ABSTRACT
The effect of gelatinized kudzu starch on the formulation and stability of oil-in-water (O/W) emulsions was investigated. The effects of gelatinization conditions (75–95°C, 5–30 min) on the interfacial tension of O/W emulsions were observed. The selected conditions included gelatinization time of 20 min and temperature of 90°C. The second part of this study investigated the effect of homogenization methods on the stability of O/W emulsions. The effects of oil types (soybean oil, medium-chain triglycerides (MCTs), and limonene), oil weight fractions (5–30% (w/w)), and kudzu starch concentrations (0–5% (w/w)) were also investigated. The results indicated that rotor-stator homogenization in combination with high-pressure homogenization was suitable for the formulation of O/W emulsions. O/W emulsions containing 10% (w/w) soybean oil could be stabilized by 3% (w/w) gelatinized kudzu starch and may be used in future emulsified products.
Introduction
Kudzu root is a radix of the Pueraria plant, which originates from China and has long been used in the food and pharmaceutical industries. Kudzu root was recorded as a medicinal herb in the Shen Nong Ben Cao Jing 2000 years ago[Citation1]. Many food products and medicines in China and Japan contain kudzu root. It could be used as a natural starch source, since its starch content is about 51.6% ((w/w), dry mass)[Citation2], and the granule size of kudzu starch varies between 2 and 20 μm[Citation3]. The viscosity of gelatinized starch solution is higher than that of corn and lotus starches[Citation4], and it demonstrates good stability during heating and cooling [Citation5].
Starch, the main energy storage component of a plant, has two main fractions, amylose and amylopectin[Citation6]. The numerous applications of starch in food emulsions include thickening agent, viscosity modifier, and texture enhancer. Starch particles increase the viscosity of the continuous phase of an emulsion system and prevent gravitational separation of the suspended matter[Citation7]. Modification of starch particles by jet steam cooking produces a very thin layer around the oil–water interface and stabilizes the soybean oil-loaded emulsion with 40% volume fraction[Citation8]. The complete solubilization and elimination of the secondary starch structure during jet cooking help stabilize the mixture of starch and oil both in dry state and in aqueous suspension[Citation9].
Starch granules cannot be dissolved in cold water; however, when starch suspended in water is heated, the granules absorb water and swell. If the temperature keeps increasing, birefringence may be lost, leading to increased viscosity of the aqueous solution and eventual gelatinization[Citation10–Citation12]. Stable emulsion including gelatinized starch dispersion and sunflower oil was prepared by adding glycerol and lecithin, and its particle size decreased with increasing concentration of glycerol[Citation13]. Thus, gelatinized starch could be used as a wall material for the encapsulation of oil.
Emulsion is a mixture of two immiscible liquids in which one liquid is dispersed as spherical droplets into the other phase. In general, emulsion-based foods must be kept stable for a certain amount of time during processing, storage, transportation, and utilization. Nevertheless, emulsions become unstable with time, temperature, and other conditions such as creaming, sedimentation, flocculation, coalescence (extensive droplet can lead to oiling-off), and phase inversion[Citation7]. Surfactants (e.g., proteins, polysaccharides, and different hydrocolloids) are generally used as stabilizers or emulsifiers in an emulsion system. These emulsifiers or stabilizers decrease the interfacial tension between the dispersed phase and the continuous phase, while increasing the steric hindrances and/or electrostatic repulsion between the droplets and in turn stabilizing the emulsion system[Citation7, Citation14–Citation15].
Kudzu starches from Vietnam, Japan, and Korea have different thermal properties. The gelatinization temperature of starch from Vietnam ranges from 78.9 to 83.4°C; that of starch from Japan ranges from 65.3 to 83.4°C; and that of starch from Korea ranges from 57.5 to 73.0°C[Citation3]. Based on these values, we selected 75–90°C as the optimum gelatinization temperature range for this study. Villamonte et al.[Citation16] found that high-pressure treated and gelatinized corn starch could formulate and stabilize emulsions due to starch fragmentation. Therefore, gelatinized kudzu starch may have the potential for emulsion stabilization without the addition of other surfactants. The purpose of the present study was to investigate the effects of gelatinized kudzu starch on the formation and stabilization of oil-in-water (O/W) emulsions for developing a new application of kudzu starch in food emulsion.
Materials and methods
Materials
Commercial kudzu root starch used in this study was procured from Ohsawa-Japan Co., Ltd. (Tokyo, Japan). Limonene, soybean oil, sodium azide (NaN3, analytical grade), Sudan IV dye, and fluorescein-4-isothiocyanate (FITC) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Medium-chain triglyceride (MCT) was purchased from the Nisshin OilliO Group, Ltd. (Tokyo, Japan). All other chemicals used were of analytical grade and were used without further purification.
Interfacial tension measurement
Interfacial tension between gelatinized kudzu starch dispersion and soybean oil was measured by the pendant drop method (DM-501, Kyowa Interface Science Co., Ltd. Saitama, Japan) at 25°C using a water bath. Prior to interfacial tension measurements, the densities of gelatinized kudzu starch dispersions (0–3%, (w/w)) and different oils were determined using a density/specific gravity meter (DA-130N, Kyoto Electronics Co., Ltd., Kyoto, Japan). For interfacial tension measurements, samples were loaded in a syringe and pushed out to form a pendant drop at the tip of a stainless-steel needle surrounded by the oil phase. Every sample was measured at least 10 times and the average values were used for analysis.
Preparation of O/W emulsions
The kudzu starch dispersion was prepared by adding different volumes of Milli-Q water. The mixture was gelatinized by constant stirring at 90°C for 20 min, following the findings of Van Hung and Morita[Citation3], and the effects of different gelatinization conditions on interfacial tension were observed. The fresh gelatinized kudzu dispersion, which was used as the continuous phase, contained 0.02% (w/w) NaN3 as an antimicrobial agent. Soybean oil, MCT, or limonene was used as the dispersed phase.
The O/W emulsions were prepared using gelatinized kudzu starch dispersions as the continuous phase, while different oils served as the dispersed phase. The coarse emulsions were made using a rotor-stator homogenizer (Polytron PT-3000, Kinematica AG, Lucerne, Switzerland) at 10,000 rpm for 5 min. The mixtures were homogenized immediately using a high-pressure homogenizer (Nano Vator NV200, Yoshida Kikai, Co., Ltd. Japan) at 100 MPa in two cycles. The effects of starch concentrations (0, 0.1, 1, 3, and 5 (w/w)) and oil weight fractions (5, 10, 20, and 30% (w/w)) on the stability of O/W emulsions were investigated. Emulsions formulated using kudzu starch, rice starch, corn starch, and wheat starch were observed in this study.
Determination of O/W emulsion particle-size and particle-size distribution
The average particle sizes of emulsions were expressed as Sauter mean diameter (d3,2) and were detected using a laser diffraction particle size analyzer (LS 13320, Beckman Coulter Ltd., Brea, USA). The d3,2 was calculated as follows:
where di is the droplet diameter and ni is the number of droplets having diameter di. Particle-size distribution was represented by the relative span factor (RSF) and was estimated as follows:
where d90, d50, and d10 are emulsion particle-size diameters corresponding to the cumulative distributions at 90%, 50%, and 10%, respectively.
Determination of creaming stability
Due to gravity and the low density of oil, the droplets in emulsions started to move upward during storage, and a creaming layer could be easily observed. The O/W emulsions were poured into test tubes, sealed with screw caps, and stored at ambient temperature for 9 days. The creaming index was calculated using the following equation:
Here, HE (mm) is the height of the creaming layer and HL (mm) is the height of the emulsions.
Morphological analysis
The morphology of emulsions stabilized by gelatinized kudzu starch dispersion was observed using an optical microscope (DFC300FX, Leica Microsystems, Wetzler, Germany). The microstructure, distribution of droplets, starch retro-gradation, and coalescence of emulsions were the major parameters observed during this study.
Confocal laser scanning microscopy
The microstructures of emulsion droplets formulated under optimized conditions were observed using a Leica TCS SP8 confocal laser scanning microscopy (CLSM, Leica Microsystems Inc., Heidelberg, Germany), which was equipped with argon ion laser and a multiphoton confocal microscope. Excitation wavelength of 488 nm was used during this study. Before homogenization, gelatinized kudzu starch dispersion was stained using a small amount of 0.002% FITC overnight at ambient temperature. All confocal fluorescence pictures were taken at 40× objective (40× objective: oil immersion, numeric aperture 1.30).
Viscosity measurement
The viscosity of gelatinized kudzu starch dispersion and different oils was measured using a Vibro Viscometer (SV-10, A&D Company Ltd., Tokyo, Japan) at room temperature. Thin sensors including sensor plates (vibrators) and a temperature sensor were immersed into a sample that was collected in a 35 mL measuring vessel. Viscosity was then determined by resonating current and amplitude[Citation17].
Statistical analysis
All experiments were carried out in at least duplicate, and the results are reported as the average and standard deviation of measurements. One-way analysis of variance (ANOVA) was performed using Statistix 8.1 software (Tallahassee, USA), and the least significant difference (LSD) was calculated at 95% probability level. The significant difference (p < 0.05) was presented using different letters.
Results and discussion
Effects of gelatinization time and temperature based on interfacial tension
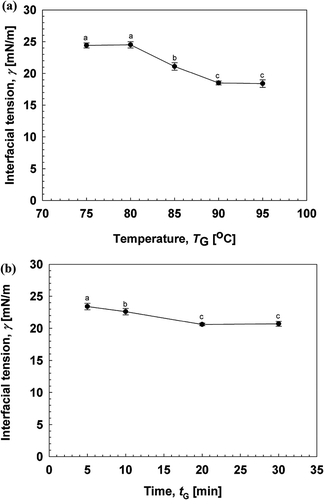
Interfacial tension reduction between oil and water was regarded as the main driving force to form O/W emulsions[Citation7]. indicates the interfacial tension (γ) between 3% (w/w) gelatinized kudzu starch dispersion and soybean oil. The γ between Milli-Q water and soybean oil was 25.5 mN/m at 25°C, while kudzu starch dispersion after gelatinization was more capable of reducing γ than the Milli-Q water system. γ decreased with increasing gelatinization temperature and decreased to 18.4 mN/m at 90°C (). Moreover, gelatinization time was an important parameter that affected γ (). Reduction of γ correlated directly with gelatinization time and maintained a constant value after 20 min of heating at 90°C. Suitable conditions for kudzu starch gelatinization include a heating time of 20 min at 90°C.
Figure 1. Effects of gelatinization conditions of kudzu starch on the interfacial tension. (a) Effect of gelatinization time on interfacial tension between soybean oil and gelatinized kudzu starch dispersion. (b) Effect of gelatinization temperature on interfacial tension between soybean oil and gelatinized kudzu starch dispersion. The different letter shows significant difference at the 95% probability level.
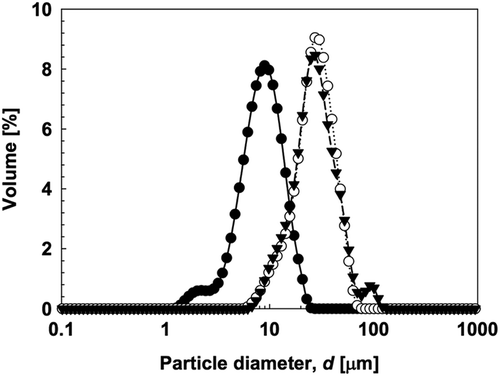
To clarify the physical properties of gelatinized kudzu starch, the particle diameter of kudzu starch dispersion was determined and compared with native kudzu starch granules (). The d3,2 of gelatinized kudzu starch dispersion exceeded that of native starch granules (7.42 μm). The increase in d3,2 was due to granule swelling. Storage time had almost no influence on the d3,2 of the gelatinized kudzu starch dispersion.
Figure 2. The particle size distribution of native kudzu starch and gelatinized kudzu starch. (![]()
Thirty percent of a starch granule mass is regarded as the crystalline region and contains helices of amylopectin, double helix of amylose and amylopectin, V helix of amylose and enclosed lipid, and free amylose and free lipid. The remaining 70% mass is regarded as the amorphous region. The amorphous regions contain amylose and amylopectin. High temperature increases the solubility of starch in water and the crystalline regions melt and form a polymer network. Reduction in γ might be due to the formation of helical inclusion complexes with the lipid during high shear and high-pressure homogenization, where these complexes might reside at the newly created oil–water phase interface. These results correlated well with the previous study of Villamonte et al.[Citation16]. Moreover, kudzu starch contains traces of protein (0.14–0.18%) [Citation3], which might play a significant role in reducing γ during gelatinization.
Effect of different homogenization techniques on the stability of O/W emulsions
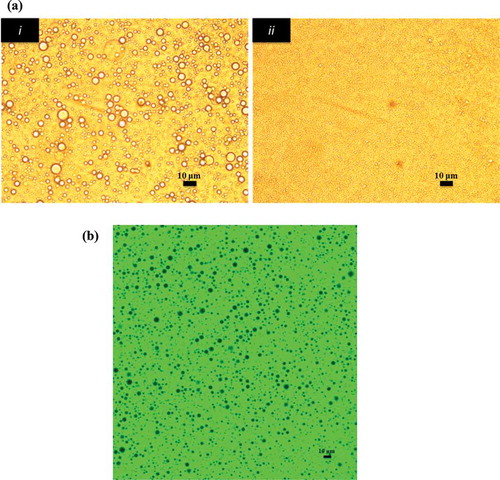
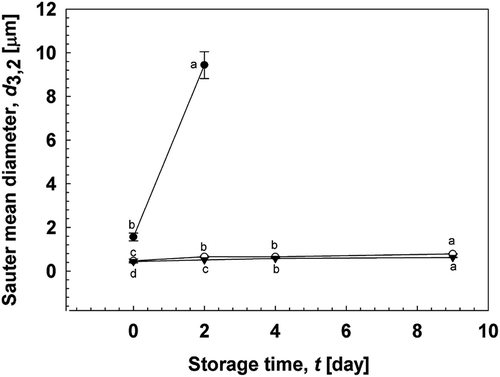
Two different homogenization techniques were used to check the stability of O/W emulsions stabilized by gelatinized kudzu starch dispersion. These techniques include the use of a rotor-stator homogenizer and a high-pressure homogenizer. The microstructure and stability of O/W emulsions were observed for 9 days. depicts the microstructure of emulsions using optical microscopy. These micrographs showed that homogenization has a pronounced effect on the particle size of emulsions. Particle size decreased to the sub-micron level with high-pressure homogenization. The stabilization process with smaller oil droplets () after high-pressure homogenization can also be observed under CLSM, which clearly shows oil droplets surrounded by gelatinized kudzu starch dispersion.
Figure 3. Microstructure of O/W emulsions. (a) Microstructure of freshly prepared O/W emulsions stabilized with gelatinized starch. (i) Formulated with rotor-stator homogenizer and (ii) formulated with high-pressure homogenizer. (b) Confocal laser scanning microscopy image of O/W emulsions stabilized by gelatinized kudzu starch dispersion.
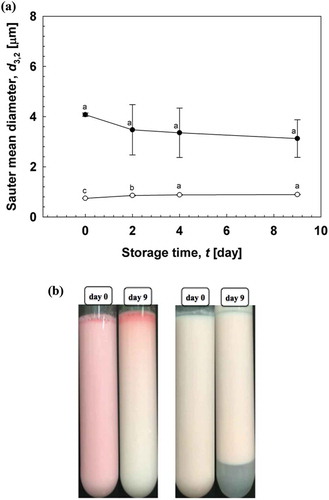
indicates the storage stability of O/W emulsions stored at ambient temperature for 9 days. The d3,2 of O/W emulsions significantly decreased with the use of the high-pressure homogenizer compared with the use of only the rotor-stator homogenizer. The d3,2 of O/W emulsions decreased from 4.01 to 0.74 μm using a combination of rotor-stator and high-pressure homogenizer. The RSF of the emulsions also decreased from 2.61 to 1.88. Oiling-off and creaming were observed in emulsions formulated using the rotor-stator homogenizer (), whereas slight phase separation was observed in emulsions formulated using the high-pressure homogenizer, especially after 9 days of storage. The particle size of emulsions below 1 μm cannot be achieved with rotor-stator systems; in contrast, mean droplet diameters below 0.2 μm can be obtained with high-pressure systems[Citation18]. Polydisperse O/W emulsions have better stability with smaller droplet sizes[Citation19]. These results confirmed that rotor-stator homogenization followed by high-pressure homogenization reduces d3,2 and may improve the storage stability of O/W emulsions stabilized with gelatinized kudzu starch.
Figure 4. Storage stability of O/W emulsions stabilized using gelatinized starch. (a) d3,2 of O/W emulsions formulated with rotor-stator homogenizer and/or high-pressure homogenizer. (![]()
Effect of kudzu starch concentration on the formulation and stability of O/W emulsions
indicates the effect of different kudzu starch concentrations (0–5% (w/w)) on the stability of O/W emulsions. The d3,2 of emulsions decreased with increasing concentration of kudzu starch, whereas d3,2 increased with storage time. Gelatinized kudzu starch dispersion has the capacity to decrease d3,2 at a specific concentration, correlating with a decrease in γ. However, d3,2 increased during storage when the concentration of kudzu starch was 5% (w/w), mainly due to the higher aggregation of starch particles.
Figure 5. (a) The d3,2 of O/W emulsions containing different kudzu starch concentrations. (![]()
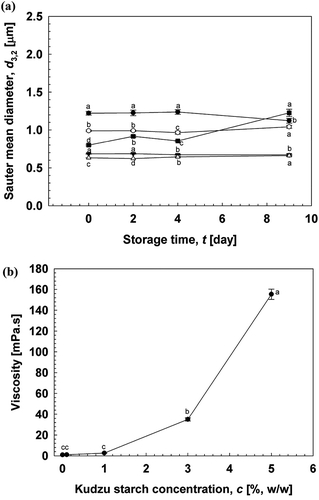
During storage (), oiling-off and creaming were observed in emulsions containing no starch dispersion. A similar destabilization phenomenon was observed in emulsions containing 0.1–1% (w/w) kudzu starch. Emulsions containing 3–5% (w/w) starch dispersion remained stable at day 1, but increase in d3,2 was observed in emulsions containing 5% (w/w) kudzu starch. The viscosity of gelatinized kudzu starch rapidly increased with increasing kudzu starch concentration (). The viscosity of 5% (w/w) gelatinized kudzu starch solution increased to 155.3 mPa.s after cooling at room temperature. A suitable viscosity range may be needed to increase the stability of O/W emulsions[Citation20–Citation21].
Table 1. The pictures of O/W emulsions containing different kudzu starch concentrations stored in test tubes at 25°C at days 0 and 9.
Effects of oil type on the formulation and stability of O/W emulsions
Oil type affects O/W emulsion formulation and stability[Citation22]. In this study, the effect of oil type (soybean oil, MCT, and limonene) on the stability of O/W emulsions was investigated. The density, viscosities, and γ between oil and Milli-Q water or gelatinized starch dispersions are listed in . Soybean oil was depicted to have high viscosity and gelatinized kudzu starch reduced γ, compared with Milli-Q water. Previous studies showed that the ratio of dispersed to continuous phase affected emulsification, and the ratio of 0.1:5 indicated good emulsion stability[Citation23].
Table 2. Physicochemical properties of dispersed phases used in the present study.
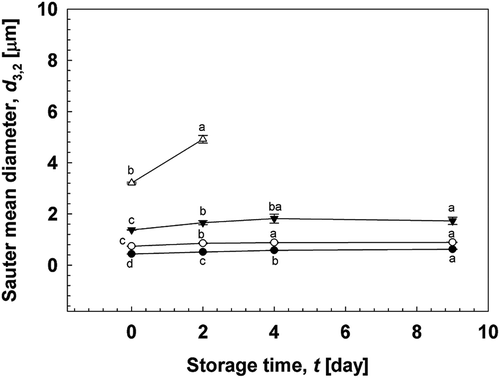
shows the d3,2 of O/W emulsions containing 5% (w/w) oil in the dispersed phase stabilized by 3% (w/w) gelatinized kudzu starch for 9 days. The d3,2 of fresh O/W emulsions containing soybean oil was 0.44 μm, that containing MCT was 0.47 μm, and that containing limonene was 1.56 μm. RSF varied between 1.42 and 2.07 with different dispersed phases. During the evaluated storage period, soybean oil or MCT oil-loaded O/W emulsions remained more stable than limonene-loaded emulsions. Oiling-off occurred in emulsions containing limonene after just 2 days, while creaming was observed in emulsions containing MCT after 4 days, and the creaming index increased from 2.0% to 5.1% during storage. These results agreed with the findings of Wooster et al.[Citation24], in which emulsions containing viscous oils, such as long-chain triglycerides (LCTs), remained stable against Ostwald ripening and were more stable than emulsions containing less viscous oils.
Figure 6. The d3,2 of O/W emulsions containing different oil types. The emulsions contained 5% (w/w) oil in the dispersed phase. (![]()
Limonene is a derivative flavour component of citrus oils. Nevertheless, soybean oil having an LCT structure and MCT are more insoluble in water than limonene. Thus, the stability of emulsions containing limonene was easily affected by Ostwald ripening and oiling-off occurred after 2 days.
Effect of oil weight fraction on the formulation and stability of O/W emulsions
To explore the emulsifying ability of gelatinized kudzu starch dispersion, emulsions containing 5–30% (w/w) soybean oil were formulated. The results are presented in . Oiling-off was observed in emulsions containing 30% soybean oil. Emulsions containing 5–10% (w/w) soybean oil remained stable during storage and had d3,2 of 1 μm, while creaming and oiling-off were observed in emulsions containing higher oil fractions (). These results were similar to the findings of Floury et al.[Citation25], in which the stability of O/W emulsions decreased linearly with the function of d3,2 and by increasing the oil phase volume. The slight increase in d3,2 might be due to the retrogradation of kudzu starch. Retrogradation of starch was observed due to the presence of water phase at the bottom of the test tube. Amylose and amylopectin may be retrograded and rearranged to reform a new crystalline shape; and water would be released during retrogradation, which might destabilize the emulsion system.[Citation26]
Figure 7. The d3,2 of O/W emulsions containing different soybean oil weight fractions. (![]()
Tabel 3. The micrographs of O/W emulsions together with pictures.
Effect of different starches on the formulation and stability of O/W emulsions
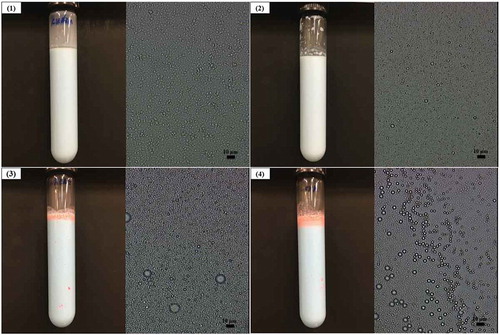
For comparative study, O/W emulsions were formulated using different gelatinized starches. The d3,2 of fresh emulsions was 0.57 μm for those containing gelatinized kudzu starch, 0.47 μm for those containing gelatinized rice starch, 0.44 μm for those containing gelatinized wheat starch, and 0.50 μm for those containing gelatinized corn starch. However, oiling-off occurred in emulsions stabilized by gelatinized rice starch, wheat starch, and corn starch stored at 25°C in just 24 h (). indicates that only emulsions containing gelatinized kudzu starch were stable. A few oil droplets on the surface of emulsions containing rice starch and their stability were better than that of those formulated by wheat starch and corn starch.
Figure 8. The microstructures of O/W emulsions kept at 25°C for 24 h stabilized by different gelatinized starches together with pictures. The oil phase was stained with Sudan IV dye, and the continuous phase was dyed with methylene blue for the observation of emulsions. (1) Emulsions stabilized with kudzu starch, (2) rice starch, (3) wheat starch, and (4) corn starch.
The emulsifying capacity of starch is improved through enzymatic treatment or acid hydrolysis, due to the reduced molecular weight of starch[Citation27–Citation28]. However, Fanta et al. found that dextran could not form an observable film at the oil–water interface, since the molecular weight was < 2 × 106, while the film was observed with a high molecular weight (5–40 × 106)[Citation8]. The weight-average molecular weights were 2.05 × 108 g/mol for kudzu starch, 8.53 × 108 g/mol for rice starch, 10.1 × 108 g/mol for wheat starch, and 11.3 × 108 g/mol for corn starch. These values fall in the range to form a stable emulsion system[Citation29–Citation30]. Moreover, the viscosity of kudzu starch was the highest after gelatinization. Hence, gelatinized kudzu starch was more suitable for stabilizing O/W emulsions than native rice starch, wheat starch, or corn starch.
Conclusion
According to the findings of this study, O/W emulsions could be stabilized using gelatinized kudzu root starch without a commercial or natural emulsifier. A combination of rotor-stator homogenization and high-pressure homogenization was suitable for formulating O/W emulsions based on gelatinized starch dispersion. Emulsions containing soybean oil were more stable than those containing low viscous MCT or limonene. The optimized gelatinized conditions were a gelatinization time of 20 min and a gelatinization temperature of 90°C. The limitation of emulsion stabilization includes a low volume fraction of oil phase. Moreover, 3% (w/w) gelatinized starch solution demonstrated better stability than the other tested concentrations. This stabilization method could be applied in food, medicine, and cosmetic industries because of the high-quality and high-value products in a cost-effective manner.
References
- Yan, B.; Wang, D.-y.; Xing, D.-m.; Ding, Y.; Wang, R.-f.; Lei, F.; Du, L.-j. The Antidepressant Effect of Ethanol Extract of Radix Puerariae in Mice Exposed to Cerebral Ischemia Reperfusion. Pharmacology Biochemistry and Behavior 2004, 78(2), 319–325.
- Zheng, L.; Furao, L.; Hui, W. Analysis of the Nutritional Components of Kudzu Vine Root. Modern Food Science and Technology 2011, 27, 1010–1019.
- Van Hung, P.; Morita, N. Chemical Compositions, Fine Structure and Physicochemical Properties of Kudzu (Pueraria lobata) Starches from Different Regions. Food Chemistry 2007, 105(2), 749–755.
- Geng, Z.; Zongdao, C.; Yimin, W. Physicochemical Properties of Lotus (Nelumbo nucifera Gaertn.) and Kudzu (Pueraria hirsute Matsum.) Starches. International Journal of Food Science & Technology 2007, 42(12), 1449–1455.
- Suzuki, A.; Hizukuri, S.; Takeda, Y. Physicochemical Studies of Kuzu Starch. Cereal Chemistry 1981, 58(4), 286–290.
- Stephen, A.M. Food Polysaccharides and their Applications; CRC Press: New York, NY, 1995; Vol. 67.
- McClements, D. Food Emulsions: Principles, Practice and Techniques; CRC Press: Boca Raton, Florida, USA, 2004; 20–50pp.
- Fanta, G.; Felker, F.; Eskins, K.; Baker, F. Aqueous Starch–oil Dispersions prepared by Steam Jet Cooking. Starch Films at the Oil–Water Interface. Carbohydrate Polymers 1999, 39(1), 25–35.
- Knutson, C.; Eskins, K.; Fanta, G. Composition and Oil-Retaining Capacity of Jet-Cooked Starch-Oil Composites. Cereal Chemistry 1996, 73, 185–198.
- Biliaderis, C. Thermal Analysis of Food Carbohydrates; Elsevier Applied Sciences: London, 1990; 168–220pp.
- Donovan, J.W. Phase Transitions of the Starch–Water System. Biopolymers 1979, 18(2), 263–275.
- Parker, R.; Ring, S. Aspects of the Physical Chemistry of Starch. Journal of Cereal Science 2001, 34(1), 1–17.
- Yılmaz, G.; Jongboom, R.; Van Soest, J.; Feil, H. Effect of Glycerol On the Morphology of Starch–Sunflower Oil Composites. Carbohydrate polymers 1999, 38(1), 33–39.
- Dickinson, E. Hydrocolloids as Emulsifiers and Emulsion Stabilizers. Food Hydrocolloids 2009, 23(6), 1473–1482.
- Rayner, M.; Timgren, A.; Sjöö, M.; Dejmek, P. Quinoa Starch Granules: A Candidate for Stabilising Food‐Grade Pickering Emulsions. Journal of the Science of Food and Agriculture 2012, 92(9), 1841–1847.
- Gina, V.; Vanessa, J.; Marie de L. Stabilizing Emulsions using High-Pressure-Treated Corn Starch. Food Hydrocolloids 2016, 52, 581–589.
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Monodisperse W/O/W Emulsions Encapsulating L-Ascorbic Acid: Insights on their Formulation using Microchannel Emulsification and Stability Studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 458, 69–77.
- Schultz, S.; Wagner, G.; Urban, K.; Ulrich, J. High‐Pressure Homogenization as a Process for Emulsion Formation. Chemical Engineering & Technology 2004, 27(4), 361–368.
- Chung, H.; Kim, T.W.; Kwon, I.C.; Jeong, S.Y. Stability of the Oil-in-Water type Triacylglycerol Emulsions. Biotechnology and Bioprocess Engineering 2001, 6(4), 284–288.
- Udomrati, S.; Khalid, N.; Gohtani, S.; Nakajima, M.; Neves, M.A.; Uemura, K.; Kobayashi, I. Effect of Esterified Oligosaccharides On the Formation and Stability of Oil-In-Water Emulsions. Carbohydrate Polymers 2016, 143, 44–50.
- Dickinson, E. Introduction to Food Colloids; Oxford University Press: London, UK; 1992.
- Qi, F.; Wu, J.; Sun, G.; Nan, F.; Ngai, T.; Ma, G. Systematic Studies of Pickering Emulsions Stabilized by Uniform-sized PLGA Particles: Preparation and Stabilization Mechanism. Journal of Materials Chemistry B 2014, 2(43), 7605–7611.
- Braginsky, L.; Belevitskaya, M. Kinetics of Droplets Breakup in Agitated Vessels. Liquid–Liquid Systems; Nova science: Commack, New York; 1996.
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24(22), 12758–12765.
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innovative Food Science & Emerging Technologies 2000, 1(2), 127–134.
- Ratnayake, W.S.; Jackson, D.S. Gelatinization and solubility of corn starch during heating in excess water: new insights. Journal of Agricultural and Food Chemistry 2006, 54(10), 3712–3716.
- Sweedman, M.C.; Tizzotti, M.J.; Sch€afer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydrate Polymers 2013, 92, 905–920.
- Yan, H.; Zhengsong, L.; Zhengbiao, G.; Yan, W.; Yansheng, P. Structure and emulsification properties of octenyl succinic anhydride starch using acid-hydrolyzed method. Starch 2016, 68, 1–9.
- Sang-Ho, Y.; Chandani, P.; Jianfu, S.; Liyang, Y.; Dong-soon, S.; Jay-lin, J. Molecular structure of selected tuber and root starches and effect of amylopectin structure on their physical properties. Journal of Agriculture and Food Chemistry 2009, 57, 1556–1564.
- David, G.S.; Atanu, B.; Jay-lin, J.; Georage, E.I. Chagens in structure and properties of starch of four botanical sources dispersed in the ionic liquid, 1-butyl-3-methylimidazolium chloride. Carbohydrate Polymers 2007, 67, 21–31.
