ABSTRACT
The effect of transglutaminase (TG) and ascorbic acid (AA) on the physicochemical properties of gluten was studied. The results showed that TG increased the gluten yield, storage modulus, and particle size distribution by forming a G-L bond in the protein structure. TG changes the three-dimensional structure of gluten by forming a G-L bond in the protein chains and partly preventing disulfide bond formation. However, in the high concentration of the enzyme, by reducing the ratio of lysine to glutamine, TG catalyzes the deamidation of glutamine residues and thus the K′, gluten yield, and particle size decrease significantly. The rheological behavior of glutenin macropolymer (GMP) gel was changed and the gluten extensibility increased. AA leads to an improvement in the aggregation of gluten and increases the particle size distribution. The results showed that AA leads to an increase in the disulfide bond formation and the strength of the gluten network. Adding AA reduced the delta value and increased G’ compared with the control.
Introduction
Wheat flour is a multicomponent system mainly composed of carbohydrates, proteins, lipids, and moisture; carbohydrates (mainly starch) and proteins (mainly gluten) are widely used in the food industry.[Citation1,Citation2] The flour components are responsible for creating the texture, taste, appearance, and other characteristics of the final product. The flour protein contains two main components: gliadin and glutenin; gliadin is the monomeric subunit of gluten and is divided into four groups according to the mobility in the electrophoresis gel. Glutenin can be divided in two groups: high-molecular-weight (HMW) glutenin and low-molecular-weight (LMW) glutenin.[Citation2–Citation4] The three-dimensional network of gluten is formed when mixing with the hydrated wheat flour protein, thus forming a bond between gliadin and glutenin. This structure is responsible for the viscoelastic properties of the dough. In addition, the interaction between gliadin and glutenin leads to beneficial properties in the final product.[Citation2,Citation5] Compounds such as potassium bromate and azodicarbonamide can be used to produce a strong gluten network; however, the use of these compounds is limited due to their carcinogenic properties. Therefore, the use of enzymes and compounds that have been recognized as safe and have no destructive effect on the human body after baking is an appropriate alternative.[Citation6] Transglutaminase (TG) is one of the enzymes used to improve the properties of protein in recent years.[Citation3] TG (EC 2.3.2.13) catalyzes the formation of an isopeptide bond between the γ-carboxyamide group of peptide-bound glutamine residues as acyl donors and a variety of primary amines as acyl acceptors, for instance, the ε-amino group of lysine residues or other amines. This crosslinking is principally formed in high-molecular-weight glutenin subunits (HMW-GS). However, this enzyme affects the low-molecular-weight glutenin subunits (LMW-GS), α-gliadin, and water-extractable proteins. In the absence of free amine groups, TG catalyzes the reaction of deamination. In this case, the water molecules act as acyl acceptors. On the other hand, by compressing the gluten structure during the formation of the isopeptide bond, the possibility of forming the G-L bond decreases and the water molecule acts as an acyl acceptor. These reactions that are catalyzed by TG could be due to the modification of the physicochemical properties of proteins.[Citation7–Citation10] Larsson and Eliasson demonstrated that 230 ppm of ascorbic acid (AA) could increase the gluten network strength. During mixing of the dough, AA was converted into dehydroascorbic acid under the influence of atmospheric oxygen and exiting natural oxidase enzymes in flour .[Citation11] Moreover, dehydroascorbic acid could oxidize the free sulfhydryl (SHf) groups and change the gluten structure by forming the disulfide bond.[Citation12,Citation13] Tsen showed that the SHf groups decreased in the presence of AA and dehydroascorbic acid.[Citation14] The formation of crosslinking as the result of AA and TG action could change the physicochemical properties of the gluten structure. The purpose of this work was modifying the physicochemical properties of gluten by adding AA or TG.
Materials and methods
Materials
Commercial wheat flour applied for all experiments was provided by Atlas Company, Isfahan, Iran. Wheat flour characteristics included 12.19% moisture content[Citation15] and 11.1% protein (Kjeldahl method, N 5.7). Optimal water absorption for flour was determined by a Farinograph. The TG was gifted by Arteezyme, Karaj, Iran, and AA was provided by Applychem, Germany. The activities of TG were 100 U/g of enzyme. (One unit of TG is defined as the amount of enzyme that releases 1 mmol of hydroxamic acid in 1 min at 37°C). The enzyme and AA doses used included: TG: 80, 200, 400, 600, and 800 ppm and AA: 50, 100, and 200 ppm. The doses used were recommended by the enzyme manufacturer and according to our pretest in the laboratory. Wheat flour without enzyme and AA was used as a control.
Physicochemical measurements
The yields of gluten, gluten index (GI), and water binding in wet gluten of the samples were determined by the AACC 38-12A method.[Citation15] For measuring the capacity of water binding, the difference between the weights of total wet gluten and total dry gluten is calculated and it gives the water bond in the wet gluten. Moreover, the content of protein recovery in the dry samples was determined by the Kjeldahl method.
Gluten rheological properties
The two parameters used were the maximum resistance to extension (Rmax) and maximum extensibility (E). Rmax is an indicator of gluten strength and higher Rmax indicates stronger gluten and hence more energy is needed to stretch the gluten. Extensibility assesses the deformation of the gluten before it is ruptured. The rheological properties of gluten were measured using a Kieffer extensibility rig fitted to a Texture Analyser as described by Kieffer and et al.[Citation16]
Characterization of GMP gel
GMP isolation
GMP isolation was conducted by the method of Wang et al.[Citation17] For defatted gluten, a freeze-dried gluten sample (3 g) was dispersed in 75 ml of petroleum ether, mixed for 20 min, and centrifuged with 18,900 g for 10 min at 5°C. All processes were repeated as in the same previous condition and the remaining petroleum ether was evaporated in a fume hood at night. Defatted gluten sample (80 mg) was dispersed in 10 ml 1.5% (w/v) SDS solution for 1 h at room temperature and ultracentrifuged with 69,000 g for 30 min at 20°C. The supernatant was discarded and the gel-like layer that was found on the pellet of the starch (called GMP) was weighed as the GMP wet weight.
Protein content of the GMP gel
The protein content of the GMP gel was determined by the Dumas method, after drying at 80°C for 2 h.
Rheometer measurements
Rheological measurements were conducted to determine the GMP gel properties by using a Physical MCR301 rheometer (Anton-paar, GmbH, Graz, Austria). Strain sweeps and frequency sweeps were tested at 20°C. The gap size was 1.0 mm. Frequency sweep measurements were performed in the range from 0.05 to 5 Hz at a constant strain (0.02). During the strain sweep measurements, strain was increased from 0.002 to 0.12 at a constant frequency (0.15 Hz).
Characterization of GMP dispersion
Viscosity measurement
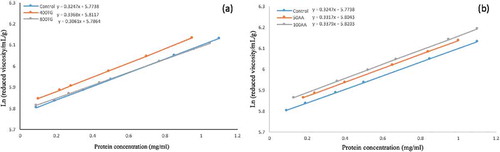
To obtain GMP dispersion, fresh GMP gel (5 g wet weight) was dispersed in 50 ml of 1.5% (w/v) SDS solution. The flow time of the GMP dispersion was measured at 25 ± 0.1°C using an Ubbelohde capillary viscometer and the protein concentration of the GMP dispersion was determined using the Dumas method. The relative viscosity (ηrel = flow time of sample/flow time of solvent) and specific viscosity (ηsp = ηrel −1) were calculated by 1.5% (w/v) SDS solution as the solvent. Reduced viscosity (ηred = ηsp /protein concentration of GMP dispersion) was plotted against the protein concentration of the GMP dispersion and extrapolated to zero concentration to determine the intrinsic viscosity [η] (ml/g).[Citation18]
Particle size distribution
The size distribution of the dispersed glutenin particles was measured using a Static Laser Particle Analyzer (Horiba, LA-930, Japan) according to Wang et al.[Citation19]
Determination of SHf groups
The contents of SHf of the gluten samples were determined according to Tuhumury et al.[Citation20]
SDS-PAGE analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Walker by using a 10% (w/v) acrylamide separating gel and 2.5% acrylamide stacking gel.[Citation21] Samples were prepared in Tris-glycine buffer (pH 8.8) containing SDS (1.5%, w/v) and β-mercaptoethanol (0.2%, v/v). Then, they were mixed and ultrasonicated for 5 min (frequency 50 kHz and 80 W). The final protein concentration of the sample solutions prepared was about 2 mg/ml.
Statistical analysis
Data were reported as mean±standard deviation (SD). SAS statistical software release 9.1 (SAS Institute Cray, NC) was used for the statistical analysis. The least significant difference (LSD) test was conducted to determine the differences between the samples at the significance level of p < 0.05.
Results and discussion
Physicochemical properties of the samples
The results of the effect of enzyme on gluten yield and protein recovery are shown in . In agreement with the results reported by Olsen[Citation22], increasing the enzyme levels to 600 ppm led to an increase in the gluten yield and protein recovery, and this might be related to the formation of the G-L bond in the gluten structure. However, at the dose of 800 ppm of TG, a significant decrease was observed in the gluten yield compared with the previous level. It appears that TG leads to an improvement in the separation of gluten from starch and increases the gluten yield by establishing an isopeptide bond between glutamine as the acyl donor and other amino acids as the acyl acceptor (such as lysine). However, at a high concentration of the enzyme, the amount of available lysine decreases and the enzyme leads to the deamination of glutamine in the gluten structure by using water molecule as an acyl acceptor. Due to this phenomenon, the negative charge density increases in the gluten structure and sustainable aggregation does not occur in the gluten structure because of the repulsive force and increasing distance between the protein chains. These results are consistent with the results observed by Steffolani et al.[Citation23] In the samples with AA, the tendency to increase the gluten yield and protein recovery was observed, so that at all levels AA caused significant changes in gluten yield compared with the control sample. It seems that SHf groups are oxidized under the influence of AA and the related compounds and form a disulfide bond; the results have been confirmed by the SHf group. By establishing the covalent bond between the protein chains, the agglomeration of gluten was improved and it led to an increase in the gluten yield and protein recovery. The same results were observed by Larson and Ellison.[Citation11] GI is a method for the qualitative analysis of wheat flour protein, where 50<GI<100 is suitable for bread-making. Increasing AA leads to increase in GI, which can be due to the new covalent bond formed under the influence of AA. A similar trend was observed as a result of using TG and the isopeptide bond formation to 600 ppm enzyme concentration. However, at the highest concentration of the enzyme, the GI was significantly decreased due to the deamidation of glutamine. Rosell et al. reported similar results.[Citation6] All levels of TG increased the water absorption in the wet gluten structure, which was consistent with the reports presented by Liu et al.[Citation5] It seems that TG causes better retention of water in the gluten structure by the formation of the G-L bond and by changing the three-dimensional protein structure. Liu et al. reported that due to the lack of amines and the deamidation of glutamine, there are more hydrophilic residues on the surface and the water absorption in the gluten structure increases.[Citation5] Increasing the level of AA led to a decrease in the water absorption in gluten slightly and insignificantly. The effect of AA on water absorption is presented in .
Table 1. Effect of TG on physicochemical properties of gluten.
Table 2. Effect of AA on the physicochemical properties of gluten.
Gluten rheological properties
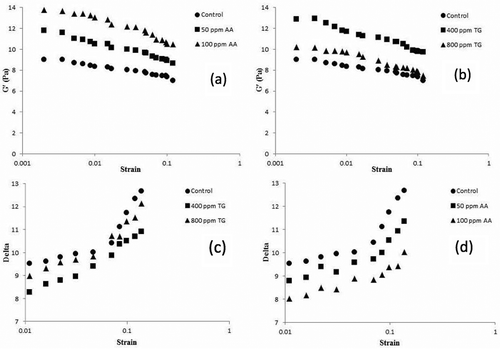
The extensibility of gluten is a good method to measure the quality of gluten and the gas retention in the dough depends on it. The ratio of gluten resistance to gluten extensibility is an indicator of gluten strength and a higher proportion implies stronger gluten. The results of the effect of TG on the rheological properties of gluten showed that the treatment with the enzyme concentration of 800 ppm had the highest gluten extensibility and the lowest resistance to extension. It could be due to an increase in the negative charge density as a result of deamidation of glutamine, which in this case seems that the stable agglomeration in the gluten structure is not proper and the gluten resistance to extension is reduced. These results were not consistent with the observations of Steffolani et al.[Citation23] In other concentrations, no significant difference was observed between the enzyme sample and the control sample. The results obtained from the effect of AA on the rheological properties of gluten are shown in . All levels of AA caused significant changes in the rheological properties of gluten so that the extensibility constantly and significantly decreased and the resistance to extension increased by increasing the concentration of AA. The results obtained were consistent with the results of Tseng et al.[Citation3], and it can be due to the disulfide bond formation and the increase in gluten strength.
Rheological properties of GMP gel
The effect of TG and AA on the rheological properties of GMP gel is shown in . Don et al.[Citation24] reported that the γ value represents the ratio of viscous behavior to elastic behavior of a gel and storage modulus (G’) represents the gel stiffness. The low γ value shows the elastic behavior of the gel and increasing the G’ represents the gel stiffness. γ value at 400 ppm enzyme concentration is less than the 800 ppm enzyme level and the control sample. Moreover, the modulus G’ in the GMP gel obtained from the concentration of 400 ppm is more than the control sample and the enzyme concentration of 800 ppm. The results of difference in the structure of the GMP gel obtained from the above-mentioned samples show that the GMP gel obtained from the 400 ppm level is more stiff and elastic than the other two samples. These changes are related to two reasons: 1) the G-L bonds formed under the effect of TG; 2) the more concentration of protein in the GMP gel. Lareh et al. had observed the same results.[Citation8] During deamidation, the weight of the GMP wet protein content is significantly decreased because of using high concentrations of the enzyme. It also seems that due to the effect of changes in the protein structure and distance of the protein chains, the bonds between the chains are changed, which possibly results in a decrease in G’ and an increase in the γ value, which represents a decrease in stiffness and elasticity of the gel at the highest TG level. Our data with AA shows that AA increases G’ and decreases the γ value. These observations indicate that AA changes the structure of the GMP gel compared with the control. It can be explained that under the influence of AA and considering the data of the SHf group, more disulfide bonds are formed in the gluten structure, which results in more stiffness and elasticity of the GMP gel.
Effect of TG and AA on the properties of GMP dispersion
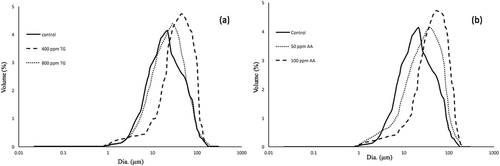
Intrinsic viscosity represents the voluminosity of the average particle size (the hydronamic volume per unit of mass) of glutenin, which is a concept in line with the average particle size. Moreover, the K’ value shows the tendency of the glutenin particles to aggregate. The effect of TG and AA on the GMP dispersion properties is shown in and and . It seems that under the influence of AA, the sulfhydryl groups are oxidized and the covalent bond is formed, resulting in an increase in particle size and intrinsic viscosity. By increasing the concentration of AA, the peak is shifted to the right and the particle size distribution becomes larger. Amiri et al. reported that Glucose oxidase (GOX) leads to an increase in the particle size 200 distribution by the formation of a disulfide bond.[Citation25] Our results show that adding 400 ppm of enzyme increases the intrinsic viscosity and the particle size disruption in the GMP gel. This can be due to the G-L bond formation and the interaction between the protein chains in the GMP gel. However, at 800 ppm concentration, the peak of the particle size distribution curve changes to the left and K’ is significantly decreased. In this enzyme concentration, the deamidation of glutamine increases the distance between the protein chains. In these circumstances, the possibility of interaction between the gluten proteins decreases, resulting in a sharp decrease in the tendency to aggregate.
Table 3. Effect of AA and TG on the intrinsic viscosity, K′, and particle size of GMP dispersion.
SHf groups determination
Sulfhydryl groups are known as important functional groups in many food proteins. Cysteine plays an important role in the gluten structure and is involved in the formation of intrachain and interchain disulfide bonds.[Citation26,Citation27] The bonds play an important role in increasing the gluten strength and protein chain folding. About 5% of the total cysteine of flour is SHf groups, representing a high proportion of disulfide bonds in the gluten structure; however, this proportion changes by oxidizing and reducing agents.[Citation28–Citation30] The results showed that increasing the concentration of TG to 600 ppm led to a significant increase in the SHf groups. The flexibility in the protein structure is reduced by the effect of enzyme and G-L bond formation. It seems that the change in the three-dimensional structure of the protein leads to a decrease in the probability of interaction of the thiol groups in the gluten structure and it results in an increase in the SHf groups. At the highest concentrations of TG, a significant decrease in the amount of SHf groups was observed compared with the previous levels. Probably at this concentration of TG, due to the deamidation of glutamine, the negative charge density in the protein chains and the protein chain flexibility increase and thus the possibility of interaction between the sulfhydryl groups and the formation of disulfide bond increase. Steffolani et al. had observed similar results earlier.[Citation23] Kohler studied the effect of AA on the oxidation of glutathione groups in wheat flour protein and observed the formation of a disulfide bond in the protein chain.[Citation31] Our data indicate that the addition of AA decreases the SHf groups drastically and significantly and it can represent the production of dehydroascorbic acid from AA. Dehydroascorbic acid causes the oxidation of sulfhydryl groups and the formation of disulfide bond due to the oxidative property. The results are available in and .
SDS-PAGE of gluten modified by TG and AA
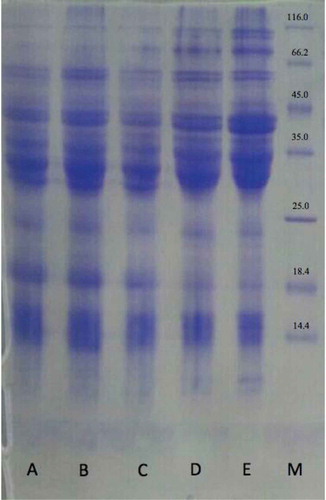
In 2003, Bauer et al. found that HMW and -gliadin are the main subunits affected by TG.[Citation32] The electrophoresis patterns of the modified gluten by TG and AA treatments are shown in . Lines 3 and 4 show the effect of 400 and 800 ppm of TG concentrations on the gluten. As can be seen, the intensity of the bands is decreased between the ranges of 66.2 and 116 kDa under the influence of the enzyme. These bands are related to HMW glutenin subunit. Due to the high levels of glutamine content in this fraction, the G-L bond is formed and polymerization occurs in the gluten network with the enzymes. It seems that HMW is the main subunit to form the bond under the influence of TG. Notably, increasing the intensity of the band between the ranges of 25 and 116 kDa is under the influence of 800 ppm compared with the previous enzyme concentration. The deamidation occurs in this enzyme concentration, which prevents the establishment of maximum bond in the gluten structure and its agglomeration. The effect of AA with the concentration of 100 and 50 ppm on gluten can be observed in lines 1 and 2. The HMW bands were removed by the treatment with AA. Moreover, the intensity of the bands of LMW,
, and γ-gliadin decreased, which can be due to the presence of cysteine in the structure of these components, the effect of AA on it, and the formation of the disulfide bond. On the other hand, AA did not lead to any change in
-gliadin, which might be due to the lack of cysteine in the sequence of
-gliadin.
Explaining the mechanism of the effect of TG and AA on the gluten formation
Our explanations are based on the formation of a gluten network according to the hyper-aggregation model. Briefly, in this model, the formation of the gluten network is presented in three stages. Gluten subunits form a larger polymer by forming the covalent bond in the first stage and forming the physical, hydrogen, electrostatic, and hydrophobic bonds in the second stage. In the final stages, other polymers and physical conditions affect the formation of the final network. In the samples with AA, the sulfhydryl groups are oxidized and form the disulfide bond. The covalent bond formed leads to an increase in the gluten yield, particle size distribution, and G’ modulus. It seems that the major effect of AA is in the first stage of hyper-aggregation. Moreover, under the influence of TG in the first stage of the hyper-aggregation model, the covalent bond is formed between the glutamine and lysine, which causes the changes in the gluten properties. However, at high concentrations of the enzyme, all possible bonds are formed between the lysine and glutamine in the first stage of hyper-aggregation. Then, by decreasing the lysine residues, the additional concentration of the enzyme causes the deamidation of glutamine and the increase in negative charge density. Under these circumstances, the possibility of forming the physical, hydrogen, electrostatic, and hydrophobic bonds is decreased in the second stage of hyper-aggregation. At high concentrations of the enzyme, the tendency to aggregate and the gluten yield are decreased and the GMP gel becomes more viscous.
Conclusion
Our observations showed that AA causes better gluten aggregation and the improvement of separation of gluten from wheat flour. It was concluded that during mixing, the AA was converted into dehydroascorbic acid and under the influence of this oxidizing compound activity, the disulfide bond is formed in the gluten structure, which results in increasing the gluten yield, forming a stronger gluten structure, and improving the gluten aggregation. Reducing the SHf group and gluten extensibility and also increasing the particle size in the GMP gel can confirm these changes. Although the wheat protein has a low lysine content, the G-L bond formation in the gluten structure by the TG enzyme can lead to the formation of a strong gluten network and change the rheological behavior. The changes in the gluten network depend on the concentration of the TG enzyme. Adding TG to 600 ppm level leads to increased gluten yield and GI. Moreover, increasing the G’ modulus and decreasing the γ value shows more elastic and stiff GMP gel under the effect of the enzyme. It seems that the formation of the G-L bond changes the three-dimensional structure of the protein so that the formation of other bonds such as disulfide changes. At the highest concentration of TG and in the absence of amine substrates, glutamine is converted into glutamic acid and the negative charge of the chain increases and the distance of the chains increases due to the resulting repulsive force. It seems that the gluten yield and particle size decrease and extensibility is increased as a result of this event.
Acknowledgment
The authors would like to thank Isfahan University of Technology of Iran (IUT) for supporting this research.
References
- Day, L.; Augustin, M. A.; Batey, I. L.; Wrigley, C. W. Wheat Gluten Uses and Industry Needs. J. Trends Food Sci. Technol. 2006, 17, 82–90. DOI:10.1016/j.tifs.2005.10.003.
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of Leavening of Bread Dough with Mathematical Modelling. J. Food Eng. 2007, 83, 142–148. DOI:10.1016/j.jfoodeng.2007.02.014.
- Tseng, S.; Lai, M. Physicochemical Properties of Wheat FlourDough Modified by Microbial Transglutaminase. J. Food Sci. 2002, 67, 750–755. DOI:10.1111/jfds.2002.67.issue-2.
- Wieser, H.; Chemistry of Gluten. J. Food Microbiol. 2007, 24, 115–119. DOI:10.1016/j.fm.2006.07.004.
- Liu, S.; Zhang, D.; Liu, L.; Wang, M.; Du, G.; Chen, J. Enhanced Water Absorption of Wheat Gluten by Hydrothermal Treatment Followed by Microbial Transglutaminase Reaction. J. Sci. Food Agric. 2010, 90, 658–663.
- Rosell, C. M.; Wang, J.; Aja, S.; Bean J., S.; Lookhart, Wheat Flour Proteins as Affected by Transglutaminase and Glucose Oxidase. J. Cereal Chem. 2002, 80, 52–55. DOI:10.1094/CCHEM.2003.80.1.52.
- Gerrard, J. A.; Newbbery, M. P.; Ross, M.; Wilson, A. J.; Fayle, S. E.; Kavale, S. Pastry Lift and Croissant Volume as Affected by Microbial Transglutaminase. J. Food Eng. Phys. Prop. 2000, 65, 312–314.
- Larré, C. S.; Denery-Papini, Y.; Popineau, G.; Deshayes, C.; Desserme,; Lefebvre, J. Biochemical Analysis and Rheological Properties of Gluten Modified by Transglutaminase. J. Cereal Chem. 2000, 77,2, 121–127. DOI:10.1094/CCHEM.2000.77.2.121.
- Kieliszek, M.; Misiewicz, A. Microbial Transglutaminase and Its Application in the Food Industry. A Review. J. Folia Microbiol. 2013, 59,3, 241–250.
- Motoki, M.; Seguro, K. Transglutaminase and Its Use for Food Processing. J. Trends Food Sci. Technol. 1998, 9, 204–210. DOI:10.1016/S0924-2244(98)00038-7.
- Larsson, H.; Eliasson, A. C. Phase Separation of Wheat Flour Dough Studied by Ultracentrifugation and Stress Relaxation. II. Influence of Mixing Time, Ascorbic Acid, and Lipids. J. Cereal Chem. 1996, 73, 25–31.
- Hruškovă, M.; Navotnă, D. Effect of Ascorbic Acid on the Rheological Properties of Wheat Fermented Dough. Czech J. Food Sci. 2003, 4, 137–144.
- Joye, I.; Lagrain, B.; Delcour, J. Endogenous Redox Agents and Enzymes that Affect Protein Network Formation during Breadmaking – A Review. J. Cereal Sci. 2009, 50, 1–10. DOI:10.1016/j.jcs.2009.04.002.
- Tsen, C. C.; The Improving Mechanism of Ascorbic Acid. J. Cereal Chem. 1965, 42, 86–97.
- American Association of Cereal Chemists. Approved Methods of the AACC. Methods. 2000, 38-12A, 44–16.
- Kieffer, R.; Wieser, H.; Henderson, M. H.; Graveland, A. Correlations of the Breadmaking Performance of Wheat Flour with Rheological Measurements on a Micro-Scale. J. Cereal Sci. 1998, 27, 53–60. DOI:10.1006/jcrs.1997.0136.
- Wang, M.-W.; Hamer, R. J.; Van Vliet, T.; Gruppen, H.; Marseille, J. P.; Weegles, P. L. Effect of Water Unextractable Solids on Gluten Formation and Properties: Mechanistic Considerations. J. Cereal Sci. 2003, 37, 55–64. DOI:10.1006/jcrs.2002.0478.
- Sakai, T.; Extrapolation Procedures for Intrinsic Viscosity and for Huggins Constant K´. J. Polym. Sci. 1968, 6, 1659–1672.
- Wang, M.; Van Vliet, T.; Hamer, R. J. Interaction of Water Unextractable Solids and Xylanase with Gluten Protein: Effect of Wheat Cultivars. J. Cereal Sci. 2005, 41, 251–258. DOI:10.1016/j.jcs.2004.07.007.
- Tuhumury, H. C. D.; Small, D. M.; Day, L. The Effect of Sodium Chloride on Gluten Network Formation and Rheology. J. Cereal Sci. 2014, 60, 229–237. DOI:10.1016/j.jcs.2014.03.004.
- Walker, J. M.; SDS Polyacrylamide Gel Electrophoresis of Proteins. In The Protein Protocols Handbook, 2nd; Humana Press Inc.: Totowa, New Jersey, 2002; pp. 61–67.
- Olsen, H.; A Method for the Separation of Wheat Flour Using A Transglutaminase Enzyme. It is a patent with patent number WO2002015713A1. 2002, WO2002015713 A1.
- Steffolani, M. E.; Ribotta, P. D.; Perez, G.; A. E., Effect of Glucose Oxidase, Transglutaminase, and Pentosanase on Wheat Proteins: Relationship with Dough Properties and Bread-Making Quality. J. Cereal Sci. 2010, 51, 366–373. DOI:10.1016/j.jcs.2010.01.010.
- Don, C.; Lichtendonk, W. J.; Plijter, J. J.; Hamer, R. J. Understanding the Link between GMP and Dough: From Glutenin Particles in Flour Towards Developed Dough. J. Cereal Sci. 2003, 38, 157–165. DOI:10.1016/S0733-5210(03)00017-1.
- Amiri, A.; Shahedi, M.; Kadivar, M. Evaluation of Physicochemical Properties of Gluten Modified by Glucose Oxidase and Xylanase. J. Cereal Sci. 2016, 71, 37–42. DOI:10.1016/j.jcs.2016.07.013.
- Li, X.; Lv, Y.; Chen, Y.; Chen, J. A Study on the Relationship between Rheological Properties of Wheat Flour, Gluten Structure, and Dumpling Wrapper Quality. Int. J. Food Prop. 2016, 19, 1566–1582. DOI:10.1080/10942912.2014.951894.
- Ahmed, J.; Thomas, L. Effect of Β-Glucan Concentrate on the Water Uptake, Rheological and Textural Properties of Wheat Flour Dough. Int. J. Food Prop. 2014, 18, 1801–1816.
- Ahmed, J.; Mulla, M. Z.; Arfat, Y. A. Particle Size, Rheological and Structural Properties of Whole Wheat Flour Doughs as Treated by High Pressure. Int. J. Food Prop. 2016, 20, 1829–1842.
- Singh, A.; Lahlali, R.; Vanga, S. K.; Karunakaran, C.; Orsat, V.; Raghavan, V. Effect of High Electric Field on Secondary Structure of Wheat Gluten. Int. J. Food Prop. 2015, 19, 1217–1226.
- Bigne, F.; Puppo, M. C.; Ferrero, C. Rheological and Microstructure Characterization of Composite Dough with Wheat and Mesquite (Prosopis Spp) Flours. Int. J. Food Prop. 2015, 19, 243–256.
- Kohler, P.; Effect of Ascorbic Acid in Dough: Reaction of Oxidized Glutathione with Reactive Thiol Groups of Wheat Glutelin. J. Agric. Food Chem. 2003, 51, 4954−4959.
- Bauer, N.; Koehler, P.; Wieser, H.; Schieberle, P. Studies on Effects of Microbial Transglutaminase on Gluten Proteins of Wheat. I. Biochemical Analysis. J. Cereal Chem. 2003, 80, 781–786. DOI:10.1094/CCHEM.2003.80.6.781.