ABSTRACT
Cumin (Cuminum cyminum L.) is one of the most important herbs known for stomach disorders. In this study, storage temperature effects on the quality of cumin essential oil were investigated. Changes in essential oils compositions were detected during storage for six months in a refrigerator (4°C), a freezer (−20°C), and at room temperature (25°C). The main constituents of the essential oil from the cumin fruits under different conditions of storage were cumin aldehyde belonging to oxygenated monoterpenes and p-cymene, and β-pinene belonging to monoterpene hydrocarbons. Results indicated that at room temperature, the proportions of compounds with lower boiling temperatures such as β-pinene (1.57–10.03%) and p-cymene (14.93–24.9%) were decreased; however, cumin aldehyde (45.45–64.31%) increased during cumin oil storage. Furthermore, the oil compositions showed the least alterations and C. cyminum kept its primary quality when stored at low temperatures.
Introduction
Essential oils are aromatic and volatile liquids obtained from various species and parts of plants, herbs, and spices such as flowers, roots, bark, leaves, seeds, peel, fruits, and wood, described by a strong and generally pleasant odor and flavor.[Citation1] For centuries, these substances have been widely utilized in medicine, perfumery, and cosmetics industries. Moreover, they have been added to foods as part of spices or herbs.[Citation2] They constitute very potent and natural biologically active agents with various properties. They possess a range of pharmaceutically relevant characteristics due to their antioxidant, antifungal, antibacterial, anti-parasitic, and insecticidal properties, which result in a wide field of applications.[Citation3–Citation5]
The essential oils are complex mixtures of several chemical components that mainly include hydrocarbons, monoterpenes, and sesquiterpenes, with the general formula (C5H8)n. In addition, oxygenated compounds include alcohols, aldehydes, esters, ethers, ketones, phenols, and oxides obtained from these hydrocarbons.[Citation2,Citation6]Cuminum cyminum L. (“Zireh-e-Sabz” Persian name means green cumin), which is a well-known herbal medicine in Iran, is an annual herb belonging to the family Apiaceae. The origin of C. cyminum is Egypt, Turkistan, and the east of the Mediterranean area. Moreover, the herb is cultivated widely in Iran, China, India, Morocco, southern Russia, Japan, Indonesia, Algeria, and Turkey.[Citation7]
The cumin fruit provides additional taste and flavor to foods, besides it has been considered to have medicinal and therapeutic properties for centuries. In folk medicine, the cumin fruit is used as a diuretic, emmanogogic, antispasmodic, carminative, stimulant, astringent as well as a remedy against indigestion, flatulence, toothache, dyspepsia, diarrhea, colic, epilepsy, and jaundice.[Citation8,Citation9] Moreover, the cumin fruits contain a yellow-colored fresh volatile oil (2–5% v/w) that imparts the characteristic aroma and medicinal value to the ripe fruits. Results of investigations[Citation7–Citation10] indicated that the essential oil from the cumin fruits have high antifungal, antibacterial, and antioxidant activities. Therefore, it is also used as a fumigant or additive in the storage of foodstuffs.[Citation11]
Many phytochemical studies have been conducted to investigate the composition of the essential oil of cumin fruits.[Citation10,Citation12] These state that the major components of cumin essential oil are oxygenated compounds, especially aldehydes, wherein the most prominent one is cumin aldehyde in different percentages.[Citation13,Citation14] Variation in the chemical composition of essential oils, although genetically controlled, may be influenced by the origin and environmental conditions, harvesting and postharvesting stages like the season and phenological stage of harvest, drying and extraction methods, storage conditions, and distillation time and type, which lead to higher essential oil content and better quality.[Citation15–Citation18] In addition, according to the previous investigations, the essential oils compositions change due to the processing and storage of the isolated oil. Several factors such as temperature, light, and oxygen availability have a very important influence on the modification processes.[Citation19] The impacts of different storage conditions on the essential oils composition of Leonurus cardiac,[Citation20] Majorana hortensis,[Citation21] Rosa damascena (petals),[Citation22] Thymus daenensis,[Citation23] and Myrtus communis[Citation24] were studied before.
There is little chemical information about the compositional changes of essential oils during storage conditions. Therefore, the aim of this study was to evaluate the effect of storage conditions on the chemical composition of essential oil of C. cyminum. In this regard, the essential oil components of C. cyminum were analyzed in different storage conditions including room temperature (25°C), refrigerator (4°C), and freezer (−20°C) during six months of storage.
Material and methods
Plant material
Cumin fruits were collected from the plants cultivated in Khorasan-e-Razavi province region, northeast Iran. The fruits were harvested at the full mature stage and stored in room temperature until oil extraction.
Isolation of essential oil and storage conditions
Cumin fruits were crashed and the essential oil isolated by hydrodistillation for 4 h, using a Clevenger-type apparatus. The distillated oils were dried over anhydrous sodium sulfate. To investigate the impacts of temperatures and different storage conditions on the compositions of distilled oils, the oil samples were poured in three dark glasses, sealed, and were subjected to different storage temperatures such as refrigerator (4°C), freezer (−20°C), and room temperature (25°C) for six continuous months. The oil analyses of all storage treatments were performed monthly. Every month, each sample was injected to Gas Chromatography with Flame lionization Detector (GC_FID) and gas chromatography and mass spectrophotometry (GC/MS) and the compounds were detected. Moreover, to determine the exact effects of storage conditions on the essential oil composition during the experimental period, the fresh extracted oil was analyzed immediately after extraction. The extracted essential oil was yellow in color and had a distinct sharp odor.
Oil analysis procedure
The composition of the essential oils was determined by GC/MS. The GC analysis was performed on an Agilent Technologies 7890 GC (Agilent Technologies, Santa Clara, CA) equipped with a single injector and a flame ionization detector (FID) using an HP–5MS capillary column (30 m × 0.25 mm, 0.25 µm film thicknesses) coated with 5% phenyl and 95% methyl polysiloxane. The carrier gas was helium (99.999% pure) at a flow rate of 0.8 mL/min. The initial column temperature was 60°C and it was programmed to increase at 4°C/min to 280°C. The injector temperature was set at 300°C. Split injection was conducted with a ratio split of 1:40. Essential oil samples of 0.1 µL were injected neat (directly).
GC-MS analyses of aromatic oil samples were performed on an Agilent Technologies 7890 gas chromatograph coupled to an Agilent 5975 C mass selective detector (MSD) and a quadrupole Electron Ionization (EI) mass analyzer (Agilent Technologies, Palo Alto, CA, USA). An HP–5MS 5% column (coated with methyl silicone) (30 m × 0.25 mm, 0.25 µm film thicknesses) was used as the stationary phase. Helium was used as the carrier gas at 0.8 mL/min flow rate. The temperature was programmed from 60 to 280°C at a 4°C/min ramp rate. The injector and the GC-MS interface temperatures were maintained at 290°C and 300°C, respectively. Mass spectra were recorded at 70 eV. The mass range was from m/z 50 to 550. The ion source and the detector temperatures were maintained at 250 and 150°C, respectively. The samples (0.1 µL) were injected neat with a 1:40 split ratio.
Identification of compounds
Constituents were identified by comparison of their retention index (RI) relative to the C5-C24 n-alkanes obtained on a nonpolar HP-5MS column by comparison of the RI, provided in the literature, by comparison of the mass spectra with those recorded by the NIST 08 (National Institute of Standards and Technology) and Willey (ChemStation data system). The individual constituents were identified by RIs and compared with constituents known from the literature.[Citation25,Citation26] The percentage composition was computed from the GC-FID peak areas without using any correction factors.
Results and discussion
Different storage conditions can influence the chemical components of the essential oil. There is little research on the storage conditions of plant secondary metabolites, especially essential oils as volatile compounds. In this investigation, the compositions of hydro-distilled essential oils of C. cyminum were determined at different storage temperatures and times. In total, 16 components were identified, which represent 92.6–99.86% of the total detected compounds in essential oil samples (–). According to the GC/MS analysis shown in the tables, the main components of the essential oils from the fruits of cumin under all storage conditions during six months were cumin aldehyde belonging to oxygenated monoterpenes and p-cymene, and β-pinene belonging to monoterpene hydrocarbons.
Table 1. Chemical composition of C. cyminum essential oils during six months of storage at room temperature (25°C)
Table 2. Chemical composition of C. cyminum essential oils during six months of storage at refrigerator temperature (4◦C)
Table 3. Chemical composition of C. cyminum essential oils during six months of storage at freezer temperature (−20◦C)
According to the results of previous studies, the major components of cumin essential oil from various regions and phenological stages were cumin aldehyde, cumin alcohol, β-pinene, ocimene, γ-terpinene, p-cymene, and safranal in different percentages.[Citation10,Citation27,Citation28] In most studies, aldehydes compounds, especially cumin aldehyde, were found to be the most prominent,[Citation29] which was the same as our report. To the best of our knowledge, the pathway for the biosynthesis of cumin aldehyde and its precursor is not understood yet and the mechanism of transformation for essential oils compound is doubtful. In addition, the fresh and spicy notes of cumin oil are related to γ-terpinene and cumin aldehyde, respectively.[Citation30]
The results of this study indicate that the concentration of components with a lower molecular weight decreased with storage time, especially at room-temperature conditions (– and –). The findings of this investigation indicated that at room temperature, the changes in the amounts of some compounds are as follows: β-pinene, as the main constituent of cumin oil, was 10.93% immediately after oil extraction, then gradually decreased to 1.57%, and then showed about 85.63% decline at the end of the storage period. The trend of β-pinene evolution following one to six months after essential oils storage was 8.23, 42.45, 51.87, 55.26, 75.85, and 85.63%, respectively.
Figure 1. Changes of the major compounds of Cuminum cyminum essential oil during six months of storage at room temperature.
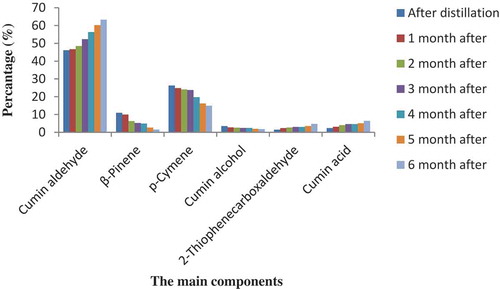
Figure 2. Percentage of the main compounds of C. cyminum essential oils after six months of storage at room temperature (25°C), refrigerator (4°C), and freezer (−20°C) conditions.
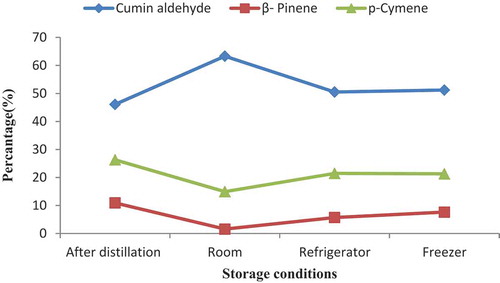
Figure 3. Percentage of the grouped components of C. cyminum essential oils after six months of storage at room temperature (25°C), refrigerator (4°C), and freezer (−20°C) conditions.
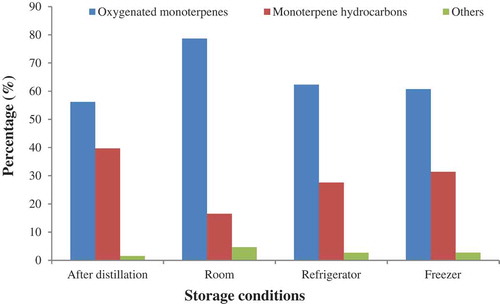
The second main component that showed the same trend with β-pinene was p-cymene, which showed a decreasing trend with storage time. The quantity of p-cymene was 26.3% at the time of oil extraction and then its amounts were 24.81, 24.09, 23.78, 19.71, 16.19, and 14.93%, after one to six months, respectively. The concentration of p-cymene decreased up to 43.23% after six months of storage ( and ). It might be due to evaporation, oxidation, and other unwanted changes in the essential oils constituents during the storage period. It is clear that with time, lower boiling point compounds have a marked decrease at room-temperature storage condition.
The most important results of the present study were the increasing trend in the quantities of cumin aldehyde by the storage time at room temperature. The cumin aldehyde was 46.10% immediately after oil extraction, then gradually reached 63.31% after six months, and showed a trend of 37.33% growth at the end of the storage period. The increasing trends of this component’s evolution following one to six months after essential oil storage were 46.67, 48.46, 52.32, 56.29, 60.21, and 63.31%, respectively ( and ). At refrigerator and freezer temperatures, the same trends were observed. β-Pinene and p-cymene showed a decreasing trend with storage time; however, cumin aldehyde, as the major constituent in cumin oil, increased during the cumin oil storage (, and ). The comparison of essential oils constituents between different conditions of storage (temperature and time) indicated that the major components amounts were intensively changed during storage at room temperature compared to other storage conditions (). Moreover, according to the results of this study, 2-thiophenecarboxaldehyde was identified in the essential oil of cumin. It seems to be a new compound for the essential oil of cumin ().
On the other hand, our findings indicated that safranal decreased during storage. In this regard, the findings of our work indicated that the concentration of safranal with a lower molecular weight decreased by prolonging the storage time (). This phenomenon can be due to evaporation, oxidation, and other unwanted changes in the essential oil components during the storage period.[Citation20] It is clear that with passing time, lower boiling point compounds markedly decreased in refrigerator and particularly at room-temperature conditions. However, it was very slight in freezing temperature.[Citation23] Furthermore, the effects of various temperatures such as room temperature (25°C), refrigerator (4°C), and freezer (−20°C) on the essential oil composition of different medicinal plants have been studied before.[Citation20,Citation23,Citation31]
The primary components of essential oils from plants are monoterpenes and the effects of many medicinal herbs have been attributed to them.[Citation32] In this study, monoterpenoids were the most common components identified in cumin essential oil. All samples at different storage conditions are mainly composed of oxygenated monoterpenes, represented exclusively by cumin aldehyde with different percentages from 45.59 to 63.31% at different times and temperatures. In addition, monoterpene hydrocarbons, including p-cymene and β-pinene, were the second group of chemical components (– and ).
Changes in the essential oil compositions of Melissa officinalis[Citation33] and Thymus daenensis[Citation23] were evaluated during storage for four months in a refrigerator (4°C), a freezer (−20°C), and at room temperature (25°C). In conformity with our results, they reported that, at room temperature, the proportions of compounds with lower boiling temperatures were decreased. Furthermore, the oil compositions showed the least alterations and retained their primary quality when stored at low temperatures, particularly at −20°C.
Variation in the chemical composition of cumin oil indicated that different chemotypes of C. cyminum L. exist in the world. In fact, several chemotypes were reported according to the origin of the studied cumin. In conformity with our findings, the results of previous studies showed the major component in the essential oil of the Spanish cumin.[Citation34] In agreement with our findings, cumin aldehyde chemotype was found by Li & Jiang[Citation11] and Oroojalian et al.[Citation12] who studied Turkish, Chinese, and Egyptian cumin fruits.
On the other hand, the essential oils should be stored in tightly closed, darkened glass containers in a cool place to ensure lasting quality.[Citation35] According to previous investigations on change in the composition of the essential oil of marjoram during storage, insignificant changes in the composition of the essential oil were observed when stored in a dark glass container for one year and its organoleptic characteristics remained largely unaffected. However, storage in a transparent container changed the composition of the oil, due to the chemical transformations of terpenoids. Insignificant changes in the content of substances stored in the dark may be attributed to their structure and reactivity. Oxidation of the most labile components of the oil was the main process.[Citation36]
Only few studies have been performed to assess the effect of storage conditions on the stability of essential oils.[Citation19,Citation36,Citation37] According to the results of previous investigations, the composition of essential oils from cardamom (Elettaria cardamomum Maton L.) and clove (Caryophyllus aromaticus L.)[Citation38] changed in storage condition, which affected their properties. The oil composition may undergo considerable changes upon processing and storage conditions. Previous reports indicated that several factors such as different harvest time, storage conditions, storage duration at different temperatures, light, and oxygen availability can affect the essential oil quality and composition.[Citation19,Citation23,Citation39–Citation41] This may lead to changing color, an increase in viscosity, or the development of an unpleasant, often sharp aroma by alternations in composition and a rise of oxidized compounds. Moreover, due to the terpenoid oxidation products, some allergic skin reactions may accrue.[Citation42–Citation44]
Conclusion
The main process during the storage of essential oil is evaporating the compounds with a lower boiling temperature, mostly monoterpene hydrocarbons. From the findings of this study, the best results of the major constituents were obtained at −20°C and 4°C (the total of 39.67% monoterpene hydrocarbons after distillation is reduced to 31.36% and 27.59%, respectively, after six months). Hence, there was a higher decrease in the oil quality during the storage period of six months at room temperature. It can be concluded that the essential oil of C. cyminum, which was stored in a refrigerator and a freezer, retained its primary quality better compared with room-temperature storage. In sum, the storage of C. cyminum oil at low temperatures can prevent the decrease in the concentrations of the oil compounds and help maintain the essential oil’s primary quality with the least alterations. These results may be extended to the storage of the essential oils of plants with the same chemical characteristics. Hence, these useful findings can help essential oil producers and consumers use this oil and its compounds in different related industries such as food, cosmetic, and medicinal products. In conclusion, the different storage conditions of plant secondary metabolites, especially essential oils, is an interesting topic, which needs further studies with various aromatic plant essential oils that constitute different chemical compounds.
References
- Sanchez, E.; Garcia, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio Cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. DOI:10.1128/AEM.03052-09.
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6(1), 39–44. DOI:10.1186/1472-6882-6-39.
- Adorjan, B.; Buchbauer, G. Biological Properties of Essential Oils: An Updated Review. Flavour. Fragr. J. 2010, 25, 407–426. DOI:10.1002/ffj.2024.
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D. I.; Domingues, F. C. Antifungal Activity of Coriandrum Sativum Essential Oil, Its Mode of Action against Candida Species and Potential Synergism with Amphotericin B. Phytomedicine. 2011, 19, 42–47. DOI:10.1016/j.phymed.2011.06.033.
- Bajpai, V. K.; Baek, K.-H.; Kang, S. C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2011, 45, 722–734. DOI:10.1016/j.foodres.2011.04.052.
- Espina, L.; Somolinos, M.; Loran, S.; Conchello, P.; Garcia, D.; Pagan, R. Chemical Composition of Commercial Citrus Fruit Essential Oils and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes. Food Control. 2011, 22, 896–902. DOI:10.1016/j.foodcont.2010.11.021.
- Tuncturk, R.; Tuncturk, M. Effects of Different Phosphorus Levels on the Yield and Quality Components of Cumin (Cuminum Cyminum L.). Res. J. Agric. Biol. Sci. 2006, 2, 336–340.
- El–Ghorab, A. H.; Nauman, M.; Anjum, F. M.; Hussain, S.; Nadeem, M. A Comparative Study on Chemical Composition and Antioxidant Activity of Ginger (Zingiber Officinale) and Cumin (Cuminum Cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. DOI:10.1021/jf101202x.
- Allahghdri, T.; Rasooli, I.; Owlia, P.; Nadooshan, M. J.; Ghazanfari, T.; Taghizadeh, M.; Astaneh, S. D. A. Antimicrobial Property, Antioxidant Capacity, and Cytotoxicity of Essential Oil from Cumin Produced in Iran. J. Food Sci. 2010, 75, H54–H61. DOI:10.1111/j.1750-3841.2009.01467.x.
- Hajlaoui, H.; Mighri, H.; Noumi, E.; Snoussi, M.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A. Chemical Composition and Biological Activities of Tunisian Cuminum Cyminum L. Essential Oil: A High Effectiveness against Vibrio Spp. Strains. Food Chem. Toxicol. 2010, 48, 2186–2192. DOI:10.1016/j.fct.2010.05.044.
- Li, R.; Jiang, Z. T. Chemical Composition of the Essential Oil of Cuminum Cyminum L. from China. Flavour. Fragr. J. 2004, 19, 311–313. DOI:10.1002/ffj.1302.
- Oroojalian, F. R.; Kasra-Kermanshahi, R.; Azizi, M.; Bassami, M. R. Phytochemical Composition of the Essential Oils from Three Apiaceae Species and Their Antibacterial Effects on Food-Borne Pathogens. Food Chem. 2010, 120, 765–770. DOI:10.1016/j.foodchem.2009.11.008.
- Sowbhagya, H. B.; Sathyendra Rao, B. V.; Krishnamurthy, N. Evaluation of Size Reduction and Expansion on Yield and Quality of Cumin (Cuminum Cyminum) Seed Oil. J. Food. Eng. 2008, 84, 595–600. DOI:10.1016/j.jfoodeng.2007.07.001.
- Bettaieb, I.; Bourgou, S.; Wannes, W. A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential Oils, Phenolics, and Antioxidant Activities of Different Parts of Cumin (Cuminum Cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. DOI:10.1021/jf102248j.
- Moghaddam, M.; Omidbaigi, R.; Sefidkon, F. Changes in Content and Chemical Composition of Tagetes Minuta Oil at Various Harvest Times. J. Essential Oil Res. 2007, 19, 18–20. DOI:10.1080/10412905.2007.9699218.
- Figueiredo, A. C.; Barroso, J. G.; Pedro, L. G.; Scheffer, J. J. C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour. Fragr. J. 2008, 23, 213–226. DOI:10.1002/ffj.1875.
- Demuner, A. J.; Barbosa, L. C. A.; Magalhaes, C. G.; Da Silva, C. J.; Maltha, C. R. A.; Pinheiro, A. L. Seasonal Variation in the Chemical Composition and Antimicrobial Activity of Volatile Oils of Three Species of Leptospermum (Myrtaceae) Grown in Brazil. Molecules. 2011, 16, 1181–1191. DOI:10.3390/molecules16021181.
- Moghaddam, M.; Khaleghi Miran, S. N.; Ghasemi Pirbalouti, A.; Mehdizadeh, L.; Ghaderi, Y. Variation in Essential Oil Composition and Antioxidant Activity of Cumin (Cuminum Cyminum L.) Fruits during Stages of Maturity. Ind. Crops. Prod. 2015b, 70, 163–169. DOI:10.1016/j.indcrop.2015.03.031.
- Nguyen, H.; Campi, E. M.; Jackson, W. R.; Patti, A. F. Effect of Oxidative Deterioration on flavor and Aroma Components of Lemon Oil. Food Chem. 2009, 112, 388–393. DOI:10.1016/j.foodchem.2008.05.090.
- Mockute, D.; Bernotiene, G.; Judentiene, A. Storage-Induced Changes in Essential Oil Composition of Leonurus Cardiaca L. Plants Growing Wild in Vilnius and of Commercial Herbs. Chemija. 2005, 2, 29–32.
- Misharina, T. A.; Polshkov, A. N.; Ruchkina, E. L.; Medvedeva, I. B. Changes in the Composition of the Essential Oil of Marjoram during Storage. Appl. Biochemistry Microbiol. 2003, 39(3), 311–316. DOI:10.1023/A:1023592030874.
- Mohamadi, M.; Mostafavi, A.; Shamspur, T. Effects of Storage on Essential Oil Content and Composition of Rosa Damascena Mill Petals under Different Conditions. J. Essent. Oil Res. 2011, 14, 430–441. DOI:10.1080/0972060X.2011.10643598.
- Rowshan, V.; Bahmanzadegan, A.; Saharkhiz, M. J. Influence of Storage Conditions on the Essential Oil Composition of Thymus Daenensis Celak. Ind. Crops. Prod. 2013, 49, 97–101. DOI:10.1016/j.indcrop.2013.04.029.
- Bahramzadegan, A.; Rowshan, V.; Saharkhiz, M. J. Essential Oil Composition of Myrtus Communis L. under Different Storage Condition. J. Essent. Oil Res. 2015, 18(6), 1467–1475. DOI:10.1080/0972060X.2014.884767.
- Adams, R. P.;. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publications: Carol Stream, IL, 2007.
- McLafferty, F. W.;. Wiley Registry of Mass Spectral Data, 9th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, 2009; pp. 662.
- Rebey, I. B.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of Drought on the Biochemical Composition and Antioxidant Activities of Cumin (Cuminum Cyminum L.) Seeds. Ind. Crops. Prod. 2012, 36, 238–245. DOI:10.1016/j.indcrop.2011.09.013.
- Moghaddam, M.; Ghasemi Pirbalouti, A.; Mehdizadeh, L.; Pirmoradi, M. R. Changes in Composition and Essential Oil Yield of Ocimum Ciliatum at Different Phenological Stages. Eur. Food Res. Technol. 2015a, 240, 199–204. DOI:10.1007/s00217-014-2320-y.
- Derakhshan, D.; Sattari, M.; Bigdeli, M. Effect of Subinhibitory Concentrations of Cumin (Cuminum Cyminum L.) Seed Essential Oil and Alcoholic Extract on the Morphology Capsule Expression and Urease Activity of Klebsiella Pneumonia. Int. J. Antimicrob. Agents. 2008, 32, 432–436. DOI:10.1016/j.ijantimicag.2008.05.009.
- Jirovetz, L.; Buchbauer, G.; Stoynanava, A.; Georgiev, E.; Damianova, S. Composition, Quality Control and Antimicrobial Activity of the Essential Oil Cumin (C. Cyminum L.) Seeds from Bulgaria that Had Been Stored for up 36 Years. Int. J. Food Sci. Technol. 2005, 40, 305–310. DOI:10.1111/j.1365-2621.2004.00915.x.
- Baydar, H.; Schulz, H.; Kruger, H.; Erbas, S.; Kineci, S. Influences of Fermentation Time, Hydro-Distillation Time and Fractions on Essential Oil Composition of Damask Rose (Rosa Damascena Mill.). J. Essent. Oil Res. 2008, 11(3), 224–232. DOI:10.1080/0972060X.2008.10643624.
- Gherlardini, C.; Galeotti, N.; Mazzanti, G. Local Anaesthetic Activity of Monoterpenes and Phenylpropanes of Essentialoils. Planta Med. 2001, 67, 564–566. DOI:10.1055/s-2001-16475.
- Najafian, S. H.;. Storage Conditions Affect the Essential Oil Composition of Cultivated Balm Mint Herb (Lamiaceae) in Iran. Ind. Crops. Prod. 2014, 52, 575–581. DOI:10.1016/j.indcrop.2013.11.015.
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Angel Perez-Alvarez, J. Chemical Composition of the Essential Oils Obtained from Some Spices Widely Used in Mediterranean Region. Acta. Chim. Slov. 2007, 54, 921–926.
- Buckle, J.;. Clinical Aromatherapy: Essential Oils in Practice, 2nd ed.; Churchill Livingstone: Edinburgh, 2003.
- Misharina, T. A.; Polshkov, A. N. Antioxidant Properties of Essential Oils: Autoxidation of Essential Oils from Laurel and Fennel and of Their Mixtures with Essential Oil from Coriander. Appl. Biochemistry Microbiol. 2005, 41, 610–618. DOI:10.1007/s10438-005-0111-8.
- Haddouchi, F.; Chaouche, T.; Lazouni, H. A.; Benmansour, A. Physicochemical Study Essential Oils of Thymus Fontanesii according to Its Conservation. Der. Pharma. Chemica. 2011, 3, 404–410.
- Gopalakrishnan, N.;. Studies on the Storage Quality of Carbon Dioxide-Extracted Cardamom and Clove Bud Oils. J. Agric. Food Chem. 1994, 42(3), 796–798. DOI:10.1021/jf00039a039.
- Kazaz, S.; Erbas, S.; Baydar, H. The Effects of Storage Temperature and Duration on Essential Oil Content and Composition Oil Rose (Rosa Damascena Mill.). Turk. J. Field Crops. 2009, 4, 89–96.
- Turek, C.; Stintzing, F. C. Evaluation of Selected Quality Parameters to Monitor Essential Oil Alteration during Storage. J. Food Sci. 2011b, 76, C1365–C1375. DOI:10.1111/jfds.2011.76.issue-9.
- Kumar, R.; Shama, S.; Sood, S.; Agnihotri, V. K.; Singh, B. Effect of Diurnal Variability and Storage Conditions on Essential Oil Content and Quality of Damask Rose (Rosa Damascena Mill.) flowers in North Western Himalayas. Sci. Hortic. 2013, 154, 102–108. DOI:10.1016/j.scienta.2013.02.002.
- Hagvall, L.; Bucktorp, C.; Svensson, S.; Nyman, G.; Borje, A.; Karlberg, A.-T. Fragrance Compound Geraniol Forms Contact Allergens on Air Exposure. Identification and Quantification of Oxidation Products and Effect on Skin Sensitization. Chem. Res. Toxicol. 2007, 20, 807–814. DOI:10.1021/tx700017v.
- Brared-Christensson, J.; Forsstrom, P.; Wennberg, A.-M.; Karlberg, A.-T.; Matura, M. Air Oxidation Increases Skin Irritation from Fragrance Terpenes. Contact Derm. 2009, 60, 32–40. DOI:10.1111/j.1600-0536.2008.01471.x.
- Brared-Christensson, J.; Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A.-T. Linalool-A Significant Contact Sensitizer after Air Exposure. Contact Derm. 2010, 62, 32–41. DOI:10.1111/cod.2010.62.issue-1.
