ABSTRACT
The structure and functionalities of rice bran protein (RBP) oxidized by peroxyl radicals were analyzed in this study. The thermal decomposition of 2,2′-azobis [2-amidinopropane] dihydrochloride (AAPH) was used to generate peroxyl radicals. Increased oxidation of RBP by AAPH gradually generated more carbonyl (COOH) groups, which resulted in a loss of protein sulfhydryl groups. Low oxidization (≤0.2 mmol/L AAPH) could cause structural unfolding with an increase in surface hydrophobicity and emulsion properties but reducing the solubility and disulfide bonding. Moderate and high oxidization (>0.2 mmol/L AAPH) could result in soluble aggregates formed by subunits with molecular weights of 53, 49, and 36 kDa, attributed to globulin, albumin, and glutelin, increasing the solubility and disulfide bonding but decreasing the surface hydrophobicity and emulsion stability. Oxidization by low concentration AAPH induced a more unordered structure and transformation from β-turn to β-sheets, while a more ordered structure increased with aggregation.
Introduction
Rice bran has approximately 15% protein content. Rice bran protein (RBP) has good digestibility and high protein efficiency ratio values of 2.0 to 2.5.[Citation1,Citation2] RBP is more nutritious than other cereals or legumes[Citation3] and contains a large amount of essential amino acids (lysine and threonine).[Citation3–Citation5] RBP is a hypoallergenic protein that has been used in infant food and has also exhibited anti-cancer activity.[Citation6–Citation8] According to Wang et al.,[Citation1] the amino acid composition of RBP can supply the recommended dietary allowance for children. However, this underutilized protein is rarely used in the food industry due to its poor functional properties and difficult extractability, and thus, enzymatic hydrolysis,[Citation9] high-temperature heat treatment,[Citation10] subcritical water treatment,[Citation11] ultrasonic treatment,[Citation12] and alkaline treatment[Citation12] have been used to improve the extraction and enhance the functional properties of RBP. Few studies have reported the effects of oxidation in modifying the structure and functional properties of RBP, although meat protein oxidation has been extensively investigated.[Citation13]
Proteins are vulnerable to oxidation, which is known to lead to the pathogenesis of relevant degenerative diseases because of their high abundance in living tissues and high susceptibility to oxidation compared with many other macromolecules.[Citation14] Protein oxidation is believed to proceed either directly by reactive oxygen species (ROS) or indirectly by reaction with a larger variety of oxidation products.[Citation15] It is known that ROS can modify target proteins, which can result in multiple structural changes, including oxidation of side-chain groups, primary chain rupture, partial denaturation, aggregation, and conformation unfolding. Oxidative modifications have been reported to reduce the content of essential amino acids and decrease digestibility, affecting the nutritional values of proteins.[Citation16–Citation18] Protein oxidation has been reported to increase protein hydrophobicity and solubility but decrease water-holding capacity, texture-forming ability, and susceptibility of protein substrates to proteolytic enzymes.[Citation19,Citation20] The abstraction of a hydrogen atom by an ROS leads to the generation of a protein carbon-centered radical, which is consecutively converted into a peroxyl radical in the presence of oxygen and an alkyl peroxide by the abstraction of a hydrogen atom from another molecule. Peroxyl radicals are both major initiating factors of lipid peroxidation chain reactions and an ROS for broad protein oxidation.[Citation21,Citation22] Peroxyl radical further reacting with HO2· leads to the generation of an alkoxyl radical and its hydroxyl derivative. Among all the free radicals generated during lipid peroxidation propagation, peroxyl radicals are the key chain-propagating species, and the reaction of peroxyl radicals continues the propagation process in protein chain oxidation reactions by abstracting hydrogen atoms from protein. However, little information is available about peroxyl radical (ROO·)-mediated RBP oxidation, whereas the study of (ROO·)-induced oxidation of RBP is necessary to elucidate the mechanisms of lipoxygenase-catalyzed lipid peroxidation of rice products.
Thermal decomposition of AAPH can generate peroxyl radicals at a known and constant rate at the stable temperature of 37°C. The amount of peroxyl radicals from the thermal decomposition of AAPH is proportional to its concentration.[Citation23] Therefore, AAPH-derived peroxyl radicals have been selected as representative free radicals of lipid peroxidation in many studies to investigate the effect of oxidation on proteins. In this study, we analyzed the effect of AAPH-derived peroxyl radicals on the protein structure and functional properties of RBP to improve our understanding of the implications of lipid and protein oxidation in rice products.
Materials and methods
Samples and materials
Defatted rice bran was purchased from Dongfang Group (Harbin, China). AAPH was purchased from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals were of analytical reagent grade and obtained in China.
RBP preparation
Deoiled rice bran was ground to pass a 60 mesh screen, and 10 g was dispersed in 100 mL of distilled water. The protein was solubilized by adjusting the pH to 9.5 using 0.1 M NaOH and shaking at 300 rpm for 2.0 h at 50°C. The remaining residue was removed by centrifugation at 3000 × g for 20 min. The protein in the supernatant was precipitated by adjusting the pH to 3.8 with 0.1 M HCl and centrifuged at 3000 × g for 20 min. The precipitated protein was centrifuged and washed with distilled water two times, and the pH was increased to 7.0 before freeze drying. This protein was labeled RBP.[Citation24]
RBP oxidation
An RBP suspension (40 mg/mL containing 0.5 mg/mL sodium azide suspended in 10 mM sodium phosphate buffer, pH 7.4) was mixed with serial concentrations of AAPH and was then incubated by continuous shaking in a water bath at 37°C in the dark for 24 h. The final concentrations of AAPH in RBP suspension were 0, 0.04, 0.2, 1, 5, and 25 mM. The reaction was stopped by immediately cooling the solution to 4°C in an ice bath and then centrifuging it at 8000 × g for 15 min at 4°C. The supernatant was dialyzed against deionized water at 4°C for 72 h to remove residual AAPH and salt. Then, the dialyzed solution was freeze-dried and stored at 4°C until use.[Citation25]
Protein carbonyl group measurement
The carbonyl groups of RBP were detected by their reaction with 2,4-dinitrophenylhydrazine (DNPH) to form protein hydrazones, and their content was estimated using the method of Huang et al.[Citation26] The results were expressed as nmol of carbonyl groups per milligram of soluble protein with a molar extinction coefficient of 22,000 M−1 cm−1.
Sulfhydryl and disulfide content measurement
The SH group contents in RBP were determined by a modification of Ellman’s method using 2,2′-dithiobis(5-nitropyridine) DTNP according to Sun et al.[Citation27] The results were expressed in nmol of SH or S–S per milligram of protein with a molar extinction coefficient of 13,600 M−1 cm−1.
Measurement of surface hydrophobicity
The surface hydrophobicity (H0) values of proteins in the samples were determined by using 1,8-anilinonaphthalenesulfonate (ANS) as a fluorescence probe. The ANS solution (20 μL, 8.0 mM) was added to 4 mL of 0.05, 0.1, 0.2, 0.5, or 1 mg/mL RBP solutions prepared in phosphate buffer solution (10 mmol/L, pH 7.0). The fluorescence intensity (FI) was measured at 390 nm (excitation) and 470 nm (emission) using a Hitachi F-7000 fluorescence spectrophotometer (Hitachi Ltd, Tokyo, Japan) with a slit width of 5 nm. The initial slope of FI versus protein concentration plot was used as the index of H0.[Citation28]
Intrinsic fluorescence emission spectroscopy
The intrinsic emission fluorescence spectra of the protein samples were obtained using a fluorescence spectrophotometer (Hitachi Ltd.) following a previous report.[Citation29] RBP dispersions (0.15 mg/mL) were prepared in 0.01 M phosphate buffer (pH 7.0). The excitation wavelength was 290 nm, and the emission spectra were recorded from 300 to 400 nm at a 10 nm/s scanning speed with a constant 5 nm slit for both excitation and emission. Each scan was performed five times.
FT-IR spectroscopy
FT-IR absorption spectra were acquired from 4,000 to 400 cm−1 in transmission mode by a Nicolet Magna IR 550 FT-IR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) continuously purged with dry air and equipped with a liquid nitrogen-cooled MCT detector. RBP samples were first freeze-dried and then produced by pressing KBr windows (1.5 mg protein to 200 mg KBr) on a Carver press at 5–6 T pressure. Each spectrum was obtained by coadding 256 interferograms at a spectral resolution of 2.0 cm−1. The decomposition of the amide I band was performed in the region of 1700–1600 cm−1. A second-derivative analysis (“peak fitting” procedure) of the IR-SD, which has been shown previously to provide reliable quantitative information, was used to obtain a quantitative analysis of the secondary structural components of RBP. The “peak fitting” procedure was applied to the linear baseline correction, the Fourier self-deconvolution, and the deconvoluted (difference) spectrum to resolve and quantify the individual component bands according to a Gaussian curve fit (GCF). The procedure maintained the initial band positions in an interval of 4 ± 1 cm−1, excluded bands with negative heights, and kept the bandwidth within the expected limits in agreement with the theoretical boundaries or predictions. The relative amounts of the different secondary structures of RBP were determined from the second derivative of the amide I band by manually computing the areas under the bands assigned to a particular substructure. The differences between the measured spectrum and the curve fit were calculated as an internal control of the success of the curve fitting process.[Citation30]
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Gel electrophoresis was conducted according to Laemmli’s method[Citation31] with a slight modification according to Jiang’s method[Citation29] using 12% and 5% acrylamide separating and stacking gels, respectively. Samples were prepared in 0.125 M Tris-HCl buffer containing 10 mg/mL SDS, 2% (v/v) 2-mercaptoethanol, 20% (v/v) glycerol, and 0.25 mg/mL bromophenol blue and were heated for 5 min in boiling water before electrophoresis. After the electrophoresis, the gel sheets were stained for protein with Coomassie brilliant blue R-250. The protein stain was destained with 10% acetic acid (v/v) containing 10% methanol (v/v).
Solubility
The solubility of RBP was determined by dispersing the protein samples in distilled water to obtain a final solution at 2 mg/mL. The pH values of the protein solution were adjusted to 7.0 with 0.5 M HCl or 0.5 M NaOH and then centrifuged at 12,000 × g for 30 min (4°C). The protein content of the resulting solution was analyzed according to Lowry’s method.[Citation32]
Emulsifying properties
The samples were dissolved in phosphate buffer solution (10 mM, pH 7.0) to obtain a final protein content of 2 mg/mL. For emulsion formation, soybean oil and RBP solution (1:3, v/v) were homogenized using a homogenizer (AE300 L-H; Shanghai Angni Instruments Co., Shanghai, China) at 24,000 rpm for 1 min. Emulsion samples (50 μL) were taken from the bottom of the beaker immediately after 0 or 10 min of homogenization and were diluted at 1:100 with 1 mg/mL SDS solution. The absorbance of the diluted emulsion was recorded at 500 nm. The emulsification activity index (EAI) and emulsification stability index (ESI) were calculated as follows:
where DF is the dilution factor (100), C is the protein concentration (g/mL), φ is the optical path (1 cm), and θ is the oil volume fraction (0.25). A0 and A10 are the absorbances of the emulsion at 0 and 10 min, respectively.[Citation28]
Statistical analysis
Each treatment was performed at least in triplicate. The results were subjected to a one-way analysis of variance according to the general linear model procedure with least square means effects. Multiple range tests were applied to determine which means were significantly different (P < 0.05) according to Fisher’s least significant differences (LSD) test. Statistical analysis was carried out using SYSTAT software (SYSTAT, Inc., Evanston, IL, USA).
Results and discussion
Protein carbonyls
As shown in , 1 mg of non-oxidized RBP (control) contained approximately 3.3 nmol of carbonyls. Oxidation caused significant increases (P < 0.05) in the protein carbonyl content with AAPH concentration since the yield of peroxyl radicals from the thermal decomposition of AAPH is positively related to its content.[Citation23]
Table 1. Protein carbonyl, free sulfhydryl, total sulfhydryl, disulfide bond, and surface hydrophobicity of RBP incubated with increasing concentrations of AAPH.
Carbonyl groups can be introduced into proteins through several pathways: (1) by direct oxidation of amino acid side chains such as those from lysine, proline, arginine, and threonine; (2) by direct attack of peptide bonds to cause protein fragmentation; and (3) by reaction with lipid oxidation products, reducing sugars or oxidized ascorbic acid.[Citation33] AAPH is a well-known water-soluble radical initiator, which has been successfully introduced as a lipid peroxidation initiator.[Citation34] In this study, peroxyl radicals decomposed by AAPH reacted with RBP at side chains and the backbone to generate carbon-centered radicals, which then converted to protein peroxyl radicals under aerobic conditions, increasing the carbonyl groups content.
Sulfhydryl and disulfide content
The carbonyl contents may not fully describe the protein oxidation in RBP. They present only the carbonyl derivatives but not other oxidation products of cysteine residues. Cysteine residues are the most susceptible amino acid residue, and the conversion of sulfhydryl groups into other sulfur-containing oxidized species is one of the earliest phenomena during protein oxidation.[Citation35] As shown in , the total and free sulfhydryl groups both significantly decreased (P < 0.05) with AAPH concentration. This phenomenon may be due to sulfur-containing amino acids such as cysteine and methionine being highly susceptible to oxidation in the presence of oxidizing lipids and yielding varied sulfur-containing compounds such as sulfone, sulfoxide, and disulfide derivatives.[Citation36] The disulfide bond contents first decreased and then increased with AAPH concentration. The decrease in disulfide bonds may be related to protein unfolding by oxidative modification, whereas the increased disulfide bond content of RBP oxidized by a high concentration of AAPH could mainly be attributed to aggregation.
Surface hydrophobicity
Surface hydrophobicity is one of the most important structure-related factors influencing the functional properties of proteins. Surface hydrophobicity is significantly correlated with protein gelation, emulsion, and foaming, which are properties critical to their application as food ingredients.[Citation37,Citation38] Surface hydrophobicity was used to detect the conformational changes between native and modified RBP. As depicted in , the surface hydrophobicity of RBP first increased and then decreased with AAPH concentration. The increased surface hydrophobicity was attributed to the exposure of hydrophobic groups, reflecting the structural changes in RBP during oxidation.[Citation26] The other possible factors during the oxidation of RBP that could increase the surface hydrophobicity include protein denaturation, dissociation of protein subunits, and expansion of peptide chains.[Citation39] Moreover, protein aggregation by hydrophobic reaction and the formation of new hydrophilic amino acid residues would decrease the surface hydrophobicity of oxidized RBP with high concentrations of AAPH.[Citation40] We previously found that the formation of insoluble or soluble aggregates decreases the surface hydrophobicity.[Citation41]
Intrinsic fluorescence emission spectroscopy
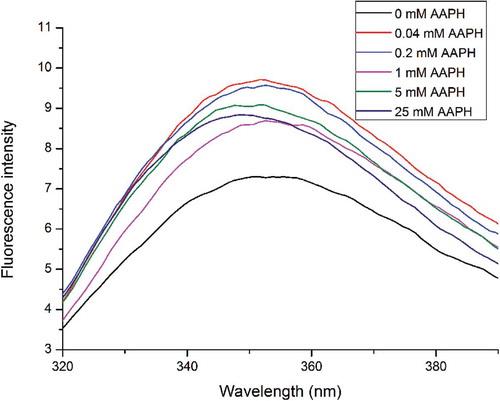
Fluorescence spectroscopy is a well-established technique to observe protein conformational changes under different micro-environmental conditions. Generally, two aromatic amino acid residues, tryptophan (Trp) and tyrosine (Tyr), are the major contributors to protein ultraviolet fluorescence. As the energy transfer from Tyr residues to Trp residues quenches the fluorescence of Tyr residues and enhances the fluorescence of Trp residues, it is generally accepted that protein fluorescence primarily results from the fluorescence of Trp residues.[Citation42]
Our previous study suggested that the fluorescence spectrum of a protein is not only determined by its chemical environment but also by the degree of exposure of hydrophobic tryptophan residues.[Citation29] As shown in , RBP exhibited a typical fluorescence emission spectrum for Trp residues in protein. In comparison, oxidized RBP had a higher FI than native RBP, which confirmed the partly unfolded structure of oxidized RBP, in agreement with the surface hydrophobicity results. The FI of oxidized RBP generally declined with AAPH concentration. It has been reported that peroxyl radical-mediated protein oxidation quenches intrinsic tryptophan fluorescence, as tryptophan residues are vulnerable to peroxyl radicals.[Citation43] Wu et al.[Citation40] found that the FI decreased when peroxyl radicals acted on tryptophan residues of soybean protein. As depicted in , oxidized RBP with ≥1 mmol/L AAPH had a significant decrease in fluorescence intensity, which might be related to protein aggregation. The λmax for native RBP was 350.6 nm, which is characteristic of the fluorescence profile of tryptophan residues in a relatively hydrophobic environment.[Citation44] The λmax of oxidized RBP first increased and then decreased with AAPH concentration. The redshifted wavelength of the maximum emission of oxidized RBP with low concentrations of AAPH suggested that Trp tended to be exposed on the protein molecular surface.
RBP oxidation resulted in flexible unfolding and exposed more amide residues initially buried intramolecularly.[Citation45] Additional hydrophobic residues of protein oxidized by high concentrations of oxidant were exposed at the molecular surface, which increased the hydrophobic interactions that contribute to the formation of protein aggregates. However, exposed Trp residues were reburied as protein aggregates formed, decreasing λmax.[Citation46]
Fourier transform infrared spectroscopy
FT-IR spectroscopy is a reliable method to detect the secondary structure of proteins. FT-IR spectroscopy permits a detailed analysis of the impact of point mutations or substrate binding on the structure and stability of proteins, using peptide backbone and side-chain “marker” bands as conformation-sensitive monitors. Another section will address the problems encountered in structural studies of membrane proteins and the specific possibilities of IR techniques in this field (infrared spectroscopy of proteins). shows the FT-IR spectra of native and AAPH-modified RBP.
Figure 2. Effect of oxidization on the FT-IR spectra of RBP (a) and deconvolution of the amide I spectra (continuous curve), the GCF bands thereof (point line), and the second-derivative spectra of RBP (b).
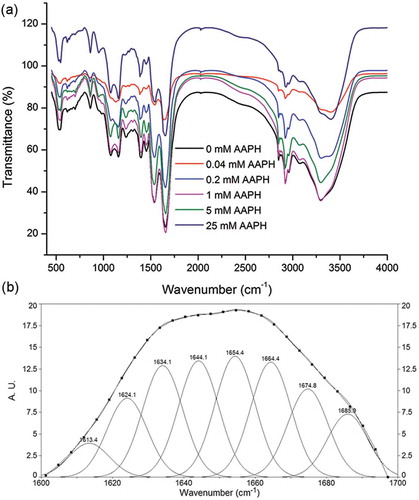
In this analysis, we combined Fourier self-deconvolution, second derivative, and GCF to quantitatively analyze the second derivative of the spectra (). Our second-derivative band positions align with previous literature data[Citation47] that reported a strong α-helix band at approximately 1650–1660 cm−1. We also obtained several bands corresponding to β-sheets in the frequency regions of 1618–1640 cm−1 and 1670–1690 cm−1.[Citation48] A series of bands corresponding to β-turns appeared in the 1660–1670 and 1690–1700 cm−1 range.[Citation47,Citation48] The random coil structure had a strong band close to 1645 cm−1.[Citation47] The percentages of α-helix, β-sheet, unordered and β-turn structures in RBP are shown in .
Table 2. Effects of the oxidization treatment on the secondary structures of RBP.
In this study, the predominant secondary structure of native RBP is β-sheet, confirming the data previously reported in our studies.[Citation24] In comparison, the levels of β-sheets in oxidized RBP were higher than in the native sample; the decreased α-helix content of oxidized protein was related to the protein structure unfolding or AAPH attacking the residues located in the α-helix region. The α-helix and β-sheet content of oxidized RBP both increased with AAPH concentration, which might be related to protein aggregate formation. Lee et al.[Citation49] reported that β-sheets play an important role in aggregation, which might be attributed to their relatively large surface areas for ordered hydrogen bonding. Antiparallel β-sheets first decreased and then increased with AAPH concentration. Choi and Ma[Citation50] noted that protein aggregation may increase the antiparallel β-sheet conformation. β-turns first decreased and then increased with AAPH concentration. This phenomenon might be due to the oxidization of RBP by low concentrations of AAPH having resulted in a transformation from β-turn to β-sheets, while the greater protein aggregation formed in the oxidized RBP by the high concentration of AAPH increased the β-turn content, as described in our previous study.[Citation41] The unordered structure content of oxidized RBP first increased, which might be due to protein unfolding, and then generally decreased with the formation of aggregation.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
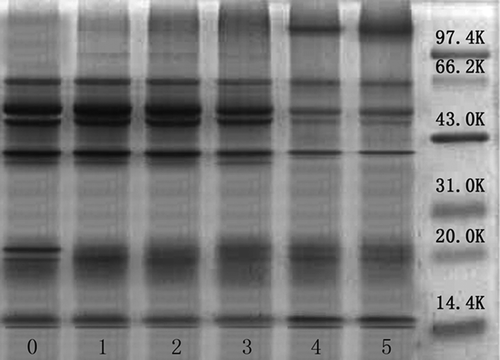
RBP consists of albumin, globulin, prolamin, and glutelin. The identified bands were subjected to extraction and purity analysis, but the contribution of each subunit of the four proteins could not be well determined. Abayomi et al.[Citation51] reported that the molecular weights of rice bran albumin had bands at approximately 31–45 kDa, while globulin showed bands at approximately 33 kDa and 53 kDa. However, Hamada[Citation52] reported molecular weights of albumin and globulin ranging from 10–100 kDa and 10–150 kDa, respectively. Wang et al.[Citation53] suggested that albumin showed protein patterns in the regions between 32 and 14 kDa, while globulin showed the following molecular mass distributions of subunits: 63, 53, 49, 36, and 22 kDa. Subunits of glutelin were mainly distributed at 54, 37, 21, and 13 kDa, whereas prolamin had two bands, at 13 and 22 kDa. The protein subunits of RBP were successfully examined by SDS-PAGE as shown in . RBP had bands at approximately 62, 53, 49, 36, 25, 21, 18, and 14 kDa. The bands at approximately 36 and 14 kDa might be attributed to albumin; those at 62, 53, 49, 36, and 21 kDa to globulin; those at 53, 36, 21, and 14 kDa to glutelin; and those at 21 and 14 kDa to prolamin. The low concentration of AAPH had no significant influence on the composition of oxidized RBP. With the AAPH concentration increasing to 25 mM, the intensity of bands near 53 kDa for globulin and glutelin, 49 kDa for globulin, and 36 kDa for albumin, globulin, and glutelin all decreased, accompanied by the increased intensity of bands attributed to protein aggregation with high molecular weight. This phenomenon suggested that the subunit with molecular weights of 53, 49, and 36 KDa could aggregate during protein oxidation.
Protein solubility
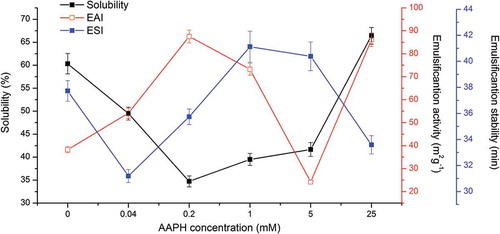
As shown in , the solubility of AAPH-modified RBP first decreased and then increased with AAPH concentration. The oxidation of RBP by low concentrations of AAPH might promote structure unfolding and peptide bond cleavage, which would disrupt their covalent and non-covalent crosslinked interactions in RBP, leading to a loss of their solubility and subsequent removal by centrifugation, thereby decreasing the protein solubility. A high concentration of oxidant reportedly could increase the formation of soluble aggregates, improving the solubility of RBP.[Citation14] Wu et al.[Citation40] used size exclusion chromatography (SEC) and found that oxidation of soy protein caused an AAPH-dose-dependent increase in fragmentation and aggregation.
In this research, the increased solubility of RBP oxidized by high concentrations of AAPH was unlike the results of the previous study.[Citation40] Since RBP has a more compact structure stabilized by disulfide bonds and non-covalent interactions,[Citation1] oxidation by low concentrations of AAPH largely attacked RBP by breaking up covalent and non-covalent interactions of subunits and smaller peptides or partly unfolding its structure instead of forming more aggregation, whereas more smaller peptide and segregative subunits formed in RBP oxidized by high concentrations of AAPH, which had been transformed into soluble aggregates.
Emulsifying properties
As an emulsifier, protein can form and stabilize small droplets. When proteins adsorb to the oil–water interface in an emulsion, they reduce the interfacial tension, therefore promoting droplet generation and forming an interfacial layer, which stabilizes droplets against flocculation or coalescence via electrostatic repulsion.[Citation54] In general, the emulsifying capability of proteins is dependent on their structural and physicochemical properties, including subunit composition, conformational stability, solubility, and hydrophobicity.[Citation55] As shown in , the EAI of oxidized RBP was much higher than that of native RBP. With the increase in AAPH concentration from 0 to 0.2 mM, the EAI of RBP significantly increased, which suggested that low oxidation promoted protein structure unfolding to expose more hydrophobic residues. However, the EAI of oxidized RBP decreased with the further increased AAPH concentration. It has been reported that highly oxidized RBP is non-flexible, which could interfere with structural rearrangement when adsorbed to oil–water interface and thus decrease the protein emulsion properties.[Citation55] Yuan et al.[Citation56] suggested that the soluble aggregates could easily adsorb to the oil–water interface and form a thicker emulsion layer, improving emulsifying properties, and thus, RBP oxidized by 25 mM AAPH had a high EAI. The ESI of RBP increased by oxidization with low and moderate concentrations of AAPH, but decreased with high AAPH concentration, which suggested that protein aggregation favored emulsion activity but disadvantaged emulsion stability.
Conclusion
The results of this research suggested that RBP oxidation induced carbonyl group generation, free sulfhydryl group degradation, and dityrosine formation. Low oxidation resulted in unfolding and structural rearrangement, exposing hydrophobic amino acids, while also increasing emulsifying activity. The soluble protein aggregates stabilized by disulfide bond formed in the RBP that was oxidized by high concentration AAPH increased the solubility. The structure–function relationship of oxidized protein and lipid peroxidation remains unknown, but further study is underway in the author’s lab.
Funding
The authors gratefully acknowledge the financial support received from Heilongjiang Province Programs for Science and Technology Development (GC13B213).
Additional information
Funding
References
- Wang, M.; Hettiarachchy, N.S.; Qi, M.; Burks, W.; Siebenmorgen, T. Preparation and Functional Properties of Rice Bran Protein Isolate. Journal of Agricultural and Food Chemistry 1999, 47, 411–416. doi:10.1021/jf9806964.
- Zhang, H.-J.; Zhang, H.; Wang, L.; Guo, X.-N. Preparation and Functional Properties of Rice Bran Proteins from Heat-stabilized Defatted Rice Bran. Food Research International 2012, 47, 359–363.
- Fabian, C.; Ju, Y.-H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Critical Reviews in Food Science and Nutrition 2011, 51, 816–827.
- Tang, S.; Hettiarachchy, N.S.; Horax, R.; Eswaranandam, S. Physicochemical Properties and Functionality of Rice Bran Protein Hydrolyzate Prepared from Heat-stabilized Defatted Rice Bran with the Aid of Enzymes. Journal of Food Science 2003, 68, 152–157.
- Mawal, Y.R.; Mawal, M.R.; Ranjekar, P. Biochemical and Immunological Characterization of Rice Albumin. Bioscience Reports 1987, 7, 1–9.
- Helm, R.M.; Burks, A. Hypoallergenicity of Rice Protein. Cereal Foods World 1996, 41, 839–843.
- Kawamura, Y.; Muramoto, M. Anti-Tumorigenic and Immunoactive Protein and Peptide Factors in Foodstuffs. 2. Anti-Tumorigenic Factors in Rice Bran, Food & Cancer Prevention 1993.
- Shoji, Y.; Takashi, M.; Isemura, M.; Tomohiro, M.; Sumihiro, H.; Isemura, S.; Aoyagi, Y. A Fibronectin-Binding Protein from Rice Bran with Cell Adhesion Activity for Animal Tumor Cells. Bioscience, Biotechnology, and Biochemistry 2001, 65, 1181–1186.
- Adebiyi, A.P.; Adebiyi, A.O.; Ogawa, T.; Muramoto, K. Purification and Characterisation of Antioxidative Peptides from Unfractionated Rice Bran Protein Hydrolysates. International Journal of Food Science & Technology 2008, 43, 35–43.
- Wiboonsirikul, J.; Kimura, Y.; Kanaya, Y.; Tsuno, T.; Adachi, S. Production and Characterization of Functional Substances from a By-product of Rice Bran Oil and Protein Production by a Compressed Hot Water Treatment. Bioscience, Biotechnology, and Biochemistry 2008, 72, 384–392.
- Wiboonsirikul, J.; Hata, S.; Tsuno, T.; Kimura, Y.; Adachi, S. Production of Functional Substances from Black Rice Bran by Its Treatment in Subcritical Water. LWT Food Science and Technology 2007, 40, 1732–1740.
- Chittapalo, T.; Noomhorm, A. Ultrasonic Assisted Alkali Extraction of Protein from Defatted Rice Bran and Properties of the Protein Concentrates. International Journal of Food Science & Technology 2009, 44, 1843–1849.
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein Oxidation in Muscle Foods: A Review. Molecular Nutrition & Food Research 2011, 55, 83–95.
- Davies, M.J. The Oxidative Environment and Protein Damage. Biochimica et Biophysica Acta, Proteins Proteomics 2005, 1703, 93–109.
- Shacter, E. Quantification and Significance of Protein Oxidation in Biological Samples. Drug Metabolism Reviews 2000, 32, 307–326.
- Hawkins, C.L.; Davies, M.J. Generation and Propagation of Radical Reactions on Proteins. Biochimica et Biophysica Acta, Bioenergy 2001, 1504, 196–219.
- Shanlin, F.; Stocker, R.; Davies, M.J. Biochemistry and Pathology of Radical-mediated Protein Oxidation. Biochemical Journal 1997, 324, 1–18.
- Santé-Lhoutellier, V.; Engel, E.; Aubry, L.; Gatellier, P. Effect of Animal (Lamb) Diet and Meat Storage on Myofibrillar Protein Oxidation and in Vitro Digestibility. Meat Science 2008, 79, 777–783.
- Davies, K.; Lin, S.; Pacifici, R. Protein Damage and Degradation by Oxygen Radicals. IV. Degradation of Denatured Protein. The Journal of Biological Chemistry 1987, 262, 9914–9920.
- Wolff, S.P.; Dean, R.T. Fragmentation of Proteins by Free Radicals and Its Effect on Their Susceptibility to Enzymic Hydrolysis. Biochemical Journal 1986, 234, 399–403.
- Duggan, S.; Rait, C.; Platt, A.; Gieseg, S.P. Protein and Thiol Oxidation in Cells Exposed to Peroxyl Radicals Is Inhibited by the Macrophage Synthesised Pterin 7, 8-Dihydroneopterin. Biochimica et Biophysica Acta, Molecular Cell Research 2002, 1591, 139–145.
- Gieseg, S.P.; Pearson, J.; Firth, C.A. Protein Hydroperoxides are a Major Product of Low Density Lipoprotein Oxidation during Copper, Peroxyl Radical and Macrophage-mediated Oxidation. Free Radical Research 2003, 37, 983–991.
- Gieseg, S.; Duggan, S.; Gebicki, J.M. Peroxidation of Proteins before Lipids in U937 Cells Exposed to Peroxyl Radicals. Biochemical Journal 2000, 350, 215–218.
- Zhou, L.; Yang, Y.; Ren, H.; Zhao, Y.; Wang, Z.; Wu, F.; Xiao, Z. Structural Changes in Rice Bran Protein upon Different Extrusion Temperatures: A Raman Spectroscopy Study. Journal of Chemistry 2016, 2016, 1–8.
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of Oxidation on the Emulsifying Properties of Soy Protein Isolate. Food Research International 2013, 52, 26–32.
- Huang, Y.; Hua, Y.; Qiu, A. Soybean Protein Aggregation Induced by Lipoxygenase Catalyzed Linoleic Acid Oxidation. Food Research International 2006, 39, 240–249.
- Sun, W.; Cui, C.; Zhao, M.; Zhao, Q.; Yang, B. Effects of Composition and Oxidation of Proteins on Their Solubility, Aggregation and Proteolytic Susceptibility during Processing of Cantonese Sausage. Food Chemistry 2011, 124, 336–341.
- Wang, Z.; Han, F.; Sui, X.; Qi, B.; Yang, Y.; Zhang, H.; Wang, R.; Li, Y.; Jiang, L. Effect of Ultrasound Treatment on the Wet Heating Maillard Reaction between Mung Bean [Vigna Radiate (L.)] Protein Isolates and Glucose and on Structural and Physico-Chemical Properties of Conjugates. Journal of the Science of Food and Agriculture 2015, 96, 1532–1540.
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship between Surface Hydrophobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. International Journal of Food Properties 2015, 18, 1059–1074.
- 475389,; Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. Journal of Chemistry 2014, 2014, 10.
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. The Journal of Biological Chemistry 1951, 193, 265–275.
- Butterfield, D.A.; Stadtman, E.R. Protein Oxidation Processes in Aging Brain. Advances in Cell Aging and Gerontology 1997, 2, 161–191.
- Niki, E.; Kawakami, A.; Saito, M.; Yamamoto, Y.; Tsuchiya, J.; Kamiya, Y. Effect of Phytyl Side Chain of Vitamin E on Its Antioxidant Activity. The Journal of Biological Chemistry 1985, 260, 2191–2196.
- Buttkus, H. The Reaction Of Malonaldehyde or Oxidized Linolenic Acid with Sulfhydryl Compounds. Journal of the American Oil Chemists' Society 1972, 49, 613–614.
- Stadtman, E.R.; Levine, R.L. Protein Oxidation. Annals of the New York Academy of Sciences 2000, 899, 191–208.
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and Foaming Properties of Transglutaminase-treated Wheat Gluten Hydrolysate as Influenced by pH, Temperature and Salt. Food Hydrocolloids 2009, 23, 72–81.
- Hua, Y.; Cui, S.W.; Wang, Q.; Mine, Y.; Poysa, V. Heat Induced Gelling Properties of Soy Protein Isolates Prepared from Different Defatted Soybean Flours. Food Research International 2005, 38, 377–385.
- Laligant, A.; Dumay, E.; Casas Valencia, C.; Cuq, J.L.; Cheftel, J.C. Surface Hydrophobicity and Aggregation Of. Beta.-Lactoglobulin Heated near Neutral pH. Journal of Agricultural and Food Chemistry 1991, 39, 2147–2155.
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative Modification of Soy Protein by Peroxyl Radicals. Food Chemistry 2009, 116, 295–301.
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. Journal of Chemistry 2014, 2014, 1–10.
- Kalapathy, U.; Hettiarachchy, N.; Rhee, K. Effect of Drying Methods on Molecular Properties and Functionalities of Disulfide Bond-Cleaved Soy Proteins. Journal of the American Oil Chemists' Society 1997, 74, 195–199.
- Campos, A.; Lissi, E.; Vergara, C.; Lanio, M.; Alvarez, C.; Pazos, I.; Morera, V.; Garcia, Y.; Martinez, D. Kinetics and Mechanism of St I Modification by Peroxyl Radicals. Journal of Protein Chemistry 1999, 18, 297–306.
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophysical Journal 2001, 80, 2093–2109.
- Saeed, S.; Gillies, D.; Wagner, G.; Howell, N.K. ESR and NMR Spectroscopy Studies on Protein Oxidation and Formation of Dityrosine in Emulsions Containing Oxidised Methyl Linoleate. Food and Chemical Toxicology 2006, 44, 1385–1392.
- Chen, N.; Zhao, M.; Sun, W. Effect of Protein Oxidation on the in Vitro Digestibility of Soy Protein Isolate. Food Chemistry 2013, 141, 3224–3229.
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR Spectra Studies on the Secondary Structures of 7S and 11S Globulins from Soybean Proteins Using AOT Reverse Micellar Extraction. Food Hydrocolloids 2008, 22, 568–575.
- Meng, G.; Ma, C.Y. Characterization of Globulin from Phaseolus Angularis (Red Bean). International Journal of Food Science & Technology 2002, 37, 687–695.
- Lee, H.-J.; Choi, C.; Lee, S.-J. Membrane-Bound Α-Synuclein Has a High Aggregation Propensity and the Ability to Seed the Aggregation of the Cytosolic Form. The Journal of Biological Chemistry 2002, 277, 671–678.
- Choi, S.-M.; Ma, C.-Y. Conformational Study of Globulin from Common Buckwheat (Fagopyrum Esculentum Moench) by Fourier Transform Infrared Spectroscopy and Differential Scanning Calorimetry. Journal of Agricultural and Food Chemistry 2005, 53, 8046–8053.
- Adebiyi, A.P.; Adebiyi, A.O.; Hasegawa, Y.; Ogawa, T.; Muramoto, K. Isolation and Characterization of Protein Fractions from Deoiled Rice Bran. European Food Research and Technology 2009, 228, 391–401.
- Hamada, J. Characterization of Protein Fractions of Rice Bran to Devise Effective Methods of Protein Solubilization. Cereal Chemistry 1997, 74, 662–668.
- 546345,; Wang, C.; Li, D.; Xu, F.; Hao, T.; Zhang, M. Comparison of Two Methods for the Extraction of Fractionated Rice Bran Protein. Journal of Chemistry 2014, 2014, 10.
- Walstra, P. Principles of Emulsion Formation. Chemical Engineering Science 1993, 48, 333–349.
- Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural Changes of Soy Proteins at the Oil–Water Interface Studied by Fluorescence Spectroscopy. Colloids Surfaces B 2012, 93, 41–48.
- Yuan, B.; Ren, J.; Zhao, M.; Luo, D.; Gu, L. Effects of Limited Enzymatic Hydrolysis with Pepsin and High-pressure Homogenization on the Functional Properties of Soybean Protein Isolate. LWT Food Science and Technology 2012, 46, 453–459.