ABSTRACT
In the present investigation, the antifungal and antimycotoxigenic activities of Boswellia serrata essential oil were evaluated in vitro and in viable maize. The GC-MS analysis of B. serrata essential oil showed a total of 29 constituents, among which, 3-carene (34.74%), β-ocimene (13.78%), D-limonene (8.25%), β-caryophyllene (6.65%) and terpinolene (5.39%) recorded the highest percentage. The B. serrata essential oil showed promising antifungal activity against 15 different field and storage fungi with percentage of mycelial inhibition ranged between 15.9–56.3% at 1µL mL˗1. Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values were ranged between 0.039–0.625 µL mL˗1 and 2.5–>10.0 µL mL˗1, respectively, against the same fungi tested. The production of aflatoxin B1 and fumonisin B1 were completely inhibited by B. serrata essential oil at 6 µL mL˗1in vitro. The ergosterol content was drastically decreased with the increasing concentration of B. serrata essential oil in vitro. Viable maize model confirmed that aflatoxin B1 and fumonisin B1 contents were significantly inhibited with increasing seedling vigour of maize. The results revealed that B. serrata essential oil could be explored for management of mould and mycotoxin contaminations in food grains and feedstuffs.
Introduction
Fungi are significant destroyers of foodstuffs during storage, rendering them unfit for human consumption by retarding their nutritive value and production of mycotoxins.[Citation1] Mycotoxins are toxic, structurally diverse, non-volatile and relatively low-molecular weight (MW <700) secondary metabolic products produced by a wide range of filamentous fungi such as species of Aspergillus, Alternaria, Fusarium and Penicillium, and they contaminate food, feed and various agricultural commodities either before harvest or under post-harvest conditions.[Citation2,Citation3] According to Food and Agricultural Organization (FAO) of the United Nations, it is estimated that around 25% of the world’s food grains are significantly contaminated with mycotoxins.[Citation4] Aflatoxins and fumonisins are the most common and wide spread contaminants in food and feedstuffs, which are mainly produced by species of Aspergillus and Fusarium, respectively.[Citation5,Citation6] Aspergillus flavus and Fusarium verticillioides are the predominant contaminants in food grains, maize in particular, by producing aflatoxin B1 (AFB1) and fumonisin B1 (FB1), respectively. The hepatotoxicity, teratogenicity, mutagenicity, immunosuppression, hemorrhage properties of AFB1 and FB1 to human has been well reported.[Citation7,Citation8] Because of their toxic properties, AFB1 and FB1 have been listed as Group 1 and Group 2B human carcinogens by the International Agency for Research on Cancer (IARC),[Citation9] respectively. The management of mould infestations and mycotoxins accumulation in food grains was generally achieved using synthetic chemicals, but residues of these chemicals cause damage to animal, plants and human.[Citation10,Citation11] The use of essential oils (EOs) for management of mould and mycotoxin contaminations in foodstuffs would be of great importance due to hazardous effects of synthetic fungicides.[Citation12,Citation13]
Boswellia serrata Roxb. ex Colebr. belongs to the family Burseraceae commonly found in India, northern Africa and Middle East.[Citation14] B. serrata has been investigated for various bioactive properties such as anti-arthritic, antiproliferative, antioxidant, analgesic, anti-inflammatory, renal and cardio protective activities.[Citation15–Citation19] Kasali et al.[Citation20] reported the various volatile constituents predominantly comprised monoterpenoids, of which α-pinene was major constituents from B. serrata bark. Antimicrobial activities of methanolic extract and resin oils of B. serrata against different human pathogenic bacteria and yeast have been previously reported.[Citation21–Citation25] Mishra and Dubey[Citation26] have reported the antifungal activity of Boswellia serrata essential oil (BSEO) against A. flavus. Although the antimicrobial activity has been previously reported, there are no reports on antimycotoxigenic property of BSEO and antifungal activities against wide range of field and storage fungi. In this article, we have reported the antifungal activity of BSEO against fifteen different field and storage fungi, and its inhibitory efficacy on AFB1 and FB1 production from toxigenic strains of A. flavus and F. verticillioides, respectively.
Materials and methods
Chemicals and culture media
All analytical grade solvents and culture media were purchased from Hi-Media (Mumbai, India). The synthetic fungicide copper oxychloride 50% WP (Fungicop-50) was purchased from Karnatak Agrochemicals Pvt Ltd (India) and zinc ethylene bisthiocarbamate 75% WP (Indofil Z-75) was purchased from Indofil Chemicals Company (India). Microtiter plates (96-well) were purchased from Axiva (New Delhi, India). AFB1 and FB1 standards were obtained from Sigma (Germany). Silica gel 60 F254 coated preparative thin layer chromatography (TLC) plates were procured from Merck (Germany).
Extraction of essential oil from B. serrata leaves
Fresh leaves of B. serrata were collected from the southern part of Karnataka (India) during 2014–16. The plant samples were authenticated by Dr. Seetharam, Professor, Department of Biological Sciences, Bangalore University, Bengaluru (India) and the authenticated voucher specimens were deposited in the Department of Microbiology and Biotechnology, Bangalore University (Bengaluru) along with a proper voucher number (Voucher numbers: BUB/MB-BT/DCM/DST-SERB/24/2014–15). BSEO was extracted by hydro-distillation method using Clevenger-type apparatus.[Citation27] The collected BSEO was stored in dark clean glass vial after removing water traces passing through anhydrous sodium sulphate and stored at 4°C for further analysis.
Chemical profile analysis of BSEO by GC-MS
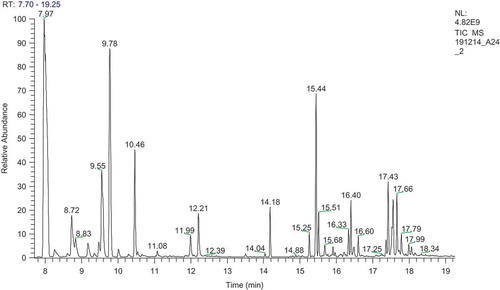
The chemical compounds present in the BSEO were identified by gas chromatography coupled to mass spectrometry (GC-MS, Thermo Electron Corporation Focus gas chromatograph, DSQ II model, Thermo Scientific, CA, USA). The GC conditions were as follows: Column, DB 5 ms with integrated guard column (Dimensions: 30mL x 0.25 mm ID x 0.25 µm film thickness); initial column temperature was 40°C for 2 min and ramp temperature was 280°C for 5 min; helium served as carrier gas; sample flow rate was 1.0 mL min˗1; injection volume was 1μL and scan mass ranged between 30 m/z–600 m/z with +ve polarity. The identification of individual component was done on the basis of retention times compared to those of authentic samples and matching spectral peaks available with National Institute of Standards and Technology (NIST, U.S Department of Commerce, Gaithersburg) spectral libraries. [Citation28]
Antifungal activity of BSEO
Fungal strains
The fungal species viz., Aspergillus flavus, A. fumigatus, A. ochraceus, A. tamarii, A. terreus, Fusarium equiseti, F. udum, F. verticillioides and Penicillium citrinum, which are associated predominantly with maize at a higher percentage, Alternaria geophila, Curvularia tetramera, F. lateritium, F. oxysporum and Penicillium expansum, which are frequently associated with sorghum at higher percentage, and Alternaria brassicicola (sunflower isolate) was collected from Department of Microbiology, University of Mysore, Mysore (India), were selected as test fungi for antifungal activity assay. The fungal species were previously isolated and reported by Thippeswamy et al.[Citation29] Mycotoxigenic strains of A. flavus and F. verticillioides were isolated from maize seeds in our previous study.[Citation5,Citation30] The isolated fungi were maintained on Sabouraud dextrose agar (SDA) and the 7-days-old cultures were used for the assays.
Determination of percent mycelial inhibition
The antifungal activity of BSEO was determined against fifteen different field and storage fungi by poisoned food technique following the procedure of Prakash et al.[Citation31] with minor modifications. The requisite amount of BSEO was dissolved in 0.5 mL of acetone and added to molten SDA to achieve final concentrations ranged from 1 to 10 µL mL˗1. A 5 mm disc of test fungi was placed at the centre of the plate and incubated at 28 ± 2°C for 7 days. The Petri dishes containing media with 0.5 mL of acetone served as control. The fungi-toxicity of the extract in terms of percentage inhibition of mycelial growth was calculated using the formula:
Where, dC = average mycelial growth in control and dT = average mycelial growth in treatment.
Determination of MICs and MFCs
The broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of BSEO following the standard procedures with some modifications.[Citation32,Citation33] Briefly, 200 μL of two-fold serially diluted BSEO in Sabouraud dextrose broth (SDB) (0.019–10.0 µL mL˗1) was added separately to the wells of a sterile 96-well microtiter plate and inoculated with 15 μL of fungal spore suspension (104 spores mL˗1) and incubated at 28 ± 2°C for 72 hours. SDB containing same concentration of copper oxychloride 50% WP and zinc ethylene bisthiocarbamate 75% WP served as positive control and SDB containing only Tween-80 served as negative control. After incubation, a 10 μL of treated broths were streaked radially onto the SDA plates and incubated at the same condition. After the incubation period, the MIC/MFC values were recorded. Parallelly, the results were also confirmed by adding a 50 μL of iodonitrotetrazolium chloride (INT; 2 mg mL˗1) to microtiter plate and incubated at 37°C for 30 min. The pale yellow-coloured INT was reduced to a pink colour which indicated the presence of fungal viability, while the yellow colour remained the same where the fungal growth was inhibited.
Effect of BSEO on AFB1 and FB1 production
The in vitro efficacy of BSEO on AFB1 production by A. flavus was determined using Sucrose-magnesium sulphate-potassium nitrate-yeast extract broth (SMKYB) medium.[Citation34] Briefly, 100 μL of spore suspension (104 spores mL˗1) of A. flavus were inoculated into SMKYB containing the requisite amount of BSEO (2.0–10.0 µL mL˗1) and incubated at 28 ± 2°C for 10 days. The broth cultures were filtered through Whatman No. 1 filter article and the obtained culture filtrate was used for AFB1 extraction by adding an equal volume of chloroform. The chloroform layer was collected and evaporated in vacuo. The AFB1 content was determined qualitatively by TLC method and quantitatively by spectrophotometric method (UV-1800, Shimadzu, Japan). The amount of AFB1 content was calculated following formula:
where, D is absorbance, M is molecular weight of AFB1 (312), E is molar extinction coefficient of AFB1 (21,800) and L is path length (1 cm).
The in vitro efficacy of BSEO on FB1 production by F. verticillioides was determined following the procedure of Bailly et al.[Citation35] with minor modifications. Briefly, 100 μL of spore suspension (104 spores mL˗1) of F. verticillioides was inoculated into SDA containing the requisite amount of BSEO (2.0 µL–10.0 µL mL˗1) and incubated at 28 ± 2°C for 10 days. To estimate FB1 production, the culture along with SDA was ground in acetonitrile-water (1:1, v/v) and filtered through 0.45 µm porous membrane filter. The extracted FB1 was visualised on eluted TLC chromatograms by spraying with 0.5% p-anisaldehyde solution followed by heating at 110°C for 10 min. The amount of FB1 was estimated using spectrophotodensitometer (Biorad, Universal Hood II, 720BR/02170, USA) at 600 nm by comparing with known concentrations of standard FB1.
Efficacy of BSEO on AFB1 and FB1 production in viable maize
The efficacy of BSEO on AFB1 and FB1 production in maize corn was determined following the procedure of Garcia et al.[Citation10] with some modifications. Briefly, freshly harvested maize samples were collected and the water activity (aw) was adjusted to 0.95 by adding sterile distilled water, based on the following formula:
where, W = volume of water required (ml), A = initial weight of the sample (g), a = initial moisture content (%) and b = required moisture content (%),[Citation36] and separated into two sets. One set of maize samples were treated with different concentrations of BSEO (2.0–10.0 µL g˗1) and inoculated with 100 μL of spore suspension (104 spores mL˗1) of A. flavus and F. verticillioides, separately. All treatments were incubated at 28 ± 2°C up to 10 days. After incubation, the A. flavus treated maize seeds (10 g) were milled and subjected for AFB1 extraction by adding an equal volume of CHCl3. After evaporation of chloroform, the residue was again re-dissolved in 1 mL of chloroform and subjected to qualitative and quantitative estimation following the standard procedures as described above.[Citation35,Citation37] Similarly, F. verticillioides treated maize seeds (10 g) were powdered using a warring blender and subjected to extraction of FB1 by adding 100 mL of acetonitrile-water (1:1, v/v), then filtered through Whatman No. 1 filter article and evaporated the acetonitrile-water on water bath. After that, the residue was re-dissolved in 1 mL of methanol, then 10 µL of sample was spotted on TLC plate along with desired different concentrations of standard FB1. Eluted the TLC plate using butanol-acetic acid-water (20:10:10, v/v/v) as a solvent system and allowed to air dry. The air-dried TLC plate was sprayed with 0.5% p-anisaldehyde in methanol-acetic acid-H2SO4 (85:10:0.5, v/v/v) solution and subjected to heat treatment at 110°C for 10 min in hot air oven (Labline, India). The amount of FB1 was estimated qualitatively and quantitatively by measuring the band intensity using spectrophoto-densitometer at 600nm and calculated the amount of FB1 by comparing with different concentrations of standard FB1. The other set was treated with 10.0 µL of BSEO and subjected to evaluation of natural seed borne mycoflora and seedling vigour of maize.[Citation37,Citation38] The percent incidences (PI) of the seed-borne fungi associated with maize seed samples were determined following the formula:
Simultaneously, to monitor the phytotoxic effect on maize, the seedling vigour was measured by recording the length of radicle and plumule of maize seedlings. The seedling vigour index (SVI) was determined following the formula.[Citation39]
Effect of BSEO on ergosterol content in fungal plasma membrane
Effect of BSEO on ergosterol content in the plasma membrane of A. flavus and F. verticillioides was detected by a method described previously by Abhishek et al.[Citation30] Fifty µL of spore suspension of A. flavus and F. verticillioides containing 106 spores mL˗1 was inoculated into respective medium (SMKYB for A. flavus and SDB for F. verticillioides) containing different concentrations of BSEO (2–10 µL mL˗1) and incubated at 28 ± 2°C for 4 days. A control set, tween-80, was kept parallel to the treatment. After incubation, mycelia were harvested and subjected to extraction of ergosterol. The extracted n-heptane layer was analysed by scanning spectrophotometry (UV-1800, Shimadzu, Japan) between 230 and 300 nm. The ergosterol content was calculated as a percentage of the mycelia wet weight by the following formula:
where, 290 and 518 are the E values (in percentages per cm) of crystalline ergosterol and 24(28)-dehydroergosterol, respectively, and pellet weight is the net wet weight (g).
Statistical analysis
The experiments were performed in triplicate and values were expressed as means ± standard error. Analysis of variance was conducted, and the differences between values were tested for deviation significance by ANOVA with the SPSS 19 (IBM, Armonk, NY, USA) programme. Differences at p ≤ 0.05 were considered statistically significant.
Results and discussion
Phytochemical characterization of BSEO
The extracted BSEO was clear to pale yellow in colour and its yield was 1.5% (v/w). The GC-MS analysis of BSEO revealed that a total of 29 phytoconstituents were present, among which 3-carene (34.74%), β-ocimene (13.78%), D-limonene (8.25%), β-caryophyllene (6.65%) and terpinolene (5.39%), were identified as major components with the highest percentage (; ). The geographical and seasonal variations have a significant effect on the yield and composition of essential oils.[Citation40] Also the plant species, parts and extraction methods that may show chemotypic variations.[Citation31,Citation41] Although some of the components of BSEO reported by the previous workers were same,[Citation42] there are variations in their percentage chemical composition in comparison with present study.
Table 1. Phytochemical constituents of BSEO.
Antifungal activity of BSEO
The growth inhibitory effect of BSEO was screened against a panel of 15 fungal pathogens containing storage and field fungi and the obtained results are presented in . The percentage of growth inhibition by the BSEO was estimated by measuring the growth diameter of colony grown on SDA with treatment and control. Most of the treated field fungi were susceptible at 1.0 µL mL˗1. Among the fungi tested, A. flavus and A. tamarii were found to be most resistant organisms with percent mycelial inhibition 15.9 and 17.5%, respectively. The field fungi such as A. brassicicola (56.3%), A. geophila (48.8%) and C. tetramera (46.9%), were found be most susceptible organisms at 1.0 µL mL˗1.The MIC and MFC values of BSEO were ranged between 0.039–0.625 µL mL˗1and 2.5–>10.0 µL mL˗1, respectively. The BSEO showed concentration-dependent growth inhibitory activity against toxigenic strains of A. flavus and F. verticillioides with percent mycelia inhibition 62.6% and 75.5%, at 10 µL mL˗1,respectively (). The MIC and MFC values of BSEO against toxigenic strains of A. flavus were 0.625 and >10 µL mL˗1, and against F. verticillioides were 0.312 and 10 µL mL˗1, respectively (). The obtained results were compared with MIC and MFC values of synthetic fungicides viz., copper oxychloride 50% and zinc ethylene bisthiocarbamate 75%. The MIC and MFC values of copper oxychloride 50% were ranged 39–1000 µg mL˗1and 625–>1000 µg mL˗1, respectively. Whereas the MIC and MFC values of zinc ethylene bisthiocarbamate 75% were ranged from 39–625 µg mL˗1 and 78–>1000 µg mL˗1, respectively. On comparative evaluation with synthetic fungicides, BSEO showed remarkable antifungal activities against different field and storage fungi tested. In antifungal evaluation, the storage fungi viz., species of Aspergillus and Penicillium, were more resistant when compared to field fungi viz., species of Alternaria, Curvularia and Fusarium. Among the methods used for antifungal activity evaluation, the better activity was observed in the broth microdilution method than poisoned food technique, because of more opportunity to test samples to come in close contact with test fungi as it has been earlier reported.[Citation43]
Table 2. Antifungal activity of BSEO and synthetic fungicides against different field and storage fungi.
Table 3. Antifungal and antimycotoxigenic activities of BSEO against toxigenic strains of A. flavus and F. verticillioides.
Management of fungal diseases in crops is generally achieved using synthetic chemicals.[Citation10,Citation44] But, residues of these chemicals in agricultural produce and by-products cause damage to animal and human health, further, continuous and indiscriminate use of chemical preservatives can lead to the development of resistance in microorganisms.[Citation1,Citation45] Essential oils, being the natural derivatives, are biodegradable and do not leave toxic residues or by-products to contaminate the environment.[Citation44,Citation46] The result of the present study confirms that BSEO showed significant antifungal activity against both field and storage fungi including mycotoxigenic strains of A. flavus and F. verticillioides.
Inhibitory efficacy of BSEO on AFB1 and FB1 production
In vitro inhibitory effects of BSEO on AFB1 production by A. flavus and FB1 production by F. verticillioides were estimated using suitable culture medium. The mycelial growth and mycotoxin production were significantly inhibited by BSEO in a dose dependant manner (). The amounts of AFB1 and FB1 contents in control were found to be 1523.6 ± 10.1 µg L˗1 and 89.7 ± 3.1 mg L˗1, respectively. AFB1 production was completely inhibited by BSEO at concentration 6 µL mL˗1 of culture medium, while FB1 was inhibited at 4 µL mL˗1. It is interesting to note that no complete inhibition of fungal biomass was observed at these concentrations.
In vivo efficacy of BSEO on AFB1 production by A. flavus and FB1 production by F. verticillioides were evaluated using viable maize as model (). The amount of AFB1 and FB1 contents extracted from untreated maize (control) were found to be 1868.5 ± 15.6 µg kg˗1 and 48.5 ± 2.1 mg kg˗1, respectively. In BSEO treated maize, AFB1 and FB1 contents were found to be 82.5 ± 1.7 µg kg˗1 and 2.6 ± 0.8 mg kg˗1, respectively, at 10 µL mL˗1. The effect of BSEO on inhibition of natural seed-borne mycoflora of maize was evaluated at 10 µL g˗1. The results revealed that species of Aspergillus (PI 95.0%), Fusarium (PI 45.0%), Penicillium (PI 80.0%), Alternaria (PI 25.0%) and Curvularia (PI 15.0%) recorded as dominant fungi, but these fungi were inhibited significantly in maize sample treated with BSEO at 10 µL g˗1 of maize. When compared to control the percent incidences species of Aspergillus, Fusarium, Penicillium, Alternaria and Curvularia were drastically reduced in maize treated with BSEO (PI 35.0, 10.0, 25.0, 0.0, 0.0%), respectively. The increasing seedling vigour index was observed in BSEO treated maize samples (2260) than control (1654), which indicates that BSEO did not show adverse effect on germination of maize seeds. The results showed that BSEO was found to be effective in control of AFB1 and FB1 production in maize.
Mycotoxin contamination of various foodstuffs and agricultural commodities is a major problem in the tropics and sub-tropics, where climatic conditions and agricultural and storage practices are favorable to fungal growth and toxin production.[Citation47] Many species of Aspergillus, Fusarium and Penicillium are not only recognised as plant pathogens but are also sources of the important mycotoxins of concern in animal and human health.[Citation48] Among the mycotoxins, aflatoxins and fumonisins are the most toxic secondary metabolites mainly produced by species of Aspergillus and Fusarium. A. flavus and F. verticillioides are two important mycotoxigenic moulds that colonise different kinds of food grains.[Citation45,Citation49] Aflatoxins are highly toxic polyketide secondary metabolites produced mainly by A. flavus. The human health impact of AFB1 exposure is widespread in developing countries.[Citation50,Citation51] Exposure to fumonisins, mainly produced by F. verticillioides, has been associated with several diseases in animals including leucoencephalomalacia in equines, pulmonary oedema in swine, liver cancer in rats and immunosuppression in poultry.[Citation52] Both aflatoxins and fumonisins are relevant in food and feedstuffs due to their widespread occurrence and co-occurrence.[Citation53] Maize, which is susceptible to common fungal infestation, was selected as model for this study. Conditions studied in vivo were closed to environment that may occur during pre- and post-harvest of maize. Results showed that the BSEO was effective in inhibition of fungal growth and toxin production in treated maize.
Effect of BSEO on ergosterol content in fungal plasma membrane
The inhibition of ergosterol content in the plasma membrane of A. flavus and F. verticillioides by BSEO is shown in . When compared to control, the percent inhibition of ergosterol content in the plasma membrane of A. flavus by BSEO was recorded to be 73.67, 79.77, 80.38, 82.21 and 83.94% at 2.0, 4.0, 6.0, 8.0 and 10.0 µL mL˗1concentrations. Similarly, the percent inhibition of ergosterol content of F. verticillioides was found to be 55.87, 56.11, 59.47, 63.07 and 65.46% at 2.0, 4.0, 6.0, 8.0, and 10.0 µL mL˗1concentrations, respectively.
Figure 2. Effect of different concentrations of BSEO on ergosterol content in the plasma membrane of A. flavus (a) and F. verticillioides (b).
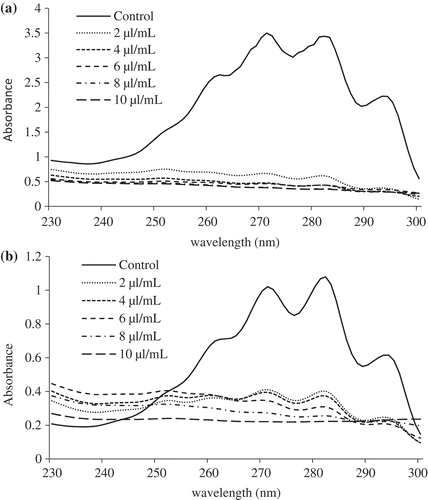
Ergosterol is specific to fungi and is the major sterol component of the fungal cell membrane.[Citation30] The major antifungal agents act by inhibition of ergosterol synthesis,[Citation54,Citation55] which leads to inhibition of fungal cell growth and reduced ergosterol synthesis, hence the measurement of ergosterol content provides information pertinent to alteration and/or disruption of normal sterol biosynthetic pathways. The lower quantity of ergosterol observed with increasing concentrations of BSEO in comparison with the control revealed that BSEO considerably impaired the biosynthesis of ergosterol.
The development of natural antifungal agents would be helpful for decreasing the negative impact of synthetic chemical agents. The EOs of higher plants are well known for their antifungal and antioxidant activities. Recent reports revealed that EOs can be explored as food preservatives to improve the shelf life and quality of stored food products.[Citation37] Due to their bioactivity in the vapour phase, this characteristic makes them attractive as botanical fumigants against moulds and mycotoxin contamination of food commodities.[Citation56] Furthermore, some EOs have been generally exempted from the toxicity data requirements by Environment Protection Agency (EPA) in view of their favourable safety profile.[Citation57] Some of the EO-based food preservatives are commercially available in European market.[Citation31] In this article, we have reported the concentration-dependent antifungal, antiaflatoxigenic and antifumonisin activities of BSEO for the first time. Hence, the present findings indicate the possible use of BSEO as alternative agents for developing plant based preservatives against post-harvest fungal infestation and mycotoxins contamination of food commodities.
Conclusion
Management of fungal contamination, biodeterioration and mycotoxin accumulation in foodstuffs is generally achieved using synthetic chemicals. However, residues of these chemicals in agricultural produce and by-products cause damage to animal and human health, further, continuous and indiscriminate use of chemical preservatives can lead to the development of resistance in microorganisms. The identification of antifungal compounds or Eos from plants is one of the promising and alternative strategies for preventing fungal-deterioration and mycotoxin contaminations. The BSEO exhibited broad spectrum antifungal activity against important field and storage moulds that would probably be a good source of antifungal agents for prevention of fungal-deterioration and mycotoxins contamination. Further, the significant antimycotoxigenic potency of BSEO offers possibilities for its application in preventing mycotoxins contamination in food and feed stuffs. The study suggests further investigations for large-scale practice in the food systems.
Additional information
Funding
References
- Al-Reza, S. M.; Rahman, A.; Ahmed, Y.; Kang, S. C. Inhibition of Plant Pathogens in Vitro and in Vivo with Essential Oil and Organic Extracts of Cestrum nocturnum L. Pesticides Biochemistry and Physiology 2010, 96(2), 86–92. 10.1016/j.pestbp.2009.09.005
- De Saeger, S. Determining Mycotoxins and Mycotoxigenic Fungi in Food and Feed; Woodhead Publishing India Private Limited: New Delhi, India, 2011; 1–396.
- Ozcakmak, S.; Gul, O. Inhibition Kinetics of Penicillium verrucosum Using Different Essential Oils and Application of Predictive Inactivation Models. International Journal of Food Properties 2017. 10.1080/10942912.2017.1308953
- Adams, M.; Motarjemi, Y. Basic Food Safety for Health Workers; World Health Organiztion: Geneva, 1999; 30.
- Thippeswamy, S.; Mohana, D. C.; Abhishek, R. U.; Manjunath, K. Efficacy of Bioactive Compounds Isolated from Albizia amara and Albizia saman as Source of Antifungal and Antiaflatoxigenic Agents. Journal of Consumer Protection and Food Safety 2013, 8(4), 297–305. 10.1007/s00003-013-0839-7
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent Food & Agriculture 2016, 2(1), 1191103. 10.1080/23311932.2016.1191103
- Magan, N.; Olsen, M. Mycotoxins in Food: Detection and Control; Woodhead Publishing limited, Cambridge, UK, 2004; 367–426.
- Domijan, A.-M.; Želježić, D.; Peraica, M.; Kovačević, G.; Gregorović, G.; Krstanac, Ž.; Horvatin, K.; Kalafatić, M. Early Toxic Effects of Fumonisin B1 in Rat Liver. Human & Experimental Toxicology 2008, 27(12), 895–900. 10.1177/0960327108100418
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans 1993, 56, 19–23pp.
- Garcia, D.; Ramos, A. J.; Sanchis, V.; Marín, S. Effect of Equisetum Arvense and Stevia Rebaudiana Extracts on Growth and Mycotoxin Production by Aspergillus flavus and Fusarium verticillioides in Maize Seeds as Affected by Water Activity. International Journal of Food Microbiology 2012, 153(1), 21–27. 10.1016/j.ijfoodmicro.2011.10.010
- Kumar, R.; Mishra, A. K.; Dubey, N. K.; Tripathi, Y. B. Evaluation of Chenopodium ambrosioides Oil as A Potential Source of Antifungal, Antiaflatoxigenic and Antioxidant Activity. International Journal of Food Microbiology 2007, 115(2), 159–164. 10.1016/j.ijfoodmicro.2006.10.017
- Houicher, A.; Hechachna, H.; Özogul, F. In Vitro Determination of the Antifungal Activity of Artemisia campestris Essential Oil from Algeria. International Journal of Food Properties 2016, 19, 1749–1756. 10.1080/10942912.2015.1107734
- Abhishek, R. U.; Mohana, D. C.; Thippeswamy, S.; Manjunath, K. Evaluation of Phyllanthus polyphyllus L. Extract and Its Active Constituent as a Source of Antifungal, Anti-Aflatoxigenic, and Antioxidant Activities. International Journal of Food Properties 2015, 18, 585–596. 10.1080/10942912.2013.853187
- El-Nagerabi, S. A. F.; Elshafie, A. E.; AlKhanjari, S. S.; Al-Bahry, S. N.; Elamin, M. R. Biological Activities of Boswellia sacra Extracts on the Growth and Aflatoxins Secretion of Two Aflatoxigenic Species of Aspergillus Species. Food Control 2013, 34(2), 763–769. 10.1016/j.foodcont.2013.06.039
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and Tolerability of Boswellia serrata Extract in Treatment of Osteoarthritis of Knee–A Randomized Double Blind Placebo Controlled Trial. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology 2003, 10(1), 3–7. 10.1078/094471103321648593
- Bhushan, S.; Kumar, A.; Malik, F.; Andotra, S. S.; Sethi, V. K.; Kaur, I. P.; Taneja, S. C.; Qazi, G. N.; Singh, J. A Triterpenediol from Boswellia serrata Induces Apoptosis through Both the Intrinsic and Extrinsic Apoptotic Pathways in Human Leukemia HL-60 Cells. Apoptosis 2007, 12(10), 1911–1926. 10.1007/s10495-007-0105-5
- Assimopoulou, A.; Zlatanos, S.; Papageorgiou, V. Antioxidant Activity of Natural Resins and Bioactive Triterpenes in Oil Substrates. Food Chemistry 2005, 92(4), 721–727. 10.1016/j.foodchem.2004.08.033
- Sharma, A.; Bhatia, S.; Kharya, M.; Gajbhiye, V.; Ganesh, N.; Namdeo, A.; Mahadik, K. Anti-Inflammatory and Analgesic Activity of Different Fractions of Boswellia serrata. International Journal of Phytomedicine 2010, 2(1), 94–99.
- Abdulmajeed, N. A. Therapeutic Ability of Some Plant Extracts on Aflatoxin B1 Induced Renal and Cardiac Damage. Arabian Journal of Chemistry 2011, 4(1), 1–10. 10.1016/j.arabjc.2010.06.005
- Kasali, A. A.; Adio, A. M.; Oyedeji, A. O.; Eshilokun, A. O.; Adefenwa, M. Volatile Constituents of Boswellia serrata Roxb. (Burseraceae) Bark. Flavour and Fragrance Journal 2002, 17(6), 462–464. 10.1002/(ISSN)1099-1026
- Aman, M.; Ravishankar Rai, V.; Samaga, P. V. Antimicrobial and Phytochemical Screening of Boswellia serrata Roxb., Rhus mysorensis Heyne, Strychnos potatorum Linn. F. and Schefflera stellata Gaertn. Medicinal and Aromatic Plant Science and Biotechnology 2010, 4(1), 69–72.
- Bhatnagar, P.; Jain, S. K. Antimicrobial Activity of Plant Extract against Fungi Associated with Monument Deterioration of Gwalior Fort in India. European Academic Research 2014, 2, 6199–6210.
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical Composition and Antimicrobial Activity of Some Oleogum Resin Essential Oils from Boswellia Spp. (Burseraceae). Annali Di Chimica 2007, 97(9), 837–844. 10.1002/(ISSN)1612-8877
- Raja, A. F.; Ali, F.; Khan, I. A.; Shawl, A. S.; Arora, D. S.; Shah, B. A.; Taneja, S. C. Antistaphylococcal and Biofilm Inhibitory Activities of Acetyl-11-Keto-β-Boswellic Acid from Boswellia serrata. BioMed Central Microbiology 2011, 11(1), 54.
- Mishra, A. K.; Dubey, N. K. Evaluation of Some Essential Oils for Their Toxicity against Fungi Causing Deterioration of Stored Food Commodities. Applied and Environment Microbiology 1994, 60(4), 1101–1105.
- Kumar, V. P.; Chauhan, N. S.; Padh, H.; Rajani, M. Search for Antibacterial and Antifungal Agents from Selected Indian Medicinal Plants. Journal of Ethnopharmacology 2006, 107(2), 182–188. 10.1016/j.jep.2006.03.013
- Srivastava, B.; Singh, P.; Srivastava, A. K.; Shukla, R.; Dubey, N. K. Efficacy of Artabotrys odoratissimus Oil as A Plant Based Antimicrobial against Storage Fungi and Aflatoxin B1 Secretion. International Journal of Food Science and Technology 2009, 44(10), 1909–1915. 10.1111/j.1365-2621.2009.01981.x
- Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, 2007.
- Thippeswamy, S.; Mohana, D.C.; Abhishek, R.U.; Manjunath, K. Inhibitory Effect of Alkaloids of Albizia amara and Albizia saman on Growth and Fumonisin B1 Production by Fusarium verticillioides. International Journal of Food Research 2014, 21, 947–952.
- Abhishek, R.U.; Thippeswamy, S.; Manjunath, K.; Mohana, D.C. Antifungal and Antimycotoxigenic Potency of Solanum torvum Swartz. Leaf Extract: Isolation and Identification of Compound Active against Mycotoxigenic Strains of Aspergillus flavus and Fusarium verticillioides. Journal of Applied Microbiology 2015, 119(6), 1624–1636. 10.1111/jam.12956
- Prakash, B.; Kedia, A.; Mishra, P. K.; Dwivedy, A. K.; Dubey, N. K. Assessment of Chemically Characterised Rosmarinus officinalis L. Essential Oil and Its Major Compounds as Plant‐Based Preservative in Food System Based on Their Efficacy against Food‐Borne Moulds and Aflatoxin Secretion and as Antioxidant. International Journal of Food Science and Technology 2015, 50(8), 1792–1798. 10.1111/ijfs.12822
- Dung, N. T.; Kim, J. M.; Kang, S. C. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil and the Ethanol Extract of Cleistocalyx operculatus (Roxb.) Merr and Perry Buds. Food and Chemical Toxicology 2008, 46(12), 3632–3639. 10.1016/j.fct.2008.09.013
- Hajji, M.; Masmoudi, O.; Souissi, N.; Triki, Y.; Kammoun, S.; Nasri, M. Chemical Composition, Angiotensin I-Converting Enzyme (ACE) Inhibitory, Antioxidant and Antimicrobial Activities of the Essential Oil from Periploca laevigata Root Barks. Food Chemistry 2010, 121(3), 724–731. 10.1016/j.foodchem.2010.01.021
- Shukla, R.; Kumar, A.; Prasad, C. S.; Srivastava, B.; Dubey, N. K. Antimycotic and Antiaflatoxigenic Potency of Adenocalymma alliaceum Miers. on Fungi Causing Biodeterioration of Food Commodities and Raw Herbal Drugs. International Biodeterioration and Biodegradation 2008, 62(4), 348–351. 10.1016/j.ibiod.2007.11.006
- Bailly, J. D.; Querin, A.; Tardieu, D.; Guerre, P. Production and Purification of Fumonisins from A Highly Toxigenic Fusarium verticilloides Strain. Revue De Médecine Vétérinaire 2005, 1(11), 547–554.
- Mohana, D.C.; Raveesha, K.A. Antimycotic, Antibiodeteriorative and Antiaflatoxigenic Potency of 2-Hydroxy-4-Methoxybenzaldehyde Isolated from Decalepis hamiltonii on Fungi Causing Biodeterioration of Maize and Sorghum Grains. Journal of Mycological Plant Pathology 2010, 40(2), 197–206.
- Shukla, R.; Kumar, A.; Singh, P.; Dubey, N. K. Efficacy of Lippia Alba (Mill.) NE Brown Essential Oil and Its Monoterpene Aldehyde Constituents against Fungi Isolated from Some Edible Legume Seeds and Aflatoxin B1 Production. International Journal of Food Microbiology 2009, 135(2), 165–170. 10.1016/j.ijfoodmicro.2009.08.002
- Agrawal, R. L. Seed Technology, 2nd ed.; Oxford and IBH Publishing Co: New Delhi, 1999; 514–590.
- McGimpsey, J. A.; Douglas, M. H.; Van Klink, J. W.; Beauregard, D. A.; Perry, N. B. Seasonal Variation in Essential Oil Yield and Composition from Naturalized Thymus vulgaris L. in New Zealand. Flavour and Fragrance Journal 1994, 9(6), 347–352. 10.1002/(ISSN)1099-1026
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. International Journal of Jood Microbiology 2004, 94(3), 223–253. 10.1016/j.ijfoodmicro.2004.03.022
- Sparg, S.; Kulkarni, M.; Light, M.; Van Staden, J. Improving Seedling Vigour of Indigenous Medicinal Plants with Smoke. Bioresource Technology 2005, 96(12), 1323–1330. 10.1016/j.biortech.2004.11.015
- Niebler, J.; Buettner, A. Frankincense Revisited, Part I: Comparative Analysis of Volatiles in Commercially Relevant Boswellia Species. Chemistry and Biodiversity 2016, 13(5), 613–629. 10.1002/cbdv.201500329
- Prakash, B.; Mishra, P. K.; Kedia, A.; Dubey, N.K. Antifungal, Antiaflatoxin and Antioxidant Potential of Chemically Characterized Boswellia carterii Birdw Essential Oil and Its in Vivo Practical Applicability in Preservation of Piper nigrum L. Fruits. LWT-Food Science and Technology 2014, 56(2), 240–247. 10.1016/j.lwt.2013.12.023
- Tripathi, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of Some Essential Oils as Botanical Fungitoxicants in Management of Post-Harvest Rotting of Citrus Fruits. World Journal of Microbiology and Biotechnology 2004, 20(3), 317–321. 10.1023/B:WIBI.0000023844.80464.59
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Antifungal, Aflatoxin Inhibition and Antioxidant Activity of Callistemon lanceolatus (Sm.) Sweet Essential Oil and Its Major Component 1, 8-Cineole against Aungal Isolates from Chickpea Seeds. Food Control 2012, 25(1), 27–33. 10.1016/j.foodcont.2011.10.010
- Marin, S.; Sanchis, V.; Ramos, A. J. Plant Products in the Control of Mycotoxins and Mycotoxigenic Fungi on Food Commodities. Natural Products in Plant Pest Management, CAB International, Oxfordshire, UK, 2011, 31–35.
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C. S.; Dubey, N. K. Assessment of Thymus vulgaris L. Essential Oil as a Safe Botanical Preservative against Post Harvest Fungal Infestation of Food Commodities. Innovative Food Science and Emerging Technologies 2008, 9(4), 575–580. 10.1016/j.ifset.2007.12.005
- Placinta, C.; D’mello, J.; Macdonald, A. A Review of Worldwide Contamination of Cereal Grains and Animal Feed with Fusarium Mycotoxins. Animal Feed Science and Technology 1999, 78(1), 21–37. 10.1016/S0377-8401(98)00278-8
- Nguefack, J.; Dongmo, J. L.; Dakole, C.; Leth, V.; Vismer, H.; Torp, J.; Guemdjom, E.; Mbeffo, M.; Tamgue, O.; Fotio, D. Food Preservative Potential of Essential Oils and Fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Mycotoxigenic Fungi. International Journal of Food Microbiology 2009, 131(2), 151–156. 10.1016/j.ijfoodmicro.2009.02.009
- Reddy, K.N.R.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An Overview of Mycotoxin Contamination in Foods and Its Implications for Human Health. Toxin Reviews 2010, 29(1), 3–26. 10.3109/15569541003598553
- Rosas-Taraco, A.; Sanchez, E.; García, S.; Heredia, N.; Bhatnagar, D. Extracts of Agave americana Inhibit Aflatoxin Production in Aspergillus parasiticus. World Mycotoxin Journal 2011, 4(1), 37–42. 10.3920/WMJ2010.1219
- Ficoseco, M. A.; Vattuone, M.; Audenaert, K.; Catalán, C.; Sampietro, D. Antifungal and Antimycotoxigenic Metabolites in Anacardiaceae Species from Northwest Argentina: Isolation, Identification and Potential for Control of Fusarium Species. Journal of Applied Microbiology 2014, 116(5), 1262–1273. 10.1111/jam.12436
- Chulze, S. Strategies to Reduce Mycotoxin Levels in Maize during Storage: A Review. Food Additives and Contaminants 2010, 27(5), 651–657. 10.1080/19440040903573032
- Tian, J.; Huang, B.; Luo, X.; Zeng, H.; Ban, X.; He, J.; Wang, Y. The Control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz Essential Oil and Its Potential Use as a Food Preservative. Food Chemistry 2012, 130(3), 520–527. 10.1016/j.foodchem.2011.07.061
- Reis, M.; Carvalho, C.; Andrade, F. A.; Fernandes, O. F. L.; Arruda, W.; Silva, M. R. R. Fisetin as a Promising Antifungal Agent against Cryptocococcus neoformans Species Complex. Journal of Applied Microbiology 2016, 121(2), 373–379. 10.1111/jam.2016.121.issue-2
- Prakash, B.; Shukla, R.; Singh, P.; Mishra, P. K.; Dubey, N. K.; Kharwar, R. N. Efficacy of Chemically Characterized Ocimum gratissimum L. Essential Oil as an Antioxidant and a Safe Plant Based Antimicrobial against Fungal and Aflatoxin B1 Contamination of Spices. Food Research International 2011, 44(1), 385–390. 10.1016/j.foodres.2010.10.002
- Dubey, N. K.; Shukla, R.; Kumar, A.; Singh, P.; Prakash, B. Prospects of Botanical Pesticides in Sustainable Agriculture. Current Science 2010, 98(4), 479–480.