ABSTRACT
This objective of this work was to produce spray-drying microencapsulated carotenoid extracts from pequi pulp using maltodextrin and gum arabic and to evaluate the influence of drying temperature on the physicochemical properties of microencapsulated extracts. Emulsions were spray-dried into powders at 150°C, 170°C, and 190°C. Morphological and physicochemical properties of the obtained product were investigated. The temperature of 190°C was that which best conserved the carotenoids and had the best observed solubility. The microspheres produced by spray drying presented an average size of 20 µm. Neither morphological nor color differences were observed for particles dried at different temperatures.
Introduction
Pequi (Caryocar brasiliense Camb.) is a species native to the Brazilian Cerrado. It produces a fruit with a remarkable flavor that is highly valued in local cuisine.[Citation1,Citation2] Pequi has a high carotenoid content and the potential to be a source of natural dyes.[Citation3] Carotenoid compounds have human health benefits because of their antioxidant activity, which shows promise for treating degenerative (e.g., cancer) and cardiovascular diseases, among others.[Citation4]
Because of their low stability to environmental conditions (e.g., exposure to high temperatures, light intensities, and oxygen levels), carotenoids – which are responsible for the typical color of pequi fruits – are generally susceptible to degradation reactions during processing and storage operations. Carotenoid extracts require special care to prevent degradation,[Citation5] which has prompted research on carotenoid preservation strategies. Various packaging techniques have been proposed to increase fruit post-harvest stability and quality.[Citation6,Citation7] Furthermore, encapsulation may allow for carotenoid dispersion in water, thus enabling their application in a wider range of products.[Citation8]
Spray drying stands out as one of the most widely used microencapsulation techniques because of apparatus availability, the ability to apply multiple encapsulating agents, good volatile compound retention, and final product stability.[Citation9] Spray drying is a process of transformation of a fluid in a dry product in a single operation with little effect on quality, being presented as a common method for encapsulation in the food industry. Spray drying has many advantages, including low operating costs, good yields, rapid capsule solubility, small capsule size, and suitable capsule stability, in addition to protecting light- and oxygen-sensitive compounds from free radical degradation.[Citation10,Citation11] This study aimed to produce spray-dried-microencapsulated carotenoid extracts from pequi pulp, as well as to assess the influence of drying temperature on the physicochemical properties of the dried extracts.
Materials and methods
Carotenoid extract preparation
Pequi pulp was dried in tray dryer with hot air (moisture content ca. 5%) at 60°C and for use in carotenoid extraction. A 1:5 (weight ratio) dried pulp:acetone suspension was left to rest for 24 h under refrigeration before being filtered. The residue was washed with acetone until colorless. The carotenoid extract was filtered and concentrated in a rotary evaporator at 35°C.
Carotenoid extract microencapsulation
The extraction of carotenoids in encapsulated powder was obtained with the help of a spray dryer. The emulsion was formulated with an encapsulating matrix composed of maltodextrin (DE 10) and gum Arabic (encapsulating and emulsifying function) at the concentration of 20% and 10%, being that the hydration of the encapsulating agents occurred at ambient temperature for 24 h. The extract containing carotenoids was then incorporated into the matrix in the proportion 3:1 (m/m) with the help of ultrasound (Unique, Brazil). The drying procedure was done on a pilot scale, single-stage spray dryer (model MSD 1.0) equipped with a 1 mm diameter spray nozzle (Labmaq, Brazil) using air and product flow rates of 35 and 1.10 L/h as well as inlet temperatures of 150°C, 170°C, and 190°C.
Physicochemical characteristics of microencapsulated carotenoid extracts
Moisture content, water activity (Aw), pH, total soluble solids (TSS), titratable acidity (TA), total carotenoids and color (L*, a*, and b*; C*, H*, and IE) of the microencapsulated powders were analyzed immediately after spray drying. Moisture content was determined through the gravimetric method proposed by the Adolfo Lutz Institute.[Citation12] Aw was determined on a thermo-hygrometer (Aqualab, Decagon. Model 3TE, Pullman, WA, USA) at 25°C. TSS and pH were determined with a tabletop refractometer (model ABBÉ) and a potentiometer (Tecnopon). TA was determined by titration, according to AOAC.[Citation13]
Carotenoid content was determined by spectrophotometry, following Rodriguez-Amaya.[Citation14] Each 5 g of previously ground pequi pulp was added to 50 mL of refrigerated acetone, filtered, and transferred to a separatory funnel, to which 40 mL of petroleum ether was added. The residue was washed four more times or until the acetone was completely removed. The petroleum ether extract was transferred to a 100 mL volumetric flask, with the volume completed with petroleum ether. Samples were read on a digital spectrophotometer (SP-200, BIOSPECTRO) at 450 nm. Total carotenoid content was calculated by the following equation:
where A is the solution absorbance at 450 nm, V is the final solution volume (mL), TC is the total carotenoid content, P is the sample weight (g), and is the β-carotene extinction or molar absorption coefficient in petroleum ether (2592). Color was evaluated on a CR-10 Color Reader (Minolta) by means of direct reflectance readings of the coordinates L*, a*, and b* (CIELAB L* scale). In addition to the basic coordinates, the following coordinates were measured:
Hue (H), corresponding to the tonality:
Chroma index (C*), expressing saturation or color intensity:
Solubility (Sol) was determined according to Eastman and Moore,[Citation15] with modifications: 1 g of the microencapsulated extract was added to 100 mL of distilled water. The mixture was mechanically stirred (AGI 103, Nova Ética, 1550g/5 min), transferred to tubes, and centrifuged (30,000g/5 min). A 25 mL supernatant aliquot was poured into previously weighed Petri dishes and dried at 70 ± 2°C and reduced pressure (≤100 mmHg = 13.3 kPa) for 5 h or until reaching a constant weight. Solubility was calculated by Eq. (4)
where S is the solubility (%), Wf is the evaporation residue mass (g), and Wa is the sample mass (g). Hygroscopicity (Hyg) was determined according to Cai and Corke,[Citation16] with modifications: approximately 1 g of each sample was packed in an air-tight container along with an NaCl-saturated solution (relative humidity 75.29%) at 25°C for a week. Samples were weighed and their Hyg values were expressed as grams of absorbed water per 100 g of sample dry mass (g/100 g).
Particle morphology
Particle morphology was studied using scanning electron microscopy (SEM) according to Souza et al.[Citation17] Magnification ranged from 500 to 2000×.
Statistical analysis
Analysis of variance (ANOVA) was applied to the data. If significant differences were observed among mean values, these were further compared by the Tukey’s test at 5% probability. All statistical treatments were done using the Statistical Analysis Systems (SAS) software, version 9.2, licensed by the Federal University of Viçosa.
Results and discussion
Characterization of the microencapsulated product
presents the mean values and mean comparison tests for the color attributes. No significant differences were observed among any of the studied color attributes in the different treatments (p > 0.05). The conclusion is that the tested temperatures did not affect the color of the microencapsulated carotenoid extracts. Pinto[Citation18] when drying a microencapsulated pequi extract at different temperatures, observed reduced L*, which may be attributed to sugar caramelization at high temperatures. Positive values were observed for a* and b* (), indicating that the obtained microencapsulated extracts presented reddish and yellowish shades. The positive b* value is mainly due to the presence of yellowish carotenoids (e.g., violaxanthin), which are the major pigments in microencapsulated pequi extracts.
Table 1. Color coordinates (L*, a*, b*, °H, and C*) of pequi carotenoid extracts microencapsulated at different drying temperatures.
shows the mean values of carotenoid and moisture contents, Aw, pH, TA, solubility, and hygroscopicity of the microencapsulated extracts. The extracts showed significantly different water activities, except those resulting from the drying temperatures of 150°C and 170°C, which did not differ statistically and presented mean Aw values of 0.13 and 0.12. These temperatures led to a different Aw outcome than that obtained at 190°C (0.2). Santana et al.[Citation19] found similar Aw values (0.06–0.3) for encapsulated pequi pulp. The values found in this study are below the limit established by Fennema[Citation20] as the stability threshold for dried powders (0.30). Silva et al.[Citation21] stated that, despite being below the stability limit, the oxidation rate increases for Aw values below and above the 0.20–0.30 range. Because pequi pulp was comprised of approximately 30% lipids, this Aw range can impair the oxidative stability of the lipids present in the products.
Table 2. Carotenoid and moisture contents, water activity (Aw), pH, titratable acidity (TA), solubility (Sol), and hygroscopicity (Hyg) of pequi carotenoid extracts microencapsulated at different drying temperatures.
Concerning moisture content, no difference (p > 0.05) was found for the microencapsulated carotenoid extracts dried at the tested temperatures. While not statistically significant, 190°C was the temperature that led to the highest water activity, corroborating the observations of Pinto.[Citation18] According to that author, a higher temperature of drying may contribute to higher approximation of the molecules of maltodextrin and other constituents, favoring strong interactions with reduction of sites available for binding with water.
The solubility values obtained ranged from 93.14% to 96.52%. Samples dried at 170°C and 190°C did not present statistically different solubility values, but those dried at 150°C did. The highest solubility was observed at 190°C (96.52%). The improved solubility was because of the solubility of the encapsulating agents. Cano-Chauca et al.,[Citation22] in their work on spray-dried mango juice, also observed solubility values around 95% for dehydrated products containing maltodextrin and gum arabic. The solubility of the atomized product depends on, among other factors, the air drying temperature: the higher the temperature, the bigger the particle and consequently, the more soluble the powder. As demonstrated in the morphological analysis (), the dry particles at 190°C are larger and have more spaces for water to penetrate more easily, which improves solubility. Even though solubility differences were found between the drying temperatures, all treatments presented high solubility.
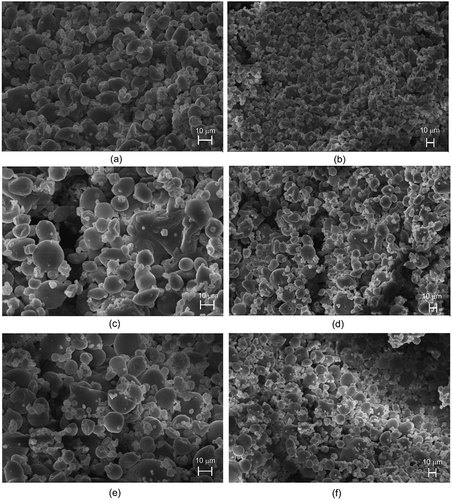
Figure 1. SEM images of pequi carotenoid extracts added by maltodextrin and gum arabic and spray-dried microencapsulated at (a) 150°C (2000×), (b) 150°C (1000×), (c) 170°C (2000×), (d) 170°C (1000×), (e) 190°C (2000×), or (f) 190°C (1000×).
shows there was no significant differences in acidity. According to Oliveira et al.,[Citation3] pequi is a low-acid fruit. We observed higher acidity than these authors, however. Our results are related to the extract/maltodextrin/gum arabic mixture, and the higher acidity is due to the components of the carotenoid extract (e.g., fatty acids) and the wall materials. Regarding pH, no significant difference was observed among the different treatments. This can also be associated with the presence of fatty acids and the wall materials.
Atomized particles are usually very hygroscopic and can easily absorb moisture. The hygroscopicity values of the microencapsulated extracts obtained here ranged from 10.43 to 11.19 g of adsorbed water/100 g of solids. This low hygroscopicity prevents particle agglomeration. There was a significant difference in hygroscopicity between the sample dried at 170°C and those dried in the other conditions. Tonon et al.[Citation23] observed that powders containing maltodextrin (10 DE) in their formulations showed less absorbed moisture regardless of drying temperature, which has to do with the number of branches containing hydrophilic groups. Thus, dehydrated products made using this material are typically less hygroscopic.
Similarly, Santana et al.,[Citation19] when studying the microencapsulation of pequi pulp, found hygroscopicity values ranging from 7.96 to 10.67 g of adsorbed water/100 g of solids. Ruiz-Cabrera et al.,[Citation24] on the other hand, observed less hygroscopicity in spray-dried passion fruit juices added by maltodextrin and lactose to act as carriers. The hygroscopicity values presented by the developed products can be attributed to the concentration of maltodextrin, since maltodextrin is a low-hygroscopicity material.
The emulsion prepared for spray drying had a concentration of 35.62 μg/g. Loss of the final carotenoid was observed at concentrations of 34.28%, 42.14%, and 28.16%, for the temperatures of 150°C, 170°C, and 190°C. This reduction may have occurred because of carotenoid exposure to oxygen, light, and temperature during the encapsulation process. During the encapsulation process, carotenoids may have been lost when they were not properly encapsulated and remained on the surface, exposed to the external effects that caused their degradation.
The content of carotenoids was higher in the extract of pequi carotenoid obtained at the temperature of 190°C, in relation to those obtained at temperatures of 150°C and 170°C, which did not differ between them. The same effect was observed by Wang et al.,[Citation6] who tested various temperatures for microencapsulating lutein and found the optimum temperature to be 190°C. According to the authors, increased temperatures are capable of volatilizing the materials located on the surface of the encapsulated particles (e.g., water and some volatiles), resulting in increased carotenoid contents.
A higher drying temperature increased drying rates and reduced the duration of the constant-rate stage of the drying process, thus increasing carotenoid retention. The increased drying temperature also increased the rate of film formation on the powder particle surfaces. Their crust is firm and acts as a protective layer, limiting core material migration toward the surface.
Particle morphology
presents SEM images of pequi carotenoid extracts microencapsulated by spray drying at 150°C, 170°C, or 190°C. The spray-dried microspheres revealed similarities in morphology, namely irregular, chiefly circular shape, toothed surface, as well as irregular, crevice-, crack-, and break-free hollows. The toothed surfaces of spray-dried samples can be attributed to their shrinkage during the drying process.[Citation25] The absence of crevices, cracks, and breaks in the microspheres plays a fundamental role in ensuring greater protection and retention of the microencapsulated extract.
Another important characteristic of the microspheres is shown in : the formation of agglomerates, that is, the occurrence of small particles located on the surface of larger particles. This feature provides the microencapsulated compound with boosted stability because the outer particles protect the inner ones and consequently their pigments.[Citation26]
Microcapsule morphology depends on several drying parameters, including formulation composition, inlet temperature, solvents used, and drying rate.[Citation27] The spray-dried microspheres had an average diameter of 20 µm (), which lies within the 0.2–5000 µm range typical of microparticles.[Citation28] The diameter of the microparticles depends on spray-drying protocols, intrinsic materials properties, concentration, viscosity, and raw material.[Citation16]
Dib Taxi et al.[Citation29] studied camu camu and reported the morphology of maltodextrin microspheres to be more heterogeneous and presenting more surface imperfections, despite the smooth surface of the spheres typical of maltodextrin capsules. Barbosa et al.[Citation30] stated that the imperfections on the microparticles can contribute to the shorter shelf-life of microencapsulated carotenoids when exposed to light in the model system.
In a study by Janiszewska and Witrowa-Rajchert,[Citation25] who evaluated flavor encapsulation using maltodextrin and gum arabic through spray drying, microcapsules similar in size and shape to those produced here were observed. The same morphology was also observed for the bixin microcapsules that comprise gum arabic.[Citation30] As observed in this study and corroborated by Ré,[Citation10] surface imperfections (e.g., roughness, cracks, or collapses) occur when there is a slow film-forming process during the drying of atomized droplets. Increasing the drying temperatures is expected to increase the rate of film-forming on the drop surface.
Conclusion
The methodology used in this study allowed for the microencapsulation of pequi carotenoid extract. Encapsulation was shown to improve key characteristics of the pequi extracts (especially carotenoid solubility), leading to reduced hydrophobicity. Furthermore, it was possible to obtain a more water-soluble dye from an oil-soluble fraction. We conclude that drying temperature did not affect the color and morphology of pequi extract, but did affect the carotenoid content. Among the tested drying temperatures, 190°C was selected for greater carotenoid conservation, with further studies recommended to improve the quality of microencapsulated pequi carotenoid extracts for dye applications.
Funding
The Minas Gerais Research Foundation (FAPEMIG) is acknowledged for funding this research.
Additional information
Funding
References
- Silva, F.H.L.; Fernandes, J.S.C.; Esteves, E.A.; Titon, M.; Santana, R.C. Populações, Matrizes e Idade da Planta na Expressão de Variáveis Físicas em Frutos do Pequizeiro. Revista Brasileira de Fruticultura 2012, 34, 806–813.
- Geőcze, K.C.; Barbosa, L.C.A.; Fidêncio, P.H.; Silvério, F.O.; Lima, C.F.; Barbosa, M.C.A.; Ismail Fyaz, M.D. Essential Oils from Pequi Fruits from the Brazilian Cerrado Ecosystem. Food Research International 2013, 54, 1–8.
- Oliveira, M.E.B.; Guerra, N.B.; Maia, A.H.N.; Alves, R.E.; Matos, N.M.S.; Sampaio, F.G.M.; Lopes, M.M.T. Características Químicas e Físico-químicas de Pequis da Chapada do Araripe, Ceará. Revista Brasileira de Fruticultura 2010, 32, 114–125.
- Santana, A.A.; Kurozawa, L.E.; de Oliveira, R.A.; Park, K.J. Influence of Process Conditions on the Physicochemical Properties of Pequi Powder Produced by Spray Drying. Drying Technology 2013, 31(7), 825–836.
- Song, J.; Wang, X.; Li, D.; Meng, L.; Liu, C. Degradation of Carotenoids in Pumpkin (Cucurbita maxima L.) Slices as Influenced by Microwave-vacuum Drying. International Journal of Food Properties, 2016, 1–9.
- Wang, Y.; Ye, H.; Zhou, C.; LV, F.; Bie, X.; Lu, Z. Study on the Spray-Drying Encapsulation of Lutein in the Porous Starch and Gelatin Mixture. European Food Research and Technology, 2012, 234, 157–163.
- Guo, H.; Huang, Y.; Qian, J.; Gong, Q.; Tang, Y. Optimization of Technological Parameters for Preparation of Lycopene Microcapsules. Journal of Food Science and Technology, 2014, 51, 1318–1325.
- Matioli, G.; Rodriguez-Amaya, D.B. Microencapsulação do Licopeno com Ciclodextrinas. Ciência e Tecnologia de Alimentos, 2003, 23, 102–105.
- Santos, A.B.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Preparo e Caracterização de Microcápsula de Oleoresina de Páprica Obtidas por Atomização. Ciência e Tecnolologia de Alimentos, 2005, 25, 322–326.
- Ré, M.I. Microencapsulation by Spray Drying. Drying Technology 1998, 16, 1195–1236.
- Begum, Y.A.; Deka, S.C. Stability of Spray Dried Microencapsulated Anthocyanins Extracted from Culinary Banana Bract. International Journal of Food Properties 2017, DOI: 10.1080/10942912.2016.1277739
- IAL. Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz, 4th edn.; Instituto Adolfo Lutz: São Paulo, 2008, 1032 pp.
- Association of Official Analytical Chemists – AOAC. Official Methods of Analysis of the AOAC. 16 edn.; Association of Official Analytical Chemists: Washington, 2000, 3000 pp.
- Rodriguez-Amaya, D.B. A Guide to Carotenoids Analysis in Food; ILSI Press: Washington, 2001, 71 pp.
- Eastman, J.E.; Moore, C.O. Cold water soluble granular starch for gelled food composition. U.S. Patent 4465702, 1984.
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. Journal of Food Science, 2000, 65, 1248–1252.
- Souza, W.; Haddad, A.; Sesso, A.; Silveira, M.; Barth, O.M.; Machado, R.D.; Souto-Padrón, T. Manual Sobre Técnicas Básicas em Microscopia Eletrônica. Sociedade Brasileira de Microscopia e Microanálise, 1989, 1, 71–79.
- Pinto, M.R.M.R. Obtenção de extratos de carotenoides de polpa de pequi (Caryocar brasiliense camb.) encapsulados pelo método de secagem em camada de espuma. Dissertação (Mestrado em Ciência e Tecnologia de Alimentos). Universidade Federal de Viçosa, 2012, 86 pp.
- Santana, A.A.; Oliveira, R.; Kurozawa, L.E.; Park, K.J. Microencapsulation of Pequi Pulp by Spray Drying: Use of Modified Starches as Encapsulating Agent. Engenharia Agrícola 2014, 34, 980–991.
- Fennema, O.R. Water and ice. In Food Chemistry; Fennema, O.R.; Ed.; Artmed: New York, 1996; 17–94.
- Silva, F.A.M.; Borges, M.F.M.; Ferreira, M.A. Métodos Para Avaliação do Grau de Oxidação Lipídica e da Capacidade Antioxidante. Química Nova 1999, 22, 94–103.
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M., Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innovative Food Science and Emerging Technologies 2005, 6, 420–428.
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubingera, M.D. Water Sorption and Glass Transition Temperature of Spray Dried Açai (Euterpe oleracea Mart.) Juice. Journal of Food Engineering 2009, 94, 215–221.
- Ruiz-Cabrera, M.A.; Espinosa-Muñoz, L.C.; Aviles-Aviles, C.; González-García, R.; Moscosa-Santillán, M.; Grajales-Lagunes, A.; Abud-Archila, M. Spray-drying of Passion Fruit Juice Using Lactose-maltodextrin Blends as the Support Material. Brazilian Archives of Biology and Technology 2009, 52, 1011–1018.
- Janiszewska, E.; Witrowa-Rajchert, D. The Influence of Powder Morphology on the Effect of Rosemary Aroma Microencapsulation during Spray Drying. International Journal of Food Science & Technology 2009, 44, 2438–2444.
- Mendes, L.G. Microencapsulação do corante natural de urucum: uma análise da eficiência da goma do cajueiro como material de parede. Dissertação (mestrado) – Universidade Federal do Ceará, Programa de Pós-Graduação em Ciência e Tecnologia de Alimentos, Fortaleza, 2012, 132 pp.
- Kosaraju, S.L.; D´ath, L.; Lawrence, A. Preparation and Characterization of Chitosan Microspheres for Antioxidant Delivery. Carbohydrate Polymers 2006, 64, 163–167.
- Barros, F.A.R.; Stringheta, P.C., 2006. Microencapsulação de Antocianinas. Biotecnologia Ciência & Desenvolvimento 2006, IX, 18–24.
- Dib Taxi, C.M.A.; Menezes, H.C.; Santos, A.B.; Grosso, C.R.F. Study of the Microencapsulation of Camu-camu (Myrciaria dubia) Juice. Journal of Microencapsulation 2003, 20, 443–448.
- Barbosa, M.I.M.J.; Borsarelli, C.D.; Mercadante, A.Z. Light Stability of Spray-dried Bixin Encapsulated with Different Edible Polysaccharide Preparations. Food Research International 2005, 38, 989–994.
