, 153, 111–119.
- Peng, Y.K.; Zhang, J.; Wang, W.; Li, Y.Y.; Wu, J.H.; Huang, H. Potential Prediction of the Microbial Spoilage of Beef using Spatially Resolved Hyperspectral Scattering Profiles. Journal of Food Engineering 2011, 102(2), 163–169.
- Tao, F.; Peng, Y.; Gomes, C.L.; Chao, K.; Qin, J. A Comparative Study for Improving Prediction of Total Viable Count in Beef based on Hyperspectral Scattering Characteristics. Journal of Food Engineering 2015, 162, 38–47.
- Shaw, G.; Manolakis, D. Signal Processing for Hyperspectral Image Explotation. IEEE Signal Processing Magazine 2002, 19( 1), 12–16.
- Wold, J.P.; Westad, F.; Heia, K. Detection of Parasites in Cod Fillets by using SIMCA Classification in Multispectral Images in the Visible and NIR Region. Applied Spectroscopy 2001, 55(8), 1025–1034.
- Siche, R.; Vejarano, R.; Aredo, V.; Velasquez, L.; Saldaña, E.; Quevedo, R. Evaluation of Food Quality and Safety with Hyperspectral Imaging (HSI). Food Engineering Reviews 2016, 8(3), 306–322.
- Tsakanikas, P.; Pavlidis, D.; Panagou, E.; Nychas, G.J. Exploiting Multispectral Imaging for Non-Invasive Contamination Assessment and Mapping of Meat Samples. Talanta 2016, 161, 606–614.
- Yeh, Y.H.; Chung, W.C.; Liao, J.Y.; Chung, C.L.; Kuo, Y.F.; Lin, T.T. Strawberry Foliar Anthracnose Assessment by Hyperspectral Imaging. Computers and Electronics in Agriculture 2016, 122, 1–9.
- Ausmus, B.S.; Hilty, J.W. Reflectance Studies of Healthy, Maize Dwarf Mosaic Virus-Infected, and Helminthosporium maydis-Infected Corn Leaves. Remote Sensing of Environment 1971, 2, 77–81.
- Hamid Muhammed, H.; Larsolle, A. Feature-Vector based Analysis of Hyperspectral Crop Reflectance Data for Discrimination Quantification of Fungal Disease Severity in Wheat. Biosystems Engineering 2003, 86(2), 125–134.
- Delwiche, S.R.; Kim, M.S. Hyperspectral Imaging for Detection of Scab in Wheat. In Biological Quality and Precision Agriculture II; DeShazer, J.A.; Meyer, G.E.; Eds.; Proceedings of SPIE, Boston, Massachusetts, 2000; 4203, 13–20.
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early Detection of Fusarium Infection in Wheat using Hyper-Spectral Imaging. Computers and Electronics in Agriculture 2011, 75(2), 304–312.
- Qin, J.; Burks, T.; Zhao, X.; Niphadkar, N.; Ritenour, M. Multispectral Detection of Citrus Canker using Hyperspectral Band Selection. Transactions of the ASABE 2011a, 54(6), 2331–2341.
- Zhao, X.; Burks, T.F.; Qin, J.; Ritenour, M.A. Effect of Fruit Harvest Time on Citrus Canker Detection using Hyperspectral Reflectance Imaging. Sensing and Instrumentation for Food Quality and Safety 2010, 4(3), 126–135.
- Qin, J.; Burks, T.; Zhao, X.; Niphadkar, N.; Ritenour, M. Development of a Two-Band Spectral Imaging System for Real-Time Citrus Canker Detection. Journal of Food Engineering 2012, 108(1), 87–93.
- Endeshaw, S.; Sabbatini, P.; Romanazzi, G.; Schilder, A.; Neri, D. Effects of Grapevine Leafroll Associated Virus 3 Infection on Growth, leaf Gas Exchange, Yield and Basic Fruit Chemistry of Vitis vinifera L. cv. Cabernet Franc. Scientia Horticulturae 2014, 170, 228–236.
- Monis, J.; Bestwick, R.K. Serological Detection of Grapevine Associated Closteroviruses in Infected Grapevine Cultivars. Plant Disease 1997, 81(7), 802–808.
- Rowhani, A.; Uyemoto, J.K.; Golino, D.A. A Comparison between Serological and Biological Assays in Detecting Grapevine Leafroll Associated Viruses. Plant Disease 1997, 81(7), 799–801.
- Polischuk, V.P.; Shadchina, T.M.; Kompanetz, T.I.; Budzanivskaya, I.G.; Sozinov, A.A. Changes in Reflectance Spectrum Characteristic of Nicotiana debneyi Plant Under the Influence of Viral Infection. Archives of Phytopathology and Plant Protection 1997, 31(1), 115–119.
- MacDonald, S.L.; Staid, M.; Staid, M.; Cooper, M.L. Remote Hyperspectral Imaging of Grapevine Leafroll-Associated Virus 3 in Cabernet Sauvignon Vineyards. Computers and Electronics in Agriculture 2016, 130, 109–117.
- Naidu, R.; Perry, E.; Pierce, F.; Mekuria, T. The Potential of Spectral Reflectance Technique for the Detection of Grapevine leafroll-associated virus-3 in Two Red-Berried Wine Grape Cultivars. Computers and Electronics in Agriculture 2009, 66(1), 38–45.
- Lee, H.; Kim, M.S.; Lim, H.S.; Park, E.; Lee, W.H.; Cho, B.K. Detection of Cucumber Green Mottle Mosaic Virus-Infected Watermelon Seeds using a Near-Infrared (NIR) Hyperspectral Imaging System: Application to Seeds of the “Sambok Honey” Cultivar. Biosystems Engineering 2016, 148, 138–147.
- Popoff, M.R.; Poulain, B. Bacterial Toxins and the Nervous System: Neurotoxins and Multipotential Toxins Interacting with Neuronal Cells. Toxins (Basel) 2010, 2(4), 683–737.
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. Journal of Saudi Chemical Society 2011, 15(2), 129–144.
- Horn, B.W. Biodiversity of Aspergillus Section Flavi in the United States: A Review. Food Additives & Contaminants 2007, 24(10), 1088–1101.
- Senthilkumar, T.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Gräfenhan, T. Detection of Fungal Infection and Ochratoxin A Contamination in Stored Barley using Near-Infrared Hyperspectral Imaging. Biosystems Engineering 2016, 147, 162–173.
- Murillo-Arbizu, M.; González-Peñas, E.; Amézqueta, S. Comparison between Capillary Electrophoresis and High Performance Liquid Chromatography for the Study of the Occurrence of Patulin in Apple Juice Intended for Infants. Food and Chemical Toxicology 2010, 48(8–9), 2429–2434.
- Williams, P.; Geladi, P.; Britz, T.; Manley, M. Investigation of Fungal Development in Maize Kernels using NIR Hyperspectral Imaging and Multivariate Data Analysis. Journal of Cereal Science 2012, 55(3), 272–278.
- Feng, Y.Z.; Sun, D.W. Near-infrared Hyperspectral Imaging in Tandem with Partial Least Squares Regression and Genetic Algorithm for Non-Destructive Determination and Visualization of Pseudomonas loads in Chicken Fillets. Talanta 2013b,109, 74–83.
- Pearson, T.C.; Wicklow, D.T.; Maghirang, E.B.; Xie, F.; Dowell, F.E. Detecting Aflatoxin in Single Corn Kernels by Transmittance and Reflectance Spectroscopy. Transactions of the ASAE 2001, 44(5), 1247–1254.
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Cleveland, T.; Bhatnagar, D. Correlation and Classification of Single Kernel Fluorescence Hyperspectral Data with Aflatoxin Concentration in Corn Kernels Inoculated with Aspergillus flavus Spores. Food Additives & Contaminants: Part A 2010, 27(5), 701–709.
- Wang, W.; Heitschmidt, G.W.; Ni, X.; Windham, W.R.; Hawkins, S.; Chu, X. Identification of Aflatoxin B1 on Maize Kernel Surfaces using Hyperspectral Imaging. Food Control 2014, 42, 78–86.
- Wang, W.; Lawrence, K.C.; Ni, X.; Yoon, S.C.; Heitschmidt, G.W.; Feldner, P. Near-Infrared Hyperspectral Imaging for Detecting Aflatoxin B1 of Maize Kernels. Food Control 2015c, 51, 347–355.
- Wang, W.; Heitschmidt, G.; Windham, W.; Feldner, P.; Ni, X.; Chu, X. Feasibility of Detecting Aflatoxin B1 on Inoculated Maize Kernels Surface using Vis/NIR Hyperspectral Imaging. Journal of Food Science 2015b, 80(1), M116–M122.
- Chelladurai, V.; Jayas, D.S.; White, N.D.G. Thermal Imaging for Detecting Fungal Infection in Stored Wheat. Journal of Stored Products Research 2010, 46(3), 174–179.
- Muir, W.E.; White, N.D.G. Microorganisms in Stored Grain. In Grain Preservation Biosytems; Muir, W.E.; Ed.; Department of Biosystems Engineering University of Manitoba, Winnipeg, Canada, 2001.
- Abramson, D.; Sinha, R.N.; Mills, J.T. Mycotoxin and Odor Formation in Moist Cereal Grain during Granary Storage. Cereal Chemistry 1980, 57(5), 346–351.
- Di Crispino, K.; Haibo, Y.; Hruska, Z.; Kori, B.; Lewis, D.; Beach, J.; Brown, R.; Cleveland, T.E. Hyperspectral Imagery for Observing Spectral Signature Change in Aspergillus flavus. In Optical Sensors and Sensing Systems for Natural Resources and Food Safety and Quality; Chen, Y.R.; Meyer, G.E.; Tu, S.I.; Eds.; Proceedings of SPIE, Boston, Massachusetts, 2005; 5996, 599–606.
- Singh, C.B.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Fungal Damage Detection in Wheat using Short-Wave Near-Infrared Hyperspectral and Digital Colour Imaging. International Journal of Food Properties 2012, 15(1), 11–24.
- Zhang, H.; Paliwal, J.; Jayas, D.S.; White, N.D.G. Classification of Fungal Infected Wheat Kernels using Near-Infrared Reflectance Hyperspectral Imaging and Support Vector Machine. Transactions of the ASABE 2007, 50, 1779–1785.
- Pearson, T.C.; Wicklow, D.T. Detection of Corn Kernels Infected by Fungi. Transactions of the ASABE 2006, 49(4), 1235–1245.
- Farag, R.S.; Khalil, F.A.; Mohsen, S.M.; Bosyony, A.E. Effect of Certain Fungi on the Lipids Contents of Wheat Kernels, Sesame and Soybean Seeds. Grasas y Aceites 1985, 36, 362–367.
- Boyacioǧlu, D.; Hettiarachchy, N.S. Changes in Some Biochemical Components of Wheat Grain that was Infected with Fusarium graminearum. Journal of Cereal Science 1995, 21(1), 57–62.
- Shahin, M.A.; Symons, S.J. Detection of Fusarium Damaged Kernels in Canada Western Red Spring Wheat using visible/Near-Infrared Hyperspectral Imaging and Principal Component Analysis. Computers and Electronics in Agriculture 2011, 75(1), 107–112.
- Shahin, M.A.; Symons, S.J. Detection of Fusarium Damage in Canadian Wheat using visible/Near-Infrared Hyperspectral Imaging. Journal of Food Measurement and Characterization 2012, 6(1), 3–11.
- Siripatrawan, U.; Makino, Y. Monitoring Fungal Growth on Brown Rice Grains using Rapid and Non-Destructive Hyperspectral Imaging. International Journal of Food Microbiology 2015, 199, 93–100.
- Müller-Fischer, N. Nutrient-Focused Processing of Rice. In Agricultural Sustainability; Bhullar, G.S.; Bhullar, N.K.; Eds.; Academic Press: San Diego, United States, 2013; 197–220.
- Carlos, C.; Marreto, D.A.; Poppi, R.J.; Sato, M.I.Z.; Ottoboni, L.M.M. Fourier Transform Infrared Microspectroscopy as a Bacterial Source Tracking Tool to Discriminate Fecal E. coli strains. Microchemical Journal 2011, 99(1), 15–19.
- De Sousa Marques, A.; Nicácio, J.T.; Cidral, T.A.; de Melo, M.C.; de Lima, K.M. The Use of Near Infrared Spectroscopy and Multivariate Techniques to Differentiate Escherichia coli and Salmonella enteritidis Inoculated into Pulp Juice. Journal of Microbiological Methods 2013, 93(2), 90–94.
- Guentzel, J.L.; Lam, K.L.; Callan, M.A.; Emmons, S.A.; Dunham, V.L. Reduction of Bacteria on Spinach, Lettuce, and Surfaces in Food Service Areas using Neutral Electrolyzed Oxidizing Water. Food Microbiology 2008, 25(1), 36–41.
- Mahmoud, B.S.M.; Bachman, G.; Linton, R.H. Inactivation of Escherichia coli O157: H7,Listeria Monocytogenes, Salmonella enterica and Shigella flexneri on Spinach Leaves by X-ray. Food Microbiology 2010, 27(1), 24–28.
- Xicohtencatl-Corte, J.; Sánchez Chacon, S.; Saldaña, Z.; Freer, E.; Girón, J.A. Interaction of Escherichia coli O157: H7with Leafy Green Produce. Journal of Food Protection 2009, 72(7), 1531–1537.
- Kim, M.S.; Chen, Y.R.; Cho, B.K.; Chao, K.; Yang, C.C.; Lefcourt, A.M.; Chan, D. Hyperspectral Reflectance and Fluorescence Line-Scan Imaging for Online Defects and Fecal Contamination Inspection of Apples. Sensing and Instrumentation for Food Quality and Safety 2007, 1(3), 151–159.
- Yang, C.C.; Jun, W.; Kim, M.S.; Chao, K.; Kang, S.; Chan, D.E.; Lefcourt, A. Classification of Fecal Contamination on Leafy Greens by Hyperspectral Imaging. In Sensing for Agriculture and Food Quality and Safety II; Kim, L.S.; Ma, S.Y.; Chao, K.; Eds.; Proceedings of SPIE, Orlando, Florida 2010; 76760F.
- Yang, C.C.; Kim, M.S.; Kang, S.; Cho, B.K.; Chao, K.; Lefcourt, A.M.; Chan, D.E. Red to Far-Red Multispectral Fluorescence Image Fusion for Detection of Fecal Contamination on Apples. Journal of Food Engineering 2012, 108(2), 312–319.
- Palou, L. Penicillium Digitatum, Penicillium Italicum (green mold, blue mold). In Postharvest Decay. Control Strategies; Bautista-Baños S.; Ed.; Academic Press, Elsevier Inc.: London, United Kingdom, 2014; 2, 45–102.
- Mehl, P.M.; Chen, Y.R.; Kim, M.S.; Chan, D.E. Development of Hyperspectral Imaging Technique for the Detection of Apple Surface Defects and Contaminations. Journal of Food Engineering 2004, 61(1), 67–81.
- Li, J.; Huang, W.; Tian, X.; Wang, C.; Fan, S.; Zhao, C. Fast Detection and Visualization of Early Decay in Citrus using Vis-NIR Hyperspectral Imaging. Computers and Electronics in Agriculture 2016, 127, 582–592.
- Lorente, D.; Blasco, J.; Serrano, A.J.; Soria-Olivas, E.; Aleixos, N.; Gómez-Sanchis, J. Comparison of ROC Feature Selection Method for the Detection of Decay in Citrus Fruit using Hyperspectral Images. Food and Bioprocess Technology 2013, 6(12), 3613–3619.
- Gómez-Sanchis, J.; Gómez-Chova, L.; Aleixos, N.; Camps-Valls, G.; Montesinos-Herrero, C.; Moltó, E.; Blasco, J. Hyperspectral System for Early Detection of Rottenness Caused by Penicillium digitatum in Mandarins. Journal of Food Engineering 2008, 89(1), 80–86.
- Hamad, S.H. The Microbial Quality of Processed Date Fruits Collected from a Factory in Al-Hofuf City, Kingdom of Saudi Arabia. Emirates Journal of Food and Agriculture 2012, 24(2), 105–112.
- Liu, G.; He, J.; Wang, S.; Luo, Y.; Wang, W.; Wu, L.; Si, Z.; He, X. Application of Near-Infrared Hyperspectral Imaging for Detection of External Insect Infestations on Jujube Fruit. International Journal of Food Properties 2016, 19(1), 41–52.
- Xing, J.; Guyer, D.; Ariana, D.; Lu, R. Determining Optimal Wavebands using Genetic Algorithm for Detection of Internal Insect Infestation in Tart Cherry. Sensing and Instrumentation for Food Quality and Safety 2008, 2(3), 161–167.
- Singh, C.B.; Jayas, D.S.; Paliwal, J.; White, N.D.G. Detection of Insect-Damaged Wheat Kernels using Near-Infrared Hyperspectral Imaging. Journal of Stored Products Research 2009, 45(3), 151–158.
- Millner, P.; Martin, P.; Reynolds, S.; Reynnells, R. Survival of E. coli O157:H7, Salmonella, and Listeria in Frass from Hornworms on Tomato Leaves. International Association of Food Protection Annual Meeting, 2011, 2–77.
- Yang, C.C.; Kim, M.S.; Millner, P.; Chao, K.; Cho, B.K.; Mo, C.; Lee, H.; Chan, D.E. Development of Multispectral Imaging Algorithm for Detection of Frass on Mature Red Tomatoes. Postharvest Biology and Technology 2014, 93, 1–8.
- Schirmer, B.C.; Langsrud, S. Evaluation of Natural Antimicrobials on Typical Meat Spoilage Bacteria in vitro and in Vacuum-Packed Pork Meat. Journal of Food Science 2010, 75(2), 98–102.
- Xiong, Z.; Sun, D.W.; Zeng, X.A.; Xie, A. Recent Developments of Hyperspectral Imaging Systems and their Applications in Detecting Quality Attributes of Red Meats: A Review. Journal of Food Engineering 2014, 132, 1–13.
- Feng, Y.Z.; ElMasry, G.; Sun, D.W.; Walsh, D.; Morcy, N. Near-Infrared Hyperspectral Imaging and Partial Least Squares Regression for Rapid and Reagentless Determination of Enterobacteriaceae on Chicken Fillets. Food Chemistry 2013, 138(2–3), 1829–1836.
- Tao, F.; Peng, Y.; Li, Y.; Chao, K.; Dhakal, S. Simultaneous Determination of Tenderness and Escherichia coli Contamination of Pork using Hyperspectral Scattering Technique. Meat Science 2012, 90(3), 851–857.
- Feng, Y.Z.; Sun, D.W. Determination of Total Viable Count (TVC) in Chicken Breast Fillets by Near-Infrared Hyperspectral Imaging and Spectroscopic Transforms. Talanta 2013a, 105, 244–249.
- Wu, D.; Sun, D.W. Potential of Time Series-Hyperspectral Imaging (TS-HSI) for Non-Invasive Determination of Microbial Spoilage of Salmon Flesh. Talanta 2013c, 111, 39–46
- Peng, Y.K.; Zhang, J.; Wu, J.H.; Huang, H. Hyperspectral Scattering Profiles for Prediction of the Microbial Spoilage of Beef. Proceeding of SPIE 7315, Sensing for Agriculture and Food Quality and Safety 2009, 73150Q–1.
- Panagou, E.Z.; Papadopoulou, O.; Carstensen, J.M.; Nychas, G.J. Potential of Multispectral Imaging Technology for Rapid and Non-Destructive Determination of the Microbiological Quality of Beef Filets during Aerobic Storage. International Journal of Food Microbiology 2014, 174, 1–11.
- Barlocco, N.; Vadell, A.; Ballesteros, F.; Galietta, G.; Cozzolino, D. Predicting Intramuscular Fat, Moisture and Warner-Bratzler Shear Force in Pork Muscle using Near Infrared Reflectance Spectroscopy. Animal Science 2006, 82(1), 111–116.
- Peng, Y.K.; Wang, W. Prediction of Pork Meat Total Viable Bacteria Count using Hyperspectral Imaging System and Support Vector Machines. In Food Processing Automation Conference Proceedings, Providence, Rhode Island, United States, 2008, Paper 701P0508cd.
- Wang, W.; Peng, Y.K.; Zhang, X.L. Study on Modeling Method of Total Viable Count of Fresh Pork Meat based on Hyperspectral Imaging System. Spectroscopy and Spectral Analysis 2010, 30(2), 411–415.
- Wang, W.; Peng, Y.K.; Huang, H.; Wu, J. Application of Hyperspectral Imaging Technique for the Detection of Total Viable Bacteria Count in Pork. Sensor Letters 2011, 9(3), 1024–1030.
- Dissing, B.S.; Papadopoulou, O.S.; Tassou, C.; Ersbøll, B.K.; Carstensen, J.M.; Panagou, E.Z.; Nychas, G.J. Using Multispectral Imaging for Spoilage Detection of Pork Meat. Food and Bioprocess Technology 2013, 6(9), 2268–2279.
- Barbin, D.F.; ElMasry, G.; Sun, D.W.; Allen, P.; Morsy, N. Non-Destructive Assessment of Microbial Contamination in Porcine Meat using NIR Hyperspectral Imaging. Innovative Food Science & Emerging Technologies 2013, 17, 180–191.
- Ma, F.; Yao, J.; Xie, T.; Liu, C.; Chen, W.; Chen, C.; Zheng, L. Multispectral Imaging for Rapid and Non-Destructive Determination of Aerobic Plate Count (APC) in Cooked Pork Sausages. Food Research International 2014, 62, 902–908.
- Tao, F.; Wang, W.; Li, Y.; Peng, Y.; Wu, J.; Shan, J.; Zhang, L. A Rapid Nondestructive Measurement Method for Assessing the Total Plate Count on Chilled Pork Surface. Spectroscopy and Spectral Analysis 2010, 30(12), 3405–3409.
- Liu, Y.; Windham, W.R.; Lawrence, K.C.; Park, B. Simple Algorithms for the Classification of Visible/NIR and Hyperspectral Imaging Spectra of Chicken Skins, Feces, and Fecal Contaminated Skins. Applied Spectroscopy 2003, 57(12), 1609–1612.
- Chao, K.L.; Yang, C.C.; Kim, M.S. Spectral Line-Scan Imaging System for High-Speed Non-Destructive Wholesomeness Inspection of Broilers. Trends in Food Science & Technology 2010, 21(3), 129–137.
- Yang, C.C.; Chao, K.L.; Chen, Y.R.; Early, H.L. Systemically Diseased Chicken Identification using Multispectral Images and Region of Interest Analysis. Computers and Electronics in Agriculture 2005, 49(2), 255–271.
- Yoon, S.C.; Park, B.; Lawrence, K.C.; Windham, W.R.; Heitschmidt, G.W. Line-scan Hyperspectral Imaging System for Real-Time Inspection of Poultry Carcasses with Fecal Material and Ingesta. Computers and Electronics in Agriculture 2011, 79(2), 159–168.
- Berrang, M.E.; Smith, D.P.; Windham, W.R.; Feldner, P.W. Effect of Intestinal Content Contamination on Broiler Carcass Campylobacter Counts. Journal of Food Protection 2004, 67(2), 235–238.
- Windham, W.R.; Smith, D.P.; Park, B.; Lawrence, K.C. Algorithm development with visible/near infrared spectra for detection of poultry feces and ingesta. Transactions of the ASAE 2003, 46, 1733–1738.
- Park, B.; Lawrence, K.C.; Windham, W.R.; Smith, D.P. Performance of Hyperspectral Imaging System for Poultry Surface Fecal Contaminant Detection. Journal of Food Engineering 2006, 75(3), 340–348.
- Park, B.; Yoon, S.C.; Windham, W.; Lawrence, K.; Kim, M.; Chao, K. Line-scan hyperspectral Imaging for Real-Time In-Line Poultry Fecal Detection. Sensing and Instrumentation for Food Quality and Safety 2011, 5(1), 25–32.
- Ye, X.; Iino, K.; Zhang, S. Monitoring of Bacterial Contamination on Chicken Meat Surface using a Novel Narrowband Spectral Index Derived From Hyperspectral Imagery Data. Meat Science 2016, 122, 25–31.
- Alexandrakis, D.; Downey, G.; Scannell, A. Rapid Non-Destructive Detection of Spoilage of Intact Chicken Breast Muscle using Near-Infrared and Fourier Transform Mid-Infrared Spectroscopy and Multivariate Statistics. Food and Bioprocess Technology 2012, 5(1), 338–347.
- Nychas, G.; Dillon, V.M.; Board, R.G. Glucose, The Key Substrate in the Microbiological Changes Occurring in Meat and Certain Meat Products. Biotechnology and Applied Biochemistry 1988, 10(3), 203–231.
- Warriss, P.D. Meat Science: An Introductory Text, 2nd ed.; Cabi Series & Modular Texts Wallingford, Oxfordshire, United Kingdom, 2010; 130–150.
- Board, R.J.; Davies, A.R. The Microbiology of Meat and Poultry; Blackie Academic & Professional: London, United Kingdom, 1998, 266–282.
- Qin, J.; Chao, K.; Kim, M.S.; Kang, S.; Cho, B.K.; Jun, W. Detection of Organic Residues on Poultry Processing Equipment Surfaces by LED-Induced Fluorescence Imaging. Applied Engineering in Agriculture 2011b, 27(1), 153–161.
- Jun, W.; Kim, M.S.; Cho, B.K.; Millner, P.D.; Chao, K.; Chan, D.E. Microbial Biofilm Detection on Food Contact Surfaces by Macro-Scale Fluorescence Imaging. Journal of Food Engineering 2010, 99(3), 314–322.
- Sone, I.; Olsen, R.L.; Sivertsen, A.H.; Eilertsen, G.; Heia, K. Classification of Fresh Atlantic Salmon (Salmo salar L.) Fillets Stored under Different Atmospheres by Hyperspectral Imaging. Journal of Food Engineering 2012, 109(3), 482–489.
- Gram, L.; Dalgaard, P. Fish Spoilage Bacteria - Problems and Solutions. Current Opinion in Biotechnology 2002, 13(3), 262–266.
- He, H.J.; Sun, D.W.; Wu, D. Rapid and Real-Time Prediction of Lactic Acid Bacteria (LAB) in Farmed Salmon Flesh using Near-Infrared (NIR) Hyperspectral Imaging Combined with Chemometric Analysis. Food Research International 2014, 62, 476–483.
- Limbo, S.; Sinelli, N.; Torri, L.; Riva, M. Freshness Decay and Shelf Life Predictive Modelling of European Sea Bass (Dicentrarchus labrax) Applying Chemical Methods and Electronic Nose. LWT - Food Science and Technology 2009, 42(5), 977–984.
- Restuccia, D.; Spizzirri, U.G.; Bonesi, M.; Tundis, R.; Menichini, F.; Picci, N.; Loizzo, M.R. Evaluation of Fatty Acids and Biogenic Amines Profiles in Mullet and Tuna Roe during Six Months of Storage at 4 °C. Journal of Food Composition and Analysis 2015, 40, 52–60.
- Lee, S.; Joo, S.T.; Alderton, A.L.; Hill, D.W.; Faustman, C. Oxymyoglobin and Lipid Oxidation in Yellow Fin Tuna (Thunnus albacares) Loins. Journal of Food Science 2003, 68(5), 1664–1668.
- Maestre, R.; Pazos, M.; Medina, I. Involvement of Methemoglobin (metHb) Formation and Hemin Loss in the Pro-Oxidant Activity of Fish Hemoglobins. Journal of Agricultural and Food Chemistry 2009, 57(15), 7013–7021.
- Shikama, K. The Molecular Mechanism of Autoxidation for Myoglobin and Hemoglobin: A Venerable Puzzle. Chemical Reviews 1998, 98(4), 1357–1374.
- Cheng, J.H.; Sun, D.W. Rapid and Non-Invasive Detection of Fish Microbial Spoilage by Visible and Near Infrared Hyperspectral Imaging and Multivariate Analysis. LWT - Food Science and Technology 2015a, 62(2), 1060–1068.
- Diaz, P.; Garrido, M.D.; Banon, S. Spoilage of Sous Vide Cooked Salmon (Salmo salar) Stored under Refrigeration. Food Science and Technology International 2011, 17(1), 31–37.
- He, H.J.; Sun, D.W. Inspection of Harmful Microbial Contamination Occurred in Edible Salmon Flesh using Imaging Technology. Journal of Food Engineering 2015b, 150, 82–89.
- He, H.J.; Sun, D.W. Selection of Informative Spectral Wavelength for Evaluating and Visualising Enterobacteriaceae Contamination of Salmon Flesh. Food Analytical Methods 2015d, 8(10), 2427–2436.
- Cheng, J.H.; Sun, D.W. Rapid Quantification Analysis and Visualization of Escherichia coli Loads in Grass Carp Fish Flesh by Hyperspectral Imaging Method. Food and Bioprocess Technology 2015c, 8(5), 951–959.
- Abdel-Nour, N.; Ngadi, M. Detection of Omega-3 Fatty Acid in Designer Eggs using Hyperspectral Imaging. International Journal of Food Sciences and Nutrition 2011, 62(4), 418–422.
- Zhang, W.; Pan, L.; Tu, S.; Zhan, G.; Tu, K. Non-destructive Internal Quality Assessment of Eggs using a Synthesis of Hyperspectral Imaging and Multivariate Analysis.
- Journal of Food Engineering 2015, 157, 41–48.
- Gole, V.; Roberts, J.; Sexton, M.; May, D.; Kiermeier, A.; Chousalkar, K. Effect of Egg Washing and Correlation between Cuticle and Egg Penetration by various Salmonella Strains. International Journal of Food Microbiology 2014, 182–183, 18–25.
- Lunadei, L.; Ruiz-Garcia, L.; Bodria, L.; Guidetti, R. Automatic Identification of Defects on Eggshell through a Multispectral Vision System. Food and Bioprocess Technology 2012, 5(8), 3042–3050.
- Carrada, T. Cisticercosis Subcutánea: Parasitosis Letal y Emergente. Piel 2005, 20(4), 177–182.
- Molina-Fernández, D.; Malagón, D.; Gómez-Mateos, M.; Benítez, R.; Martín-Sánchez, J.; Adroher, F.J. Fishing Area and Fish Size as Risk Factors of Anisakis Infection in Sardines (Sardina pilchardus) from Iberian Waters, Southwestern Europe. International Journal of Food Microbiology 2015, 203, 27–34.
- Pozio, E. Searching for Trichinella: Not All Pigs are Created Equal. Trends in Parasitology 2014, 30(1), 4–11.
- Sivertsen, A.H.; Heia, K.; Stormo, S.K.; Elvevoll, E.; Nilsen, H. Automatic Nematode Detection in Cod Fillets (Gadus morhua) by Transillumination Hyperspectral Imaging. Journal of Food Science 2011, 76(1), S77–S83.
- Gonzalez, M.; Jaramillo, E. The Association between Mulinia edulis (Mollusca, Bivalvia) and Edotea magellanica (Crustacea, Isopoda) in Southern Chile. Revista Chilena de Historia Natural 1991, 64, 37–51.
- Coelho, P.A.; Soto, M.E.; Torres, S.N.; Sbarbaro, D.G.; Pezoa, J.E. Hyperspectral Transmittance Imaging of the Shell-Free Cooked Clam Mulinia edulis for Parasite Detection. Journal of Food Engineering 2013, 117(3), 408–416.
- Gómez-De-Anda, F.; Dorantes-Álvarez, L.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Calderón-Domínguez, G.; Martínez Labat, P.; de-la-Rosa-Arana J.L., Determination of Trichinella spiralis in Pig Muscles using Mid-Fourier Transform Infrared Spectroscopy (MID-FTIR) with Attenuated Total Reflectance (ATR) and Soft Independent Modeling of Class Analogy (SIMCA). Meat Science 2012, 91(3), 240–246.
- Organización Panamericana de la Salud ( OPS). Riesgos a la salud por la crianza de cerdos alimentados en sitios de disposición final de residuos sólidos en América Latina y el Caribe. Investigación bibliográfica. Lima, Perú, 2007.
- Holman, H.Y.; Miles, R.; Hao, Z.; Wozei, E.; Anderson, L.M.; Yang, H. Real-time Chemical Imaging of Bacterial Activity in Biofilms using Open-Channel Microfluidics and Synchrotron FTIR Spectromicroscopy. Analytical Chemistry 2009, 81(20), 8564–8570.
- Goel, M.; Whitmire, E.; Mariakakis, A.; Saponas, T.S.; Joshi, N.; Morris, D.; Guenter, B.; Gavriliu, M.; Borriello, G.; Patel, S.N. HyperCam: Hyperspectral Imaging for Ubiquitous Computing Applications In UbiComp 2015 - Proceedings of the 2015. ACM International Joint Conference on Pervasive and Ubiquitous Computing, 2015, 145–156.
- Malorny, B.; Paccassoni, E.; Fach, P.; Bunge, C.; Martin, A.; Helmuth, R. Diagnostic real-time PCR for Detection of Salmonella in Food. Applied and Environmental Microbiology 2004, 70(12), 7046–7052.
- Golmohammadi, M.; Cubero, J.; Penalver, J.; Quesada. J.; Lopez, M.; Llop, P. Diagnosis of Xanthomonas Axonopodis pv. citri, Causal Agent of Citrus Canker, in Commercial Fruits by Isolation and PCR-based Methods. Journal of Applied Microbiology 2007, 103(6), 2309–2315.
- Levin, R.E. PCR detection of Aflatoxin Producing Fungi and its Limitations. Review. International Journal of Food Microbiology 2012, 156(1), 1–6.
- Kaliramesh, S.; Chelladurai, V.; Jayas, D.; Alagusundaram, K.; White, N.; Fields, P. Detection of Infestation by Callosobruchus maculatus in Mung Bean using near-Infrared Hyperspectral Imaging. Journal of Stored Products Research 2013, 52, 107–111.