ABSTRACT
This work developed a fluorescence polarization (FP) assay for simultaneous monitoring BPA, BPF, BADGE, and BFDGE in canned tuna. The assay was based on the competitive binding between bisphenol analogues (BPs) and dexamethasone fluorescein (Dex-fl) for mouse peroxisome proliferator-activated receptor α ligand binding domain (mPPARα-LBD*). The soluble form of mPPARα-LBD* was expressed in Escherichia coli strain Rosetta (DE3), as a recognition element on detection of BPs. Under optimized conditions, four bisphenol analytes were detected at concentrations corresponding to the specific migration limits (SMLs) as required by the European Union. The FP assay showed IC50 values of 2.80, 6.51, 1.72, and 4.28 mg L−1 with a limit of detection of 0.35, 0.08, 0.10, and 0.49 mg L−1 for BPA, BPF, BADGE, and BFDGE, respectively. The analysis of spiked canned tuna samples showed the acceptable recoveries ranged from 87.9% to 77.3%, with variation coefficients ranging from 9.0% to 15.8%. With the high sensitivity and wide-range affinities to BPs, the developed assay based on mPPARα-LBD* exhibited the potential to be a screening assay for fast detection of BPs in canned tuna.
Introduction
Endocrine disrupting chemicals (EDCs) are natural or synthetic compounds that are able to enter the body and alter endocrine functions often through mimicking or blocking endogenous hormones.[Citation1] Therefore, the migration of EDCs in the production of polymers for lining commercial cans is an essential food safety issue. Bisphenol A (BPA) and Bisphenol F (BPF) are primarily used as monomers in industrial applications. They work as monomers in the manufacture of epoxy resins and polycarbonates, as antioxidant in some plasticizers, and as an inhibitor of polymerization of vinyl chloride in the production of soft PVC.[Citation2] Bisphenol A diglycidyl ether (BADGE) and bisphenol F diglycidyl ether (BFDGE) are, respectively, generated by the reaction of BPF and BPA with epichlorohydrin.[Citation3] Both of them are used as starting substances for the protective coatings of food cans and as additives in PVC organosol resins in order to remove excess hydrochloric acid during reaction.[Citation4] The moderately high lipophilicity and incomplete polymerization conditions can result in the occurrence of residual bisphenol analogues (BPs) that has the possibility to transfer into the environmental compartments, foods or food containers, consumer products, and human specimens.[Citation5] Because of the keen interest on the use of these compounds as internal coating materials for canned products, the detections of BPs are of particular concern in food safety field.[Citation6]
Similar to BPA, the other bisphenol analogues can be absorbed by oral route via the food chain owing to the use in epoxy based lacquers of food containers. The BPs distributed to the whole organism including the reproductive tracts and the fetuses by crossing the placental barrier.[Citation5] To assess the health risk, the toxicity of BPs was investigated in the past decade or so. From the available toxicity data, the vitro assays and laboratory animal studies have shown that BPs have the potential to disrupt normal cell function by acting as estrogen agonists and androgen antagonists and thus influence the neural networks, cardiovascular, metabolic, and immune systems.[Citation7] More critically, it is well documented that some important diseases such as cardiovascular diseases and some cancers are closely related to diet. Because of their high bioavailability, environmental persistence, and bioaccumulation potential in food chains, regulations were published to restrict the use of certain epoxy derivatives in material and articles intended to come into contact with foodstuffs. The specific migration limits (SMLs) in Europe by Commission Directive 2011/8/EU and 2002/16/EC stipulate the respective limit: 0.6 mg kg−1 of food for BPA, 1 mg kg−1 of food for BADGE and 1 mg kg−1 of food for BFDGE.
In order to determine traces of analytes in complex matrices, highly selective spectroscopic and immunoassay methods have already been applied to actual sample analysis. Traditional methods for BPs residue analysis, such as high-performance liquid chromatography,[Citation6,Citation8,Citation9] ultra-performance liquid chromatography,[Citation2] liquid chromatography–mass spectrometry,[Citation10] LC–MS/MS,[Citation11] and ELISA,[Citation7,Citation12,Citation13] are sensitive and reliable. Besides, electrochemical approaches based on various shapes of nanomaterial and composite materials for quantification and monitoring of BPs have also garnered more and more attention.[Citation14–Citation16] However, most of these methods either require expensive instruments or are time consuming, which inhibits a large-scaled application. Simplifying the assay and minimizing the analysis time are the primary goals in developing screening methods for large numbers of samples. An alternative method to testing techniques mentioned above is fluorescence polarization (FP) assay. The FP is an extensively used immunochemical detection strategy for determination of small molecular compounds. This homogeneous technique perfectly meets the requirements of an easy-to-operate, reliable, fast, and cost-effective analysis.
Apparently, for efficient surveillance purposes, an assay that can detect multi-BPs instead of each individual BP would be preferable. In the case of BPs, we used receptor proteins as recognition element on detection of analytes. Compared with the antibodies, two remarkable advantages of receptor proteins are short production period and broad-specificity recognition for small molecular compounds.[Citation17] Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor transcription factor super family and generally played key roles in the regulation of lipid metabolism, inflammation, cellular growth, and differentiation.[Citation18] Currently, three isoforms of PPARs, namely PPARα, PPARγ, and PPARβ/δ have been identified and reported since the PPAR was first discovered in 1990.[Citation19] Moreover, PPARα has been reported to bind to an even wider range of ligands than either PPARβ or PPARγ.[Citation20] Lines of evidence showed that PPARα could be activated by BPs.[Citation21,Citation22]
The objective of this work was to build a competitive FP assay based on the mouse peroxisome proliferator-activated receptor α ligand binding domain (mPPARα-LBD*) for simultaneous monitoring BPA, BPF, BADGE, and BFDGE. The soluble form of mPPARα-LBD* was expressed in Escherichia coli strain Rosetta (DE3), as a recognition element in this detective system. Conditions for several typical factors on the FP assay performance were optimized, and the quantitative measurements with canned tuna matrix were established by performing spike and recovery.
Materials and methods
Reagents and chemicals
4,4′-Isopropylidenediphenol (BPA), 4,4′-dihydroxydiphenylmethane (BPF), bisphenol A diglycidyl ether (BADGE), and bisphenol F diglycidyl ether (BFDGE) were purchased from Aladdin (Shanghai, China) and Sigma-Aldrich (St. Louis, MO, USA). Unstained protein molecular weight marker was from Thermo Fisher Scientific (San Jose, CA, USA). Dexamethasone fluorescein (Dex-fl) was purchased from Invitrogen Molecular Probes (Eugene, OR, USA). All other reagents used were of analytical grade.
Protein purification and characterization
In the present work, mPPARα-LBD*was utilized for all competitive binding assay. The mPPARα-LBD* was expressed in Escherichia coli strain Rosetta (DE3), as a six-histidine fusion protein. The cDNA encoding amino acids 202 to 266 of mPPARα-LBD* was cloned into the pET28a expression vector, which was under the control of an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible T7 promoter. Expressed His-tagged proteins from the bacterial extracts were purified on Ni-NTA affinity chromatography. Stock protein solutions were aliquoted and stored at −20°C. Purified proteins were analyzed by SDS-PAGE and Western blotting according to standard protocols. Moreover, the concentration of the purified proteins was measured by the BCA method.
Competitive FP assay
The FP assay was developed in a competition format. The whole procedure was carried out as described below. Dexamethasone fluorescein (Dex-fl) concentration was selected such that the total final fluorescence intensity was at least 10 times higher than the background of buffer, while the optimal mPPARα-LBD* dilution was determined according to the Kd tested before. To optimize the incubation time, a kinetic curve was obtained by plotting FP value versus incubation time. The time that provided a stable FP signal was selected as optimum.[Citation23] The competitive binding assay was conducted in a customized cuvette, with 8 nM of Dex-fl, 8 nM of diluted mPPARα-LBD*, and different concentrations of BPs. After an incubation at room temperature, the FP values were measured by fluorescence spectrophotometer (F-7000, Hitachi, Tokyo, Japan) at λex = 484, and λem = 520. Data analysis was performed using GraphPad Prism 5 (GraphPad Software, USA).
Determination of analytical parameters
The IC50 value, the dynamic range, and the limit of detection (LOD) were served as criteria for evaluating the FP assay. These characteristics represent the analyte concentrations that provide tracer-binding inhibitions in the FP assay of 50%, 20–80%, and 90%, respectively. Cross-reactivity (CR) was calculated according to the following equation, where IC50 is the concentration at which 50% of the tracer is bound to the analyte:
Investigation of matrix effect
Two grams of homogenized canned tuna was extracted with 8 mL of acetonitrile, and the mixture was vortex mixed for 15 min at room temperature. After centrifugation at 8000 g for 10 min, the supernatant was collected. Six millilitres of the supernatants were dried under a gentle stream of nitrogen gas at 40°C. The organic phase residue was redissolved with 0.5 mL assay buffer/methanol 70:30 (v/v). The extract was fortified at different levels and analyzed by the FP assay.
Study of recovery
To evaluate the accuracy and repeatability of the developed FP assay, the canned tuna samples were pre-spiked with three concentrations based on the SMLs as required by European Commission and then analyzed according to the detailed procedure mentioned above. As 1/2 SML of BADGE is higher than its own LOD, we tested three BPs except BADGE on this recovery study. Each sample was evaluated with three replicates to verify repeatability.
Results and discussions
Principle of fluorescence polarization assay
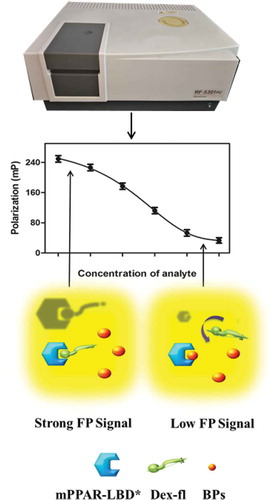
The FP assay is amenable to study molecular interactions by monitoring changes in the apparent size of fluorescently labeled molecules. It is based on the observation that when a fluorescent molecule is excited with plane-polarized light, the remaining polarization of the emitted light depends on the rotational rate of the fluorescent molecule in solution that is inversely related to its molecular weight.[Citation24] The basic principle of fluorescence polarization is depicted in .
Based on this detection principle, the light emitted by Dex-fl rotates quickly in solution and is highly depolarized and, therefore, the polarization value is low. If the mPPARα-LBD* is covalently attached to Dex-fl, the large complex rotates more slowly in solution, while the emitted light remains polarized, so the polarization value is higher. Importantly, FP assay has a number of advantages as an assay technology. It is conducted in solution phase, so it is neither radioactive nor requires any separation of bound and free ligand and it is readily adaptable to low volumes, which makes it well suited for high-throughput screening.[Citation25,Citation26]
Protein characterization
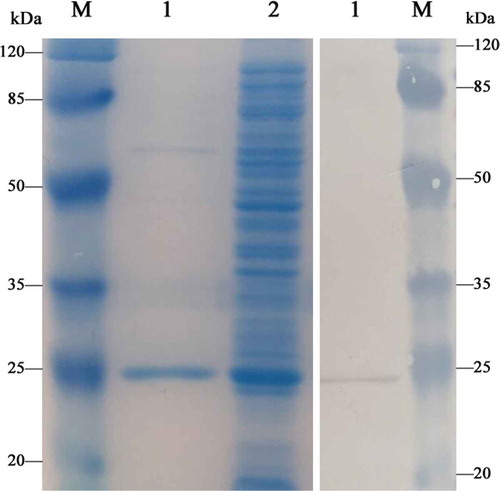
The mPPARα-LBD* was synthesized and purified as described in the experimental section. Expressed His-tagged proteins were confirmed by SDS-PAGE, which showed a dominant connection with a molecular weight of approximately 24.4 kDa (). Western blotting anti-His mouse monoclonal antibody followed by peroxidase-labeled rabbit anti-mouse IgG was also performed. The data from Western blotting analysis for total protein were in good agreement with the expected molecular mass of 24.4 kDa (). Both SDS-PAGE and Western blotting results confirmed the successful synthesis of mPPARα-LBD*. Moreover, the final yield of the 24.4-kDa protein was approximately 0.72 mg mL−1, indicating that the expression system under the appropriate condition was highly efficient.
Optimization of FP assay
The lowest possible tracer concentration would yield higher sensitivity and effectively minimize the interference to competition. In general, the optimal tracer concentration was determined by its fluorescence intensity at least ten times higher than the background signal from working buffer.[Citation27] Thus, 8 nM of Dex-fl was selected as the final working concentration in this study. With a fixed concentration of tracers, the working concentration of mPPARα-LBD* was determined according to the Kd of 7.04 nM. Twofold amounts of Dex-fl and mPPARα-LBD* at their optimal concentrations were mixed together in equal volumes to obtain working solutions for, the future use in the competitive assay.
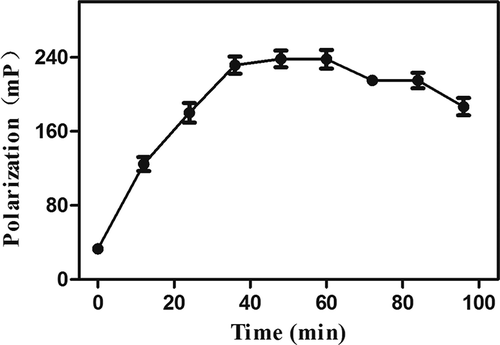
As reported previously, the time given to the competitive reaction might also have a direct effect on the sensitivity of the assay. Data presented in showed that the FP value increased sharply at the first 40 min and then diminished modestly after reaching the maximum FP values. Therefore, an incubation time of 40 min was selected for the competitive step in this study.
Analytical parameters
As the assay principle described above, the broad-spectrum activity of the BPs to mPPARα-LBD* were assessed by performing competitive assays under the optimized conditions. The IC50, the dynamic range and the LOD of the assay for BPs detection were summarized in . The analytical characteristics, IC50 in particular, are quite important index of sensitivity for the assay.[Citation28] Lower IC50 values indicated higher affinity of the BPs and a more sensitive assay. The values of IC50 were 2.80, 6.51, 1.72, and 4.28 mg L−1 for BPA, BPF, BADGE and BFDGE, respectively. Four BPs could be detected with LODs well below the SMLs established by the European Union. Data presented in also showed that the FP assay was capable of detecting 4 BPs with a satisfied detection range.
Table 1. Chemical structures and analytical characteristics of the four test bisphenol analytes
The cross-reactivity of four bisphenol analogues were determined using the improved FP assay with BPA as the reference compound (CR = 100%). As presented in , the assay based on mPPARα-LBD* was highly broad-specific to BPA, with cross-reactivity of 64.15%, 142.95%, and 89.61% for BPF, BADGE, and BFDGE, suggesting that the recognition ability of mPPARα-LBD* was floating within a small range.
Sample treatment
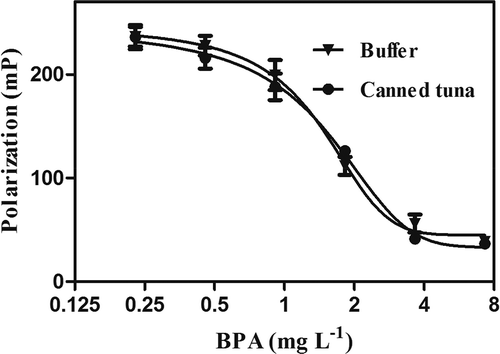
Although the improved FP assay is a comparatively simple and rapid analytical technique, canned fish is such a complex matrix that different components can strongly interfere with the analytical determination. FP assay is susceptible to some of these components in the food matrix. Therefore, sample preparation is a crucial step.[Citation29] For the purpose of acquiring information on matrix effects of canned tuna samples, calibration curves were performed in both working buffer and canned tuna extracts with standard solutions of BPs. As shown in , calibration curves were superposed to a great extent, indicating that the matrix interference could be basically eliminated by sample pretreatment.
Recovery
Spiking matrix samples with several quantities of analyte is a common practice to perform a preliminary evaluation of analytical assay reliability.[Citation30] The accuracy and precision of the assay, represented by recovery and coefficient of variation (CV), were assessed by BPs for which SMLs had been set by European Commission. The results revealed that the mean recoveries were 78.1–85.2% for BPA, 77.3–84.7% for BPF, and 79.8–87.9% for BADGE, with the corresponding coefficients of variation ranging from 10.5–15.8%, 9.8–14.1%, and 9.0–15.5%, which was well within an acceptable range, respectively (). The results of accuracy and precision with the known concentrations of BPs in canned tuna matrix confirmed the reliability of FP receptor assay.
Table 2. Recovery of BPs from spiked samples by FP assay (n = 3)
Conclusion
This work developed a competitive FP assay based on the mPPARα-LBD* for simultaneous monitoring BPA, BPF, BADGE, and BFDGE in canned tuna. The sensitivity of the assay was improved by optimizing some physical and chemical conditions. Under the optimized conditions, four bisphenol analytes could be detected below the SMLs. As expected, satisfactory recoveries with low CVs were observed in the analysis of spiked canned tuna. In conclusion, the results presented that this method is a promising alternative approach to the commonly employed analytical methods of BPs detection. However, the stability of this method was not enough, and we expect that the stability of the assay could be improved by follow-up research.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFD0300303), the National Natural Science Foundation of China (31601534), and the Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2017JQ006 and CXGC2017JQ010).
References
- Rogers, J. A.; Metz, L.; Yong, V. W. Review: Endocrine Disrupting Chemicals and Immune Responses: A Focus on Bisphenol-A and Its Potential Mechanisms. Mol. Immunol. 2013, 53, 421–430. DOI: 10.1016/j.molimm.2012.09.013.
- Gallo, P.; Di Marco Pisciottano, I.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G. D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in Canned Energy Drinks by Molecularly Imprinted Polymer Cleaning up and Uplc with Fluorescence Detection. Food. Chem. 2017, 220, 406–412. DOI: 10.1016/j.foodchem.2016.10.005.
- Oca, M. L.; Sarabia, L. A.; Herrero, A.; Ortiz, M. C. Optimum pH for the Determination of Bisphenols and Their Corresponding Diglycidyl Ethers by Gas Chromatography-Mass Spectrometry. Migration Kinetics of Bisphenol A from Polycarbonate Glasses. J. Chromatogr A. 2014, 1360, 23–38. DOI: 10.1016/j.chroma.2014.07.063.
- Wang, L.; Xue, J.; Kannan, K. Widespread Occurrence and Accumulation of Bisphenol A Diglycidyl Ether (BADGE), Bisphenol F Diglycidyl Ether (BFDGE) and Their Derivatives in Human Blood and Adipose Fat. Environ. Sci. Technol. 2015, 49, 3150–3157. DOI: 10.1021/acs.est.5b00096.
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y. L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. DOI: 10.1021/acs.est.5b05387.
- Cabado, A. G.; Aldea, S.; Porro, C.; Ojea, G.; Lago, J.; Sobrado, C.; Vieites, J. M. Migration of BADGE (Bisphenol A Diglycidyl-Ether) and BFDGE (Bisphenol F Diglycidyl-Ether) in Canned Seafood. Food Chem. Toxicol. 2008, 46, 1674–1680. DOI: 10.1016/j.fct.2008.01.006.
- Lu, Y.; Peterson, J. R.; Gooding, J. J.; Lee, N. A. Development of Sensitive Direct and Indirect Enzyme-Linked Immunosorbent Assays (Elisas) for Monitoring Bisphenol-A in Canned Foods and Beverages. Anal. Bioanal. Chem. 2012, 403, 1607–1618. DOI: 10.1007/s00216-012-5969-8.
- Munguia-Lopez, E. M.; Peralta, E.; Gonzalez-Leon, A.; Vargas-Requena, C.; Soto-Valdez, H. Migration Bisphenol (BPA) Epoxy Can. Coatings Jalapeño Peppers Acid Food Simulant J Agric Food Chem. 2002, 50, 7299–7302.
- Er, B.; Sarimehmetoglu, B. The Investigation of Bisphenol A Presence in Canned Tuna Fish Using High-Performance Liquid Chromato. J. Anim. Vet. Adv. 2011, 10, 2859–2862.
- Pedersen, S. N.; Lindholst, C. Quantification of the Xenoestrogens 4-Tert.-Octylphenol and Bisphenol a in Water and in Fish Tissue Based on Microwave Assisted Extraction, Solid-Phase Extraction and Liquid Chromatography–Mass Spectrometry. J. Chromatogr. 1999, 864, 17–24.
- Míguez, J.; Herrero, C.; Quintás, I.; Rodríguez, C.; Gigosos, P. G.; Mariz, O. C. A LC–MS/MS Method for the Determination of BADGE-related and BFDGE-related Compounds in Canned Fish Food Samples Based on the Formation of [M+Nh4]+ Aducts. Food. Chem. 2012, 135, 1310–1315. DOI: 10.1016/j.foodchem.2012.05.099.
- Zheng, J.; Zhao, S. Q.; Xu, X. T.; Zhang, K. Detection of Bisphenol A in Water Samples Using ELISA Determination Method. Water. Sci. Technol. 2011, 11, 55.
- Kim, A.; Li, C. R.; Jin, C. F.; Lee, K. W.; Lee, S. H.; Shon, K. J.; Park, N. G.; Kim, D. K.; Kang, S. W.; Shim, Y. B.; Park, J. S.; Sensitive, A. Reliable Quantification Method for Bisphenol A Based on Modified Competitive ELISA Method. Chemosphere. 2007, 68, 1204–1209. DOI: 10.1016/j.chemosphere.2007.01.079.
- Soomro, R. A.; Ibupoto, Z. H.; Sirajuddin,; Abro, M. I.; Willander, M. Electrochemical Sensing of Glucose Based on Novel Hedgehog-Like Nio Nanostructures. Sens. Actuators. B. Chem. 2015, 209, 966–974. DOI: 10.1016/j.snb.2014.12.050.
- Soomro, R. A.; Nafady, A.; Hallam, K. R.; Jawaid, S.; Al Enizi, A.; Sherazi, S. T.; Sirajuddin,; Ibupoto, Z. H.; Willander, M. Highly Sensitive Determination of Atropine Using Cobalt Oxide Nanostructures: Influence of Functional Groups on the Signal Sensitivity. Anal. Chim. Acta. 2016, 948, 30–39. DOI: 10.1016/j.aca.2016.11.015.
- Rajar, K.; Soomro, R. A.; Ibupoto, Z. H.; SirajuddinBalouch, A. Tannic Acid Assisted Copper Oxide Nanoglobules for Sensitive Electrochemical Detection of Bisphenol A. Int. J. Food Properties. 2017, 20, 1359–1367. DOI: 10.1080/10942912.2016.1209776.
- Liang, X.; Wang, Z.; Wang, C.; Wen, K.; Mi, T.; Zhang, J.; Zhang, S. Proof-of-Concept Receptor-Based, A. Assay for Sulfonamides. Anal. Biochem. 2013, 438, 110–116. DOI: 10.1016/j.ab.2013.03.028.
- Zhang, L.; Ren, X. M.; Wan, B.; Guo, L. H. Structure-Dependent Binding and Activation of Perfluorinated Compounds on Human Peroxisome Proliferator-Activated Receptor Gamma. Toxicol. Appl. Pharmacol. 2014, 279, 275–283. DOI: 10.1016/j.taap.2014.06.020.
- Ajith, T. A.; Jayakumar, T. G. Peroxisome Proliferator-Activated Receptors in Cardiac Energy Metabolism and Cardiovascular Disease. Clin. Exp. Pharmacol. Physiol. 2016, 43, 649–658.
- Zhang, J.; Xing, X. J.; Sun, Y. H.; Li, Z. L.; Xue, P. Y.; Wang, T. Y.; Li, T. Z. Characterization of the Binding between Phthalate Esters and Mouse Pparα for the Development of a Fluorescence Polarization-Based Competitive Binding Assay. Anal. Methods. 2016, 8, 880–885. DOI: 10.1039/C5AY03053F.
- Bhatti, R.; Singh, J.; Nepali, K.; Ishar, M. P. S. Possible Involvement of Ppar-Γ in the Anticonvulsant Effect of Aegle Marmelos (L.) Correa. Neurochem. Res. 2013, 38, 1624–1631. DOI: 10.1007/s11064-013-1064-6.
- Zheng, S.; Shi, J. C.; Hu, J. Y.; Hu, W. X.; Zhang, J.; Shao, B. Chlorination of Bisphenol F and the Estrogenic and Peroxisome Proliferator-Activated Receptor Gamma Effects of Its Disinfection Byproducts. Water Res. 2016, 107, 1–10. DOI: 10.1016/j.watres.2016.10.048.
- Tian, X.; Dong, Y.; Wang, Y.; Song, Z.; Meng, M.; Eremin, S. A.; Deng, C.; Yin, Y.; Xi, R. Quantification of Diethyl Phthalate by a Rapid and Homogenous Fluorescence Polarization Immunoassay. Anal. Lett. 2015, 48, 2843–2855. DOI: 10.1080/00032719.2015.1060600.
- Maza, S.; Mar Kayser, M.; Macchione, G.; Lopez-Prados, J.; Angulo, J.; De Paz, J. L.; Nieto, P. M. Synthesis of Chondroitin/Dermatan Sulfate-Like Oligosaccharides and Evaluation of Their Protein Affinity by Fluorescence Polarization. Org. Biomol. Chem. 2013, 11, 3510–3525. DOI: 10.1039/c3ob40306h.
- Jameson, D. M.; Croney, J. C. Fluorescence Polarization: Past, Present and Future. Comb. Chem. High Throughput Screen. 2003, 6, 167–176. DOI: 10.2174/138620703106298347.
- Lea, W. A.; Simeonov, A. Fluorescence Polarization Assays in Small Molecule Screening. Expert. Opin. Drug. Discov. 2011, 6, 17–32. DOI: 10.1517/17460441.2011.537322.
- Huang, P. N.; Zhao, S. Q.; Eremin, S. A.; Zheng, S. W.; Lai, D.; Chen, Y. S.; Guo, B. A. Fluorescence Polarization Immunoassay Method for Detection of the Bisphenol A Residue in Environmental Water Samples Based on A Monoclonal Antibody and 4′-(Aminomethyl)Fluorescein. Anal. Methods. 2015, 7, 4246–4251. DOI: 10.1039/C5AY00818B.
- Zhang, J.; Wang, Z.; Wen, K.; Liang, X.; Shen, J. Penicillin-Binding Protein 3 of Streptococcus Pneumoniae and Its Application in Screening of Beta-Lactams in Milk. Anal. Biochem. 2013, 442, 158–165. DOI: 10.1016/j.ab.2013.07.042.
- Mi, T.; Wang, Z.; Eremin, S. A.; Shen, J.; Zhang, S. Simultaneous Determination of Multiple (Fluoro)Quinolone Antibiotics in Food Samples by a One-Step Fluorescence Polarization Immunoassay. J. Agric. Food Chem. 2013, 61, 9347–9355. DOI: 10.1021/jf403972r.
- Lei, H. T.; Xue, G.; Yu, C. F.; Haughey, S. A.; Eremin, S. A.; Sun, Y. M.; Wang, Z. H.; Xu, Z. L.; Wang, H.; Shen, Y. D.; Wu, Q. Fluorescence Polarization as a Tool for the Detection of a Widely Used Herbicide, Butachlor, in Polluted Waters. Anal. Methods. 2011, 3, 2334. DOI: 10.1039/c1ay05347g.