ABSTRACT
This work aimed to investigate the properties of emulsions prepared with milk fat globule membrane (MFGM) and its components, which are milk fat globule membrane proteins (MFGMPs) and milk fat globule membrane lipids (MFGMLs). The results demonstrated that the particle size of emulsions prepared with MFGM and MFGMPs decreased with increasing concentrations (from 1% to 4%); however, these droplet sizes were smaller than those prepared with MFGMLs of the same concentration. In addition, the emulsion prepared with MFGMLs showed a clear phase separation, as opposed to those from MFGM and MFGMPs. These results indicated that MFGMLs alone could not contribute to the stability of the emulsions. The microscopic images also confirmed that the droplet sizes of emulsions prepared with MFGM and MFGMPs were smaller than those prepared with MFGMLs. Moreover, the emulsions prepared with MFGM and MFGMPs had lower shear stress than those prepared with MFGMLs. The MFGM and MFGMPs were found to be sensitive to pH; thus, their physical stabilities behaved according to their pH. The heat treatment results showed that the emulsions prepared with MFGM were stable over the heat of temperature ranging from 35°C to 85°C, whereas those prepared with MFGMPs were stable to heat of temperature ranging from 35°C to 65°C and became unstable at the temperature of over 65°C.
Introduction
Milk fat globule membrane (MFGM), a complex biological membrane surrounding the fat globules in raw milk, is composed of polar lipids (PLs) and specific membrane fat globule proteins (MFGMPs). There has been a current growing trend to substitute synthetic emulsifiers for others obtained from natural sources.[Citation1] Because MFGM and each of its component possess amphiphilic properties, they not only can serve as natural emulsifying agents but also are considered good emulsifiers.[Citation2,Citation3]
In recent years, MFGM and its components have attracted wide attention owing to their various nutritional functions that have been reported, and among these functions, the most important properties involving effects to health are antibacterial,[Citation4] antiviral, and antiadhesive properties.[Citation5–Citation7]
MFGM can be separated either from fresh milk or from dairy by-products such as buttermilk and butter serum, and particularly in the dairy by-products, which are rich in MFGM materials. Therefore, isolation and proper use of the MFGM properties can greatly increase the value-added to the dairy products. Moreover, the emulsion properties of MFGM are linked to its compositions, which are highly dependent on the separation methods and the source materials.[Citation8,Citation9]
Although there have been reports focusing on the emulsion of MFGM materials, and the combination of MFGM proteins and PLs,[Citation9,Citation10] the emulsifying activities of whole MFGM and each of its components, such as milk fat globule membrane proteins (MFGMPs) and milk fat globule membrane lipids (MFGMLs), are still unclear. To better understand the participation of MFGM and its components, further research is necessary to test their emulsion properties. Therefore, this study aimed to evaluate the emulsion properties of MFGM and its components.
Materials and methods
Materials
Soybean oil (Shangdong Xiwang Foodstuffs Co, Ltd, Shandong Province, China) was purchased from the local market. MFGM and its components were isolated from fresh bovine milk as described next.
Isolation of the MFGM and its components
The complete MFGM, MFGMP, and MFGML were isolated from fresh bovine milk. The fresh bovine milk was fractionized using a 9NDS-50A cream separator (Qinghai Kangping, China). The resultant cream was then isolated, washed with phosphate buffer (PBS, 0.1 M, pH 6.8), and centrifuged at 1500 × g for 10 min. The supernatant was collected and washed three times with phosphate buffer. The washed cream was then subjected to the isolation of MFGM, MFGMP, and MFGML as follows. (1) Separation of complete MFGM using the acidification method previously reported by Kanno and Kim.[Citation11] The washed cream was allowed to crystallize for 4 h at 4°C prior to churning in a mixer. The resulting butter and buttermilk were separated using a sieve. The pH of the buttermilk fraction was then adjusted to 4.8 using 1 M hydrochloric acid to precipitate the complete MFGM. The solutions were then centrifuged at 10000 × g, and both the pellet containing MFGM and supernatant were collected. After that, the pH values of the pellet that was resuspended in water and of the supernatant were adjusted to 6.8 by 1 M sodium hydroxide. Finally, the isolated complete MFGM was subjected to freeze-drying. (2) Fractionation of MFGMP, which was based on the procedure reported previously by Lu et al.[Citation12] The washed milk cream was first mixed with 0.4% sodium dodecyl sulfate (SDS) (1:1, v/v) and then sonicated for 1 min. The mixture was subsequently centrifuged at 1500 × g for 10 min. The resulting MFGMP (bottom layer) was separated from fat (top layer), and dialyzed against deionized water, followed by freeze-drying. (3) Separation of MFGML based on the procedure reported by Tang et al.[Citation13] Following (2), the top layer containing fat was separated by centrifugation at 6000 × g for 15 min. The sample was then stirred at 100 r/min for 30 min and further centrifuged at 3000 × g for 15 min to get rid of butter. The MFGML was then collected from the bottom layer, which was subsequently concentrated by a rotatory evaporator.
Composition analysis of the MFGM and its components
The content analyses of MFGM and its components were carried out using official AOAC methods, and moisture (14.004), ash (14.006), and crude protein (47.021) were determined.[Citation14] Nitrogen to protein conversion factor of 6.25 was used in the crude protein determination. The lipid content was calculated by subtraction of the percentage of moisture, protein, and ash from the whole mass. All experiments were conducted in triplicates.
Transmission Electron Microscopy
MFGM samples were visualized by transmission electron microscopy (TEM), and prepared according to Lopez et al.[Citation15] The samples were fixed overnight at room temperature with 25 g/kg glutaraldehyde in 0.1 M sodium cacodylate, washed four times, each for 15 min by 0.1 M sodium cacodylate, pH 7.2, and washed five times with distilled water, each for 5 min. Subsequently, they were post-fixed in 1% osmium tetroxyde in 0.1 M sodium cacodylate for 1 h at room temperature, and washed four times, each for 15 min with the 0.1 M sodium cacodylate, pH 7.2 and washed five times, 5 min each, with distilled water. The samples were then dehydrated by a series of ethyl alcohol concentrations (determined by grades) as follows: 50° for 30 min, 70° for 1 h, 80° for 1 h, 95° for 1 h, and three times of 100° for 30 min each, and then were left soaking in 100° ethyl alcohol overnight at room temperature. Finally, the samples were infiltrated with Epon (Electron Microscopy Sciences Epon Kit 14120) in hydroxyl-propyl methacrylate (HPMA) and embedded in Epon with 2.5% v/v benzyldimethylamine (BDMA) resin mixture prior to polymerization at 60°C for 24 h. The samples were sectioned into ultrathin slides, each with a thickness of 90 nm using a Leica ultra-microtome and stained with 4% uranyl acetate for 1 h. These slides were then visualized by a JEOL 1400 Transmission Electron Microscope operated at 120 kV. The images were taken by a camera Gatan Orius SC 1000.
SDS-PAGE of MFGMPs
The MFGMPs were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDA-PAGE) as described previously by He et al.[Citation16] The samples were suspended in 0.5 mL of reducing buffer (6% Tris-0.5 M, 10% glycerol, 5% β-mercaptoethanol, 2% SDS, and 0.05% bromophenol blue), heated in boiling water for 5 min, and then centrifuged at 2500 × g for 30 min in order to remove fat. Then, 10 mL of the supernatants was loaded onto 12% SDS-polyacrylamide gels along with markers with molecular masses ranging from 14.4 to 116 kDa (TransGen Biotech, Co., Ltd., China). The voltage was set to 200 V. The gels were stained in a solution containing Coomassie Brilliant Blue R-250 to visualize the protein bands. Finally, the gels were destained in a solution containing methanol and glacial acetic acid at concentrations of 160 and 10 mL /L, respectively.
Emulsions preparation
Oil-in-water emulsions were prepared by mixing 10% soybean oil and MFGM or its components according to the procedure described by Phan et al.[Citation9] MFGM or its components (MFGMP or MFGML) were dispersed in soybean oil and sodium phosphate buffer, pH 7.0 at ratios of 0, 1, 2, 3, and 4 g MFGM (or its components) per 100 g emulsion. The mixtures were then stirred for at least 3 h using a magnetic stirrer and were left overnight at 4°C to allow complete hydration. Then, the soybean oil and aqueous phase were mixed and heated at 50°C for 10 min. After that, the mixtures were homogenized using a high-speed homogenizer (high-speed homogenizer, AD200-H, Angli Corporation, Shanghai, China) at 13,000 rpm for 5 min, followed by a high-pressure homogenizer (APV-1000 homogenizer, Wilmington, MA, USA) at 100 MPa for three times. The resulting emulsions were stored at 4°C.
Characterization of emulsions
Measurement of particle size distribution
The average particle diameter was determined by the laser scattering method using a Malvern Mastersizer 2000 (Malvern Instruments Ltd., Worcestershire, UK). Prior to measurement, the emulsions were dispersed in water or 1% SDS solution. The volume weighted mean diameter D4,3 was the parameter used to monitor changes in the droplet size distribution. Each measurement was carried out in triplicate and the average values were calculated.
Microscopic observation
Microscopic observation of the emulsions was carried out using an Olympus Color View camera microscope (Olympus, Aartselaar, Belgium). The emulsions were diluted 20 times in deionized water to reduce their density. Each emulsion sample was carefully dropped on a glass slide and covered with a coverslip, while ensuring that there was no air trapped inside. The slides were allowed for equilibration for 3 min prior to imaging at 50× magnification.
Rheological behavior of emulsions
The rheological behaviors of the emulsions were measured using a Kinexues rheometer (Malvern Instruments,England). The emulsion samples were gently mixed and passed through a syringe to ensure homogeneity. Samples of 20 g (each) were transferred into the cup of the rheometer. Flow curves were measured at a temperature of 25°C with increasing shear rates of 0.1 to 100 s−1. The experimental data obtained were then fitted with the power law equation: Shear stress = K × (shear rate)n
where K is the consistency index and n is the flow behavior index.
Influence of various conditions on emulsion stability
Influence of storage time
To determine the influence of storage time on phase separation, 15 mL of emulsion was transferred into graduated tubes of 10 mm diameter and quiescently stored for 10 d at 4°C. The volume of the serum layer formed at the bottom of the tube was recorded every 2 d. The influence of storage time on emulsion stability was expressed as a percentage of separation as follows:
Influence of pH
The pH of each emulsion sample was adjusted to 2, 4, 6, 8, and 10 using HCl or NaOH solutions. The samples were then diluted by 100-fold with the phosphate buffer (to avoid multiple scattering) prior to measurement of their zeta-potentials.
Influence of temperature
The emulsions were heated for 30 min in water baths that were set to different temperatures (35°C, 45°C, 55°C, 65°C, 75°C, and 85°C). The heated emulsions were then cooled down to room temperature using ice water. Particle size and zeta-potential were then measured.
Statistical analysis
The statistical differences between mean values were analyzed by the one-factor analysis of the variance and the least significant difference (LSD) at a significance level of 0.05. The LSD values were calculated based on Tukey’s post hoc test using the Statistical Package for the Social Sciences v. 10.0 (SPSS, Chicago, IL, USA).
Results and discussion
Microstructure and composition analysis of MFGM fractions
As shown in , the TEM image of the ultrathin sections of MFGM showed the presence of MFGM fragments. The image showed elongated structures of MFGM fragments, indicating that after the milk fat globules were disrupted by churning, the MFGM fell off from the surface of the fat globules. In addition, proteins were seen to connect two MFGMs. This microstructure of the MFGM fragments observed in this work was in good agreement with the TEM images previously observed in buttermilk[Citation17–Citation19] and industrial butter serums.[Citation19–Citation22]
Figure 1. Transmission electron microscopy (TEM) images of the milk fat globule membrane: (a) dispersion; and (b) SDS-PAGE.
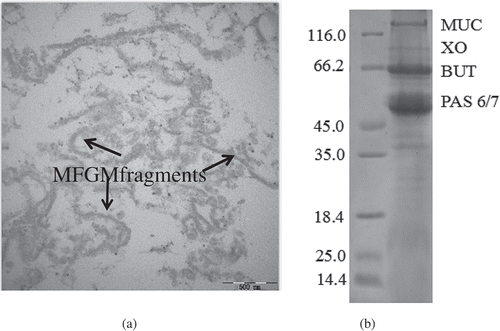
The MFGMPs isolated from fresh bovine milk was found to contain about 85% protein and 12% lipids. While the complete MFGM was composed of 73.41% lipid and 25.24% proteins, those of the isolated MFGML contained 94.47% lipid and 0.35% protein. The SDS-PAGE showed that the MFGMPs were composed of mucin 1 (MUC), xanthine oxidase (XO), butyrophilin (BTN), and periodic acid-Schiff 6 and 7 (PAS6 and PAS7). The result corresponded to that previously reported.[Citation2,Citation22] Moreover, no casein and whey proteins were found in the MFGM samples ().
Particle size distribution of emulsions
The particle size distribution of emulsions prepared with MFGM and its components is shown in . When the emulsions were diluted with water, the emulsions of MFGM and MFGMP had narrower particle size distributions compared with that of MFGML, indicating that the oil droplets were aggregated in the emulsion of MFGML. In addition, the particle size distribution of the MFGM and MFGMP emulsions became narrower when their concentrations increased. The average particle sizes (d4,3) of emulsions from MFGM, MFGMP, and MFGML after being diluted in water or in 1% SDS solution to different concentrations (1–4%) are shown in . The results showed that the emulsions of MFGML had larger droplets size than those of MFGM and MFGMP of the same concentrations. This may be due to that the MFGML contains low content of proteins, which could lead to the low repulsive barrier so that bridging flocculation of the droplets and phase separation occurred quickly. Phan et al. also reported that emulsions prepared with MFGMPs generally had smaller droplet sizes than those prepared with PLs when compared at the same concentrations.[Citation10] The droplet size of emulsions prepared with MFGM and MFGMP decreased with increasing concentrations. MFGM primarily consists of membrane-specific proteins and MFGMLs, which can be used as emulsifying agents owing to their amphiphilic natures. Because the PLs of MFGM may lower the interfacial tension, the combination of MFGMPs and PLs could strengthen the stability of emulsions prepared with MFGM.[Citation10,Citation23,Citation24] Therefore, the emulsions prepared with MFGM had significantly lower droplet size. Moreover, the droplet size of emulsions prepared with MFGMPs was significantly lower than that prepared with MFGML. This could be due to that the MFGMP fractions such as XO and BTN may have good emulsifying properties, which helped maintain emulsion stability.[Citation24] Furthermore, the droplets may be preserved by MFGMP at the oil droplet surfaces as a result of a high repulsive barrier of steric and electrostatic forces, thus preventing aggregation of the fat droplets.[Citation10]
Table 1. Mean diameter d4,3 in μm of the emulsion prepared with MFGM, MFGML, and MFGMP after being diluted in water or 1% SDS solution.
Figure 2. Particle size distribution of emulsions prepared with MFGM (a), MFGMP (b), and MFGML (c). The concentrations of MFGM, MFGMP, and MFGML were varied from 1% to 4%. The measurements were carried out after dilution in water.
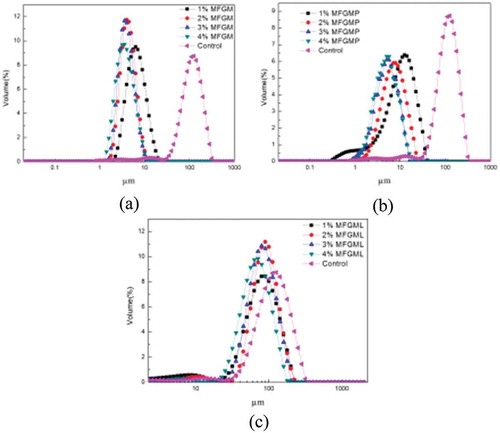
The average particle size (d4,3) of emulsions prepared with different concentrations of MFGM, MFGMP, and MFGML diluted in water was significantly larger than that diluted in SDS. SDS can serve as a small molecular weight surfactant and displace the proteins at the interface, leading to the disruption of bridging flocculation. Roesch et al. reported that emulsions diluted with SDS in solution could prevent the droplets from aggregation.[Citation23] Thus, when emulsions are diluted in SDS solution, the possible size distribution is based on individual droplet size rather than the size of the aggregated or flocculated fat.[Citation23] The emulsions prepared with 4% of either MFGM or MFGMPs had small droplet size in both water and 1% SDS. Therefore, 4% of MFGM or MFGMPs was selected as the optimum concentration in the preparation of emulsions.
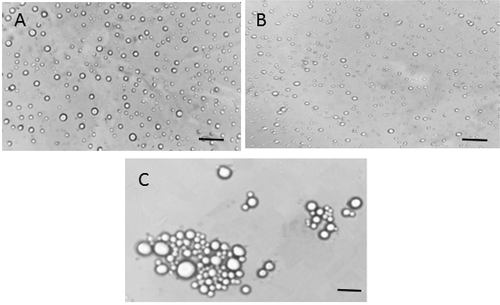
Microscopic observation
The microscopic images of emulsions prepared with MFGM and its components are presented in . The images showed that emulsions prepared with MFGML contained many large aggregates (). This could be due to that the MFGML had unprotected protein, which was unable to prevent the fat droplets from aggregation. In the absence of or in a low concentration of MFGMP, the emulsion contained insufficient active agents to cover the entire freshly created surfaces; thus the larger size of oil droplets might be formed.[Citation25] These results are in agreement with the fact that emulsions prepared with MFGML showed large particle size distribution. The sizes of fat globule droplets in emulsions prepared with MFGM and MFGMP were smaller than that prepared with MFGML, indicating that MFGM and MFGMP had good emulsifying properties ( and ). Thus, it could be concluded that both MFGM and MFGMP were required to stabilize oil-water emulsion, which were dependent not only on the ratio of lipids to proteins, but also on the compositions of MFGMPs.
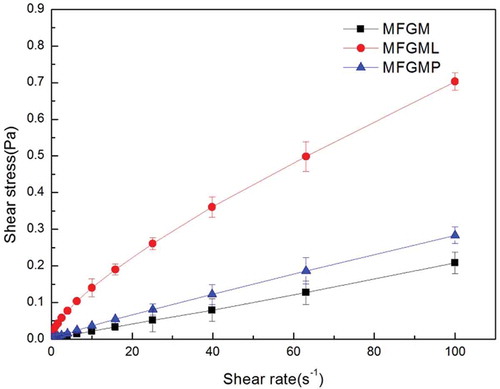
Rheological behaviour of emulsions
The curves of shear stress versus shear rate of MFGM, MFGMPs, and MFGMLs are shown in , and the parameters (in the emulsions preparation) contributed to a change in rheological behaviour with the coefficients of determination (R2), which are listed in . It could be seen from that the flow behaviors of emulsions prepared with MFGM and MFGMP showed very low shear stress against shear rate, similar to that of Newtonian flow. On the other hand, emulsions prepared with MFGML showed a high shear stress against shear rate. The higher shear stress observed from the MFGML emulsions indicated that they had higher viscosity compared with those of MFGM and MFGMP emulsions. As shown in , the consistency index (K) of MFGML emulsions was significantly higher than those of MFGM and MFGMP. Furthermore, the power law indices of MFGM and MFGMP emulsions were significantly lower than that of MFGML. No significant difference was observed in the power law indices between the MFGM and MFGMP emulsions. Emulsions prepared with MFGM had low shear stress and fluid-like flow behaviour (as indicated by the index n, which was close to 1). This may be because MFGM contains both MFGMP and PLs, which could contribute to the increase of emulsion fluidity.
Table 2. Flow curve parameters of emulsions prepared with 4% each of MFGM, MFGMP, and MFGML.
Stability of emulsions
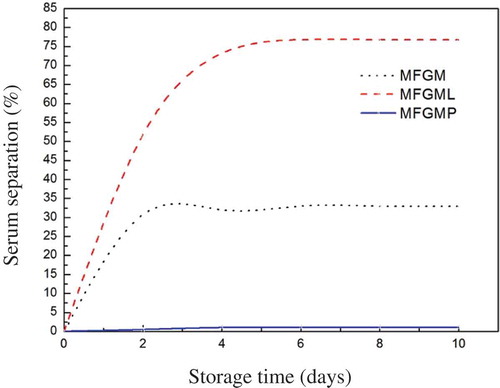
Influence of storage time on emulsions stability
Emulsion stability is important for shelf life, consumer acceptability, and food structure required for pleasant feeling during consumption.[Citation26] Many factors can influence the stability of emulsions, and these can include MFGM and its components, pH, and temperature. shows the stability of emulsions prepared with MFGM, MFGMP, and MFGML. The results demonstrated that there was no visible phase separation after 10 d of storage from emulsions prepared with MFGMP. Such excellent stability of this emulsion could be attributed to the presence of proteins, which could form a thick viscoelastic membrane around the oil droplet surface, acted as a protective coating, and retarded droplet coalescence by both electrostatic and steric stabilization.[Citation10] In contrast, the emulsions prepared with MFGML showed obvious phase separation, which may be the result of coalescence or bridging flocculation. Phan et al. reported that the degree and rate of phase separation of emulsions prepared with PLs were higher compared with those prepared with proteins.[Citation10] In addition, the degree and rate of phase separation of emulsions prepared with MFGM were higher than that prepared with MFGMP. This could be attributed to the ratio of lipids to proteins, in which MFGM contained large amounts of the PLs that did not contribute to emulsion stability.[Citation10] Moreover, in a low content of MFGMP environment, the fat globule lacked adsorbed proteins, causing the globules to combine by bridging flocculation and leading to phase separation. Although MFGM could contribute to small fat droplet size of the emulsion, phase separation may still occur in MFGM emulsion after a long period of storage.
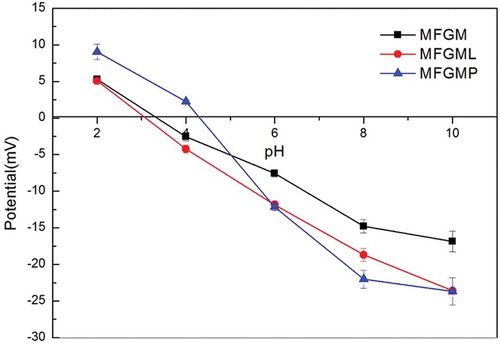
Zeta-potential of emulsions as a function of pH
shows the effect of pH on the zeta-potential of MFGM, MFGMP, and MFGML emulsions. The zeta-potentials in all emulsions were positively charged at pH 2 and negatively charged at pH 10.0. Zero charges were observed around pH values of 3.0, 3.2, and 4.2 for MFGML, MFGM, and MFGMP, respectively. These results showed that the compositions of MFGM were sensitive to pH; as a result, its physical stability was dependent on the behaviors of both MFGMP and MFGML as functions of pH. The magnitude of the zeta-potential of MFGMP was slightly higher than those of MFGM and MFGML either at high or low pH, which could be ascribed to the differences between the numbers of anionic and cationic groups presented in the samples.
Effect of temperature on emulsions stability
Emulsions are thermodynamically unstable systems that consist of two immiscible liquid phases, wherein one of these is dispersed within the other as small, spherical droplets of diameters typically ranging from 0.1 to 100.0 μm.[Citation27] The effects of heat treatment (35–85°C, 30 min) on the zeta-potential and mean particle diameter of the emulsions prepared with MFGM and its components are shown in . The zeta-potential of emulsions prepared with MFGM, MFGML, and MFGMP were only slightly fluctuated, and no significant changes were observed after the heat treatment over the temperature range studied (). These results indicated that the numbers or locations of the charged groups in the droplet were only slightly altered by the heat. This may be due to the repulsive interactions (electrostatic and steric), such as van der Waals and hydrophobic were strong enough to overcome other attractive interactions.
Figure 7. Effect of temperature (35–85°C) on zeta-potential (a) and the mean particle diameter (b) of emulsions prepared with 4% each of MFGM, MFGML, and MFGMP.
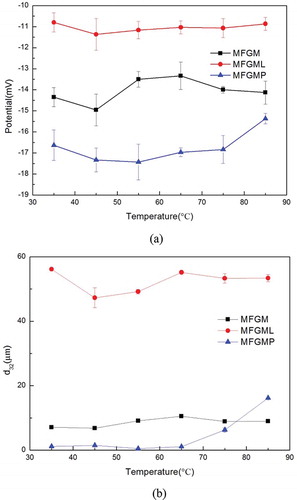
The mean particle diameter of the emulsions prepared with MFGML was the highest after heat treatment at 35°C in all temperatures (). This may be due to that lipids were more settled, which could easily lead to flocculation at low temperature, while they had better mobility at high temperature. There was no significant difference of the mean particle diameters among the MFGM emulsions after heat treatment at different temperatures. This suggested that the emulsions prepared with MFGM were stable to heat so that the oil droplet aggregation could be prevented. Moreover, the incorporation of MFGMP and MFGML caused the stability over heat of a wide range of temperatures. The mean particle diameter of the emulsions prepared with MFGMP was almost constant over the temperature ranging from 35 to 65°C (). However, the mean particle diameter of droplets significantly increased when the temperature was higher than 65°C. This may be due to that the adsorbed globular proteins underwent a conformational change upon heating, which led to exposures of nonpolar and sulfhydryl groups that were previously imbedded in the hydrophobic interior of the protein.[Citation28] Consequently, the surface hydrophobicity of the oil droplets increased, thus promoting strong hydrophobic interactions between the droplets. Thus, the thermal denaturation temperature,[Citation29] which led to instability of the MFGMP emulsions, was about 65°C.
Conclusion
This work demonstrated that the emulsions prepared with MFGM and MFGMPs had smaller droplets size than that prepared with MFGMLs. Those prepared with MFGM and MFGMP had low viscosity, which is similar to that of the Newtonian flow behaviour. The MFGML in the absence of proteins did not significantly contribute to emulsion stability. The emulsions prepared with MFGM showed heat stability to heat treatment at temperatures ranging from 35 to 65°C, whereas those prepared with MFGMPs are unstable when the heat temperature was over 65°C.
Funding
This work was supported by China Postdoctoral Science Foundation Grant (Grant nos 2012M520756 and 2014T70360) from the Chinese Postdoctoral Science Foundation Commission and the Fundamental Research Funds for the Central Universities (Grant no. HIT.NSRIF.2014094).
Additional information
Funding
References
- Perrechil, F.A.; Ramos, V.A.; Cunha, R.L. Synergistic Functionality of Soybean 7S and 11S Fractions in Oil-in-Water Emulsions: Effect of Protein Heat Treatment. International Journal of Food Properties 2015, 18, 2593–2602.
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Camp, J.V. Nutritional and Technological Aspects of Milk Fat Globule Membrane Material. International Dairy Journal 2008, 18, 436–457.
- Kanno, C. Emulsifying Properties of Bovine Milk Fat Globule Membrane in Milk Fat Emulsion: Conditions for the Reconstitution of Milk Fat Globules. Journal of Food Science 1989, 57, 1534–1539.
- Martin, H. M.; Hancock, J.T.; Salisbury, V.; Harrison, R. Role of Xanthine Oxidoreductase as an Antimicrobial Agent. Infection and Immunity 2004, 72(9), 4933–4939.
- Matsumoto, M.; Hara, K.; Kimata, H.; Benno, Y.; Shimamoto, C. Exfoliation of Helicobacter Pylori from Gastric Mucin by Glycopolypeptides from Buttermilk. Journal of Dairy Science 2005, 88, 49–54.
- Matsumoto, M.; Tani, H.; Ono, H.; Ohishi, H.; Benno, Y. Adhesive Property of Bifidobacterium Lactis LKM512 and Predominant Bacteria of Intestinal Microflora to Human Intestinal Mucin. Current Microbiology 2002, 44, 212–215.
- Saeland, E.; de Jong, M.A.W.P.; Nabatov, A.A.; Kalay, H.; Geijtenbeek, T.B.H.; Van Kooyk, Y. MUC1 in Human Milk Blocks Transmission of human Immu-nodeficiency Virus from Dendritic Cells to T Cells. Molecular Immunology 2009, 46, 2309–2316.
- Rombaut, R.; Van Camp, J.; Dewettinck, K. Analysis of Phospho- and Sphingolipids in Dairy Products by a New HPLC Method. Journal of Dairy Science 2005, 88, 482–488.
- Phan, T.T.Q.; Asaduzzaman, M.; Le, T.T.; Fredrick, E.; Van der Meeren, P.; Dewettinck, K. Composition and Emulsifying Properties of a Milk Fat Globule Membrane Enriched Material. International Dairy Journal 2013, 29, 99–106.
- Phan, T.T.Q.; Le, T.T.; Walle, D.V.D.; Van der Meeren, P.; Dewettinck, K. Combined effects of Milk Fat Globule Membrane Polar Lipids and Protein Concentration on the Stability of Oil-in-water Emulsions. International Diary Journal 2016, 52, 42–49.
- Kanno, C., Kim, D.-H. A simple Procedure for the Preparation of Bovine Milk Fat Globule Membrane and a Comparison of Its Composition, Enzymatic Activities, and Electrophoretic Properties with those Prepared by Other Methods. Agricultural and Biological Chemistry 1990, 54, 2845–2854.
- Lu, J.; Wang, X.Y.; Zhang, W.Q.; Liu, L.; Pang, X.Y.; Zhang, S.W.; Lv, J.P. Comparative Proteomics of Milk Fat Globule Membrane in Different Species Reveals Variations in Lactation and Nutrition. Food Chemistry 2015, 196, 665–672.
- Tang, H.S., He, S.H., Peng, F.S., Wang, R.C., Li Q., Ma, Y. The Effects of Milk Fat Globule Membrane and Its Individual Components on Dough Properties and Bread Quality. RSC Advances 2016, 6, 102617–102625.
- AOAC. Official Methods of Analysis, Association of Official Analytical Chemists, 15th edn.; Washington DC, 1990.
- Lopez, C.; Cauty, C.; Rousseau, F.; Blot, M.; Margolis, A.; Famelart, M. Lipid Droplets Coated with Milk Fat Globule Membrane Fragments: Microstructure and Functional Properties as a Function of pH. Food Research International 2017, 91, 26–37.
- He, S.H.; Ma, Y.; Wang, J.Q.; Li, Q.M.; Tang, S.H.; Zhao, C.H.; Li, H.M.; Maubois, J.L. Characterization of Fat Globules and Milk Fat Globule Membrane Proteins in Milk of Different Yak Breeds. Dairy Science &Technology 2010, 90, 601–609.
- Corredig, M.; Dalgleish, D.G. Isolates from Industrial Buttermilk: Emulsifying Properties of Materials Derived from the Milk Fat Globule Membrane. Journal of Agricultural and Food Chemistry 1997, 45, 4595–4600.
- Morin, P.; Jiménez-Flores, R.; Pouliot, Y. Effect of Processing on the Composition and Microstructure of Buttermilk and Its Milk Fat Globule Membranes. International Dairy Journal 2007, 17, 1179–1187.
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Organization of Lipids in Milks, Infant Milk Formulas and Various Dairy Products: Role of Technological Processes and Potential Impacts. Dairy Science and Technology 2015, 95, 863–893.
- Gallier, S.; Vocking, K.; Post, J.A.; van de Heijning, B.; Acton, D.; van der Beek, E.M.; van Baalen, T. A Novel Infant Milk Formula Concept: Mimicking the Human Milk Fat Globule Structure. Colloids and Surfaces B: Biointerfaces 2015, 136, 329–339.
- Lambert, S.; Leconte, N.; Blot, M.; Rousseau, F.; Robert, B.; Camier, B.; Gésan-Guiziou, G. The Lipid Content and Microstructure of Industrial Whole Buttermilk and Butter Serum Affect the Efficiency of Skimming. Food Research International 2016, 83, 121–130.
- Ye, A.; Singh H.; Taylor M.W.; Anema S. Characterization of Protein Components of Natural and Heat-Treated Milk Fat Globule Membranes. International Dairy Journal 2002, 12, 393–402.
- Roesch, R.R.; Rincon, A.; Corredig, M. Emulsifying Properties of Fractions Prepared from Commercial Buttermilk by Microfiltration. Journal of Dairy Science 2004, 87, 4080–4087.
- Vanderghem, C.; Bodson, P.; Danthine, S.; Paquot, M.; Deroanne, C.; Blecker, C. Milk Fat Globule Membrane and Buttermilks: from Composition to Valorization. Biotechnologie, Agronomie, Société et Environnement 2010, 14, 485–500.
- Urbina-Villalba, G.; Garcia-Sucre, M. Influence of Surfactant Distribution on the Stability of Oil/Water Emulsions towards Flocculation and Coalescence. Colloids and Surfaces A-Physicochemical and Engineering Aspects 2001, 190, 111–116.
- Ercelebi, E.A.; Ibanoglu, E. Stability and Rheological Properties of Egg Yolk Granule Stabilized Emulsions with Pectin and Guar Gum. International Journal of Food Properties 2010, 13, 618–630.
- Faria, J.T.D.; Oliveira, E.B.D.; Minim, V.D.R.P.; Minim, L.A. Emulsifying Properties of β-Lactoglobulin and QuillajaBark Saponin Mixtures: Effects of Number of Homogenization Passes, pH, and NaCl Concentration. International Journal of Food Properties 2016, 1–12.
- Day, L., Zhai, J.L., Xu, M., Jones, N.C., Hoffmann, S.V., Wooster, T.J. Conformational Changes of Globular Proteins Adsorbed at Oil-in-Water Emulsion Interfaces Examined by Synchrotron Radiation Circular Dichroism. Food Hydrocolloids 2014, 34, 78–87.
- Kanno, C.; Shimomura, Y.; Takano, E. Physicochemical Properties of Milk Fat Emulsions Stabilized with Bovine Milk Fat Globule-Membrane. Journal of Food Science 1991, 56, 1219–1223.