ABSTRACT
Density, surface tension and viscosity of five food oils were experimentally measured using the Archimedean method, Pendant drop method, and Brookfield viscometer respectively. Measurements were performed from 23 ± 1°C to the oils’ smoke point at intervals of every 20°C. Density and surface tension decreased linearly with increasing temperature, whereas the viscosity decreased exponentially. Density was modeled using the modified Rackett equation, surface tension using the Eötvös equation, and viscosity by the modified Andrade equation. The oil type influenced the density and viscosity of oil, but did not affect surface tension.
Introduction
Deep fat frying is one of the oldest and the most commonly used techniques to process foods, which involves submerging a food in hot oil (150–200°C) for a short period of time until it reaches a safe minimum internal temperature. [Citation1] It is a relatively low cost process in which the combination of temperature and heat affect the organoleptic properties of food such as flavor, texture, and color. [Citation2,Citation3] Frying is a simultaneous heat and mass transfer process which causes dehydration of the product due to high oil temperature, thus, resulting in a product which has a porous and crispy exterior and a soft and moist interior. [Citation4] The main reactions involved in this process are starch gelatinization, protein denaturation, aromatizing, and coloring via Maillard reactions, rapid cooking, and texture and flavor development. [Citation5–Citation7] Frying also causes changes in the flavor and stability of oils by hydrolysis, oxidation, and polymerization. [Citation6,Citation8] The most commonly used oils for frying include: Canola, corn, cottonseed, olive, palm, peanut, soybean, safflower, and sunflower. [Citation9] Selection of oil for frying is based on long frying stability, fluidity, bland flavor, low tendency to foam or form smoke, low tendency to polymerize, oxidative stability of the oil in the fried food during storage, and the cost. [Citation9–Citation11]
According to the US Department of Agriculture [Citation12], world vegetable oil consumption has increased from 151.68 to 177.16 million metric tons from 2011/12 to 2015/16. Out of the total production, 73.4% of the oil is used for food purposes [Citation12]; 20% of which is for shallow and deep fat frying. [Citation9] Fat plays an important role in enhancing the flavor of foods. Frying of food gives it a unique-flavor and texture combination which is very desirable to the consumers, making fried foods one of the most popular products. [Citation11] However, oil consumption poses significant health problems such as coronary heart diseases, cancer, diabetes, and hypertension, and is irreconcilable with consumer’s awareness toward the consumption of healthier and low fat food products. [Citation13] There is an increasing consumer trend for less greasy and healthier products. [Citation7] Considering the health impact and the popularity of fried foods, it is important to gain a better understanding of the principles and factors governing oil absorption so that they can be better controlled.
Deep fat frying is one of the most complex transport problems in the food industry. The main mechanisms proposed to govern the oil absorption during frying are water escape and oil uptake; capillary pressure; vapor pressure and vacuum effect; diffusion, adherence and drainage of oil on the food surface. [Citation7,Citation14] Heat is transferred by convection from the oil to the product surface followed by conduction from the surface to the interior of the product. [Citation15] Mass transfer during frying is characterized by the simultaneous movement of water in the form of vapor from the food into the oil, and the movement of oil into the food. [Citation1] Several factors such as moisture content, crust microstructure, product geometry, frying temperature and time, oil type, oil aging and pre and post frying treatments have shown to affect oil absorption during frying. [Citation7,Citation11,Citation16] Frying is known to affect the physical properties of oil such as density, viscosity and surface tension which can in turn affect the transport rate of oil, and thus the rate of oil absorption. Hence, the focus of this study is to understand the change in the oil physical properties at high temperatures, to better predict the effect of parameters affecting oil absorption at frying temperatures.
Density is an important factor which influences oil absorption as it affects the drainage rate after frying and also the mass transfer rate during the cooling stage of frying. [Citation17,Citation18] Density has been experimentally shown to be linearly dependent on temperature. [Citation19,Citation20] However, there is no mathematical equation to predict the effect of temperature on density, especially at high temperatures at which frying is conducted. Frying occurs at temperatures around 180°C; however the densities of commonly used oils are known till around 110–140°C. [Citation19,Citation20] Hence to accurately determine transport rates, density need to be determined and modeled at the frying temperatures. A liquid pycnometer [Citation19], hydrometer with temperature controller [Citation20] are the most commonly used methods to determine density of a liquid at temperatures above room temperature. Both the methods involve use of glass apparatus, hence glass expansion coefficient needs to be accounted for. Also, the assembly of the apparatus is complex. Archimedean method has been successfully used to determine density of liquids at high temperatures (molten igneous rocks) [Citation21]; however it has not been used to measure densities of food oils.
Ziaiifar et al. [Citation7] reported that the viscosity of oil is one of the main factor which governs oil absorption and drainage. The higher is the oil viscosity, the slower is the oil drainage. Oil viscosity depends on oil type as well as frying temperature and oil quality. Kim et al. [Citation13], Yilmaz [Citation18], Esteban et al. [Citation20], and Noureddini et al. [Citation22] measured viscosities of vegetable oils from room temperature up to maximum of 130°C using a glass capillary viscometer. Correlations were developed for viscosity with temperatures using empirical equations as well as relating fatty acid composition to the viscosity change. These studies give valuable information on the effect of temperature on oil degradation but fail to develop models which can be used as predictors of oil viscosity, especially at the frying temperatures. Viscometer and Rheometer are the most commonly used equipment’s to measure oil viscosity at high temperatures. [Citation7,Citation18,Citation20,Citation23] Modeling viscosity at frying temperatures can give a better understanding of the flow behavior of the oil during drainage, and thus help to characterize oil absorption.
Surface and interfacial tension (IFT) play a key role in mechanisms of oil uptake, as frying oil moves into the food’s tiny pores, which are filled with water and/or steam. [Citation4,Citation24] Surface tension of oils is measured at room temperature, however, frying process is conducted at temperatures between 150–180°C. For many pure liquids, an increase in temperature causes a linear decrease in the surface tension. Investigation of surface and interfacial tensions at high temperature is thus a key element required to characterize and predict the oil absorption by the capillary mechanism. Kalogianni et al. [Citation23] measured the interfacial tension of olive oil and palm oil at room temperature after repeated frying cycles using the pendant drop technique, and found a dynamic decrease in interfacial tension values for both oil types. Thus, frying has a significant effect on surface tension of oil and the effect needs to be predicted at high temperatures to gain a better understanding of oil absorption. Surface tension can be measured by many methods including: Pendant drop, Du Nouy ring, Wilhelmy plate, Maximum bubble pressure, and Contact angle. [Citation25] The Pendant drop technique is commonly used to measure the IFT of hydrocarbons and crude oils at high temperature [Citation26,Citation27] but has not been used to measure the surface tension of food oils at high temperatures.
While oil viscosity and density as affected by temperature and oil composition have received some attention, the surface tension of oils at frying temperatures in pure or mixed systems has not been studied to a great extent. [Citation28] The current study seeks to address this gap. The objectives of this study are to determine and mathematically model surface tension, density and viscosity of commonly used food oils at high temperatures. The models developed will help to gain a better understanding of oil absorption at the frying temperatures.
Materials and methods
Materials
Canola oil (Wesson, ConAgra Foods Inc.; Omaha NE, USA), extra virgin olive oil (Filippo Berio, SALOV North America Corp.; Lyndhurst NJ, USA), soybean oil (Crisco, The J.M. Smucker Co.; Orrville OH, USA), peanut oil (LouAna, Ventura Foods, LLC; Brea CA, USA) and corn oil (Mazola, ACH Food Companies, Inc.; Memphis TN, USA) were purchased from a local market. Decane was purchased from TCI America (Portland OR, USA). Acetone was purchased from VWR (Radnor, PA, USA). Stainless steel wire (diameter: 0.76 mm) was bought from Hobart Welders (Northern Tool; Raleigh NC, USA). A stainless steel ball (diameter: 25.4 mm) was obtained from the Precision Instrument Machine Shop (North Carolina State University Raleigh, NC, USA).
Density measurements
Oil density was determined by the Archimedean method as described by White. [Citation21] This method uses a solid object of known volume and mass suspended in the test liquid by a wire hanging from a scale. A stainless steel ball welded to a stainless steel wire was used as the reference material for this study. The density () was calculated using the following equation:
where is the buoyancy,
is the volume of the reference object,
is the surface tension effect between the liquid and the wire, and
is the volume of immersed wire. For the purpose of this study,
was assumed negligible,
was determined to be 8.58 cm3, and
equal to 0.01 cm3. The buoyancy was determined by:
where and
are the mass of the reference object plus immersed wired suspended in vacuo, and in the test fluid respectively. The value determined for
was 69.67 g. Measurements were performed at room temperature (22 ± 1°C), 40°C and at increasing intervals of 20°C until the smoke point of each oil was reached (200°C; except for olive oil, 180°C). [Citation29,Citation30] Prior to each measurement, the oil was placed inside a beaker and heated under constant agitation until the desired temperature was reached. To maintain a uniform temperature, the wire/ball was also heated along with the oil in the beaker. Measurements were performed in triplicate.
Surface tension measurements
The surface tension of the five vegetable oils in air was determined by pendant drop method using a KRÜSS (Model- DSA30B, KRÜSS GmBH, Hamburg, Germany) as well as ramé-hart goniometer (ramé-hart, Advanced Goniometer, model 300, Succasunna, NJ). The surface tension was measured using Drop Shape Analysis software and DropImage software respectively. An elevated temperature syringe with a 22 gauge stainless steel needle was used to form the drops inside an environmental chamber equipped with a temperature control system (P/N 100–11, P/N 100–10-20, P/N 100–10-12–22, and P/N 100–07 respectively; ramé-hart instrument co.; Succasunna NJ, USA) with air as the surrounding medium. The environmental chamber and the high temperature syringe, both equipped with a temperature control system (P/N 100–50; ramé-hart instrument co.; Succasunna NJ, USA) and SOLO Temperature Controller Configuration software (AutomationDirect; Cumming GA, USA) were used. Additionally, temperature was monitored at the vicinity of the needle where the drop was generated, using a thermocouple (P/N KMQSS-062U, OMEGA Engineering, Inc; Stamford CT, USA) attached to a data logger with Logger Lite software (LabQuest Mini, Vernier Software & Technology, LLC.; Beaverton OR, USA). Acetone was used to clean all surfaces before and after each experiment to avoid contamination; surfaces were allowed to dry for 1 min before starting a new measurement. Similar to density experiments, measurements were performed at room temperature (22 ± 1°C), 40°C and at increasing intervals of 20°C until the smoke point of each oil was reached (200°C; except for olive oil, 180°C). Three measurements were performed at each temperature.
Viscosity measurements
The dynamic viscosity () of the vegetable oils was determined by a Brookfield viscometer equipped with a thermo-container and programmable temperature controller (LV-DVIII, HT-60, HT-110FR respectively; Brookfield Engineering Laboratories, Inc.; Middleboro MA, USA). The thermo-container was equipped with a sample chamber, and a spindle (HT-2 and SC4-18 respectively; Brookfield Engineering Laboratories, Inc.; Middleboro MA, USA). The system was cooled using a cooling plug assembly (HT-26Y, Brookfield Engineering Laboratories, Inc.; Middleboro MA, USA) attached to pressurized air nozzle. The heating profile for the temperature controller was set up as: heat from 23°C until the smoke point of each oil (200°C, except olive oil 180°C) in increments of 20°C and holding at each temperature for 5 min. Dynamic viscosity measured was then converted into kinematic viscosity (υ, Eq. 12) using the density of each oil, for comparison with data from mathematical models. The measurements were performed at different RPM settings to confirm the Newtonian behavior of the five oils studied (data not shown).
Mathematical modeling
Density
Density as a function of temperature was predicted based on the modified Racket equation (Eq. 3) developed by Spencer and Danner [Citation31] which estimates the molar volume of a saturated pure liquid ():
where is in
,
is the critical temperature (
),
is the critical pressure (
),
is the ideal gas constant (
),
is the Rackett parameter which is unique to each compound, and
is the reduced temperature (
). Halvorsen et al. [Citation32] presented a variation of Eq. (3) to estimate the density of vegetable oils (Eq. 4), which considers the mixture of fatty acids and a correction factor to account for triglycerides.
where is the mole fraction of each component,
the molecular weight (
) of each component, and
is the correction factor. Values of
,
,
, and
corresponding to each fatty acid present in the composition of each oil used in this study are reported in . The reduced temperature (
) was obtained using the temperature (
) at which the estimation was done and a molar average of the critical temperatures as pseudocritical temperature (Eq. 5). [Citation32]
Table 1. Molecular weight, critical properties and Racket parameters of fatty acids.
The correction factor () ranges from 0.02 to 0.04 and only depends on oil type, more specifically the molecular weight. When the molecular weight of the oil (
) was lower than 875
,
was calculated using Eq. (6); however, when it was higher, Eq. (7) was used.
was calculated based on the fatty acid composition using Eq. (8).[Citation32]
Surface tension
Surface tension was predicted by the Eötvös equation (Eq. 9) as a direct function of temperature for data obtained from both the goniometers:
where is the liquid-gas surface tension (
),
is the oil’s molar mass (
),
is the oil’s density (
),
is the Eötvös constant (
),
is the oil’s critical temperature (
), and
is the temperature (
) at which
is estimated.
is the measure of entropy of the surface, and was determined using the method of least squares for each of the systems. It is known that geometrically complex molecules have higher
values (e.g., tripalmitine,
), than those almost spherical molecules (e.g., mercury,
). [Citation33] Hence,
values were determined for each oil type. The surface tension data was also modeled using the predicted density values obtained from the Rackett equation and fitting them in the Eötvös equation.
Viscosity
Viscosity as a function of temperature was modeled using the modified Andrade equation (Eq. 10) which is derived from the Arrhenius equation, and was used by Esteban et al. [Citation20], Noureddini et al. [Citation22], and Yilmaz [Citation18] to understand the viscosities of vegetable oils and fatty acids.
where is the oil kinematic viscosity (
),
is the temperature (
),
,
and
are correlation constants which are calculated using the method of least squares. Accuracy of the mathematical models for each of the physical properties measured, was determined from the percent error.
Statistical analysis
Measurements were performed in triplicate and data was analyzed using Minitab 17 (Minitab Inc., State College PA, USA). Regression (REG) analysis was performed on measured viscosity, density and surface tension for all five oils. Tukey’s test (α = 0.05) was used to determine differences among measured physical properties with temperature for each oil.
Results and discussion
Effect of temperature and oil type on physical properties of oils
Density
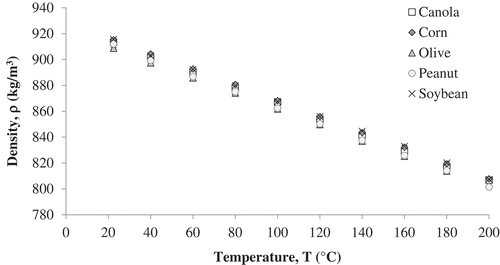
Density of soybean, canola, corn, peanut and olive oil was measured by the Archimedean method from room temperature to 200°C (except olive oil, 180°C) (). The density values obtained were similar to those previously reported in literature.[Citation19] Regression analysis showed that there was a linear decrease in density with temperature for all oils (). The intercept and slopes for each oil are reported in . Statistical analysis using General linear model (GLM) showed significant effect (p < 0.05) of temperature and oil type on density.
Table 2. Comparison of the average density values between five vegetable oils at different temperatures.
Table 3. Intercept (), slope (
) and correlation coefficient (
) values corresponding to the empirical equation to predict density of each vegetable oil.
Surface tension
Surface tension of soybean, canola, corn, peanut and olive oil was measured using a KRÜSS and ramé-hart goniometer from room temperature to 200°C (except olive oil 180°C) (). Regardless of the oil type or the equipment used, there was a significant decrease (p < 0.05) in surface tension as the temperature increased; shows data obtained using the KRÜSS goniometer. The results obtained agree with data previously reported in literature.[Citation34] Regression analysis showed that there was a linear decrease in surface tension with temperature for all oils. The intercept and slopes for each oil are reported in . There was a difference in values for the surface tension measured with KRÜSS vs ramé-hart equipment mainly because of the numerical method used by each equipment in calculating surface tension from the Young’s equation.
Table 4. Comparison of the average surface tension values between five vegetable oils at different temperatures measured using KRÜSS and ramé-hart goniometers.
Table 5. Intercept (), slope (
) and correlation coefficient (
) values corresponding to the empirical equation to predict surface tension of each vegetable oil.
Figure 2. Surface tension values of five vegetable oils from room temperature to each oil’s smoke point determined using a KRÜSS goniometer.
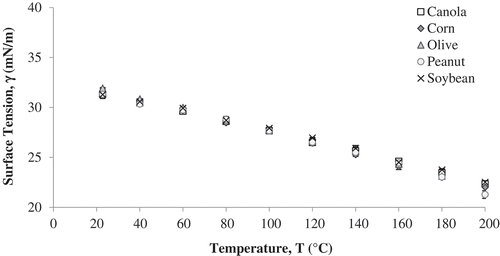
Statistical analysis showed significant effect (p < 0.05) of temperature and oil type on the surface tension values. However, the difference in surface tension values for different oil types is within the range of accuracy of each equipment and the effect can be ignored. Thus, temperature was the only significant factor affecting surface tension. This finding agrees with the result from Xu et al. [Citation34] where no effect of oil type on surface tension was observed, and the effect could be attributed to the presence of long-acyl chains in all oils, whose surface tensions are not very different from each other.
Viscosity
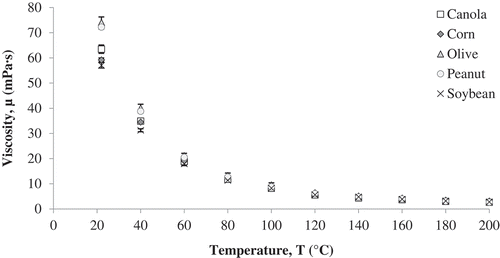
Viscosity of soybean, canola, corn, peanut and olive oil was measured using a viscometer from room temperature to 200°C (). All the oils used in the study showed Newtonian behavior at the range of temperatures studied. Regardless of the oil type, there was a significant decrease (p < 0.05) in viscosity as the temperature increased (). In the current study, viscosity was found to have a power law relation of the second degree with temperature (Eq. 11), irrespective of the type of oil used.
Table 6. Comparison of the average viscosity values between five vegetable oils over a range of temperatures.
Figure 3. Viscosity values determined for five vegetable oils from room temperature to the smoke point of each oil.
where is the dynamic viscosity
,
is the temperature (°C),
is the coefficient, and
is the power of the equation obtained by regression analysis (). The viscosity values are similar to those obtained by Noureddini et al.[Citation22], Miller et al. [Citation28] and Ziaiifar et al. [Citation7] It was observed that the viscosity values were a quadratic function of temperature for all the oils. Statistical analysis using showed significant effect (p < 0.05) of temperature and oil type on the viscosity values; differences between oils were more noticeable at temperatures below 100°C.
Table 7. Parameters and
, and correlation coefficient (
) values corresponding to the empirical equation to predict viscosity for each vegetable oil.
Mathematical modeling of physical properties of oils at high temperatures
Density
Density was modeled as a function of temperature using the modified Rackett equation (Eq. 4). In order to predict the density of a vegetable oil using this equation, it is necessary to know the critical temperature, critical pressure, molecular weight, and Rackett parameter of each fatty acid present in the oil (), as well as the molar ratios at which the fatty acids are present. Rackett parameter is estimated using these values and is a measure of the molar volume of a saturated pure liquid. [Citation35] The ,
and
were calculated as the sum of the corresponding property for each fatty acid present in the oil weighted with its correspondent molar fraction;
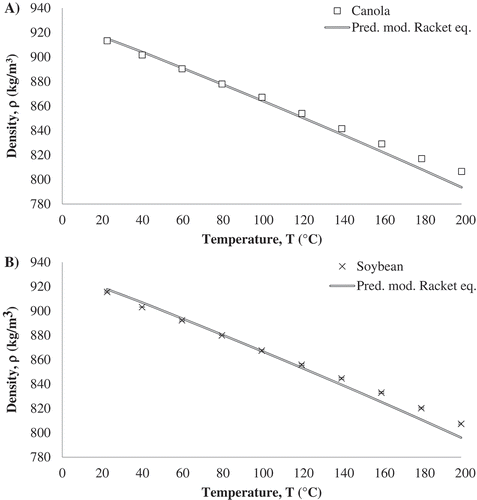
was calculated using Eq. (9) (). Similar trends were observed between experimental and predicted data with increasing temperature (). The predicted values and error (
) at the temperatures at which experiments were conducted for all five oils used in the study are reported in . The error increased with increasing temperature, but the overall error was not higher than two percent for the temperature range used in the study.
Table 8. Molecular weight, critical temperature, and critical pressure of five vegetable oils.
Table 9. Predicted density values for each vegetable oil by the modified Rackett equation and their corresponding percentage error.
Surface tension
Surface tension was modeled as a function of temperature using the semi-empirical Eötvös equation (Eq. 3). [Citation33,Citation36] It is necessary to know the value of critical temperature of oil and the molecular weight to make predictions using Eötvös equation. Eötvös constant () is the measure of entropy of the surface and its value was calculated using the method of least squares. The experimental and predicted data were observed to follow a similar trend with increase in temperature (). For all the oils used in the study,
of 6.2 dynes∙cm/mol2/3∙K fit the data using the KRÜSS goniometer and
of 5.9 dynes∙cm/mol2/3∙K fit the data using the ramé-hart goniometer. Same Eötvös constant values could be used to fit the data for all oils studied with one equipment. The Eötvös constant depends on the geometry of the molecule, and the similarity in values irrespective of oil type was as expected. The difference in Eötvös constant with respect to equipment was mainly because of the differences in numerical method used for surface tension calculation by the equipment, which affected the fit of the model. Models presented in the manuscript are from the data obtained using the KRÜSS goniometer.
Figure 5. Comparison between surface tension experimental values obtained by KRÜSS goniometer of A) Canola oil, and B) Soybean oil and their corresponding predicted values by the Eötvös equation and the modified Racket- Eötvös equations.
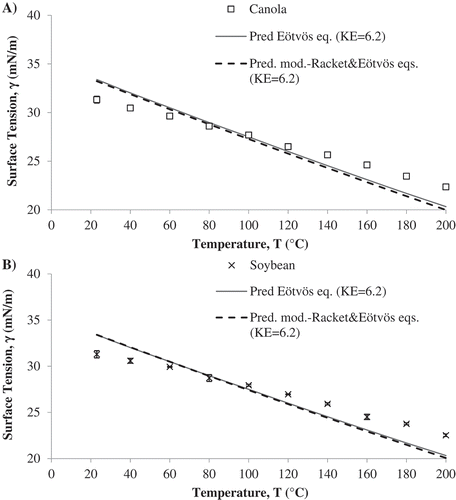
The predicted values and error () at the temperatures at which experiments were conducted for all five oils used in the study are reported in . The error was less than ten percent for all five oils at the temperature range studied; this was true for predicted values compared with data obtained from both equipment, when using their corresponding
. According to the data, the Eötvös equation has a higher accuracy of prediction of surface tension between 60–100°C, as the error was less than five percent in this temperature range for all five oils. Overall, the Eötvös equation overestimates the surface tension value at lower temperatures (23 and 40°C) and underestimates the surface tension at higher temperatures (140–200°C). Surface tension was also modeled using a combination of Eötvös and modified Rackett equation (). The model can help to predict the values of surface tension of oils at high temperature if the free fatty acid composition, density and critical parameters of the oil are known.
Table 10. Predicted surface tension values for each vegetable oil by the Eötvös equation (KE = 6.2 dynes∙cm/mol2/3∙K) and their corresponding percentage error comparing with experimental data obtained using KRÜSS goniometer.
Viscosity
Viscosity of the oils was modeled up to 200°C using the modified Andrade equation (Eq. 10). The coefficients a, b and c were obtained using regression analysis. The Andrade equation is a form of the Arrhenius equation and uses kinematic viscosity values to establish relation between viscosity and temperature. Conversions between dynamic and kinematic viscosities were done using the following equation:
where is the kinematic viscosity (
),
is the dynamic viscosity (
), and
the density (
) at the selected temperature. The values of
,
and
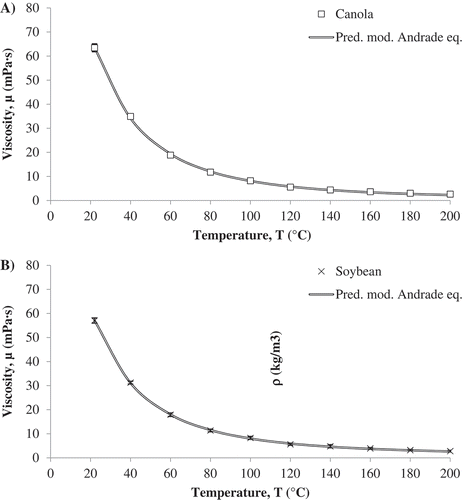
were obtained using the method of least squares (). Similar trends to previous studies [Citation18,Citation20,Citation22] were observed in the current study using a different assembly. According to the data, there is a high degree of accuracy between the experimental and the predicted data for all the temperatures used in the study (). The error percentage increased with increasing temperature, however the experimental and predicted data had similar trends for change in viscosity with temperature ().
Table 11. Parameters ,
and
corresponding to the modified Andrade equation used to predict the viscosity of each vegetable oil.
Table 12. Predicted dynamic viscosity values for each vegetable oil by the modified Andrade equation and their corresponding percentage error.
Conclusion
Based on the results, temperature had a significant effect on all three physical properties of oil measured. Oil type was shown to have a significant effect on viscosity and density, but did not influence surface-tension. Surface tension and density decreased linearly with increasing temperature, whereas the decrease in viscosity followed a power law model. The trends for all the three physical properties were similar to those reported in literature, and thus results could be corroborated. Mathematical models built to predict the change in surface tension (the Eötvös equation and modified Rackett-Eötvös equations), density (the modified Rackett equation), and viscosity (the modified Andrade equation) seemed to agree well with the experimental data. The error percentage for mathematical models increased with increasing temperature, however the model and experimental values followed the same trend at the range of temperatures studied. The mathematical models developed can thus be used to predict the change in physical properties of oil at high temperatures; specially for controlling process parameters during frying, spray drying, dairy processing or atomization during biodiesel production. Understanding of transport rate of oil at high temperatures (up to 200°C) when different types oils are used can be used for varied applications such as a better/more accurate prediction of the oil absorption rates during frying, and understanding of drying rate during spray drying.
Acknowledgments
We would like to thank Dr. Owen Jones, Dr. Rituraj Borghain, Dr. Oswaldo Campanella and Dr. Cordelia Running for granting us access to use the equipment and facilities in their lab.
Additional information
Funding
References
- Farkas, B. E.; Singh, R. P.; Rumsey, T. R. Modeling Heat and Mass Transfer in Immersion Frying. I, Model Development. Journal of Food Engineering 1996, 29(2), 211–226. 10.1016/0260-8774(95)00072-0.
- Bouchon, P.; Pyle, D. L. Modelling Oil Absorption during Post-Frying Cooling: I: Model Development. Food and Bioproducts Processing 2005, 83(4), 253–260.
- Saguy, I. S.; Dana, D. Integrated Approach to Deep Fat Frying: Engineering, Nutrition, Health and Consumer Aspects. Journal of Food Engineering 2003, 56(2–3), 143–152.
- Dana, D.; Saguy, I. S. Review: Mechanism of Oil Uptake during Deep-Fat Frying and the Surfactant Effect-Theory and Myth. Advances in Colloid and Interface Science 2006, 128–130, 267–272.
- Chang, S. S.; Peterson, R. J.; Ho, C.-T. Chemical Reactions Involved in the Deep-Fat Frying of Foods1. Journal of the American Oil Chemists’ Society 1978, 55(10), 718–727.
- Choe, E.; Min, D. B. Chemistry of Deep-Fat Frying Oils. Journal of Food Science 2007, 72(5), R77–R86.
- Ziaiifar, A. M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of Mechanisms, Conditions, and Factors Involved in the Oil Uptake Phenomenon during the Deep-Fat Frying Process. International Journal of Food Science & Technology 2008, 43(8), 1410–1423.
- Xu, T.-T.; Li, J.; Fan, Y.-W.; Zheng, T.-W.; Deng, Z.-Y. Comparison of Oxidative Stability among Edible Oils under Continuous Frying Conditions. International Journal of Food Properties 2015, 18(7), 1478–1490.
- Aydar, A. Y.; Rodriguez-Martinez, V.; Farkas, B. E. Determination and Modeling of Contact Angle of Canola Oil and Olive Oil on a PTFE Surface at Elevated Temperatures Using Air or Steam as Surrounding Media. LWT - Food Science and Technology 2016, 65, 304–310.
- Kochhar, S. P. Stabilisation of Frying Oils with Natural Antioxidative Components. European Journal of Lipid Science and Technology 2000, 102(8–9), 552–559. 10.1002/(ISSN)1438-9312.
- Bouchon, P. Understanding Oil Absorption During Deep-Fat Frying. In: Advances in Food and Nutrition Research; Steve, L. T., Editor; Elsevier Inc., Atlanta, GA: 2009, 57, 209–234.
- United States Department of Agriculture. Oilseeds: World Markets and Trade; Foreign Agricultural Service, Economics, Statistics and Market Information System. Washington, DC. December 9, 2015.
- Kim, J.; Kim, D. N.; Lee, S. H.; Yoo, S.-H.; Lee, S. Correlation of Fatty Acid Composition of Vegetable Oils with Rheological Behaviour and Oil Uptake. Food Chemistry 2010, 118(2), 398–402.
- Mellema, M.;. Mechanism and Reduction of Fat Uptake in Deep-Fat Fried Foods. Trends in Food Science & Technology 2003, 14(9), 364–373.
- Hubbard, L. J.; Farkas, B. E. Influence of Oil Temperature on Convective Heat Transfer during Immersion Frying. Journal of Food Processing and Preservation 2000, 24(2), 143–162.
- Moreira, R. G.; Sun, X.; Chen, Y. Factors Affecting Oil Uptake in Tortilla Chips in Deep-Fat Frying. Journal of Food Engineering 1997, 31(4), 485–498.
- Bouchon, P.; Pyle, D. L. Modelling Oil Absorption during Post-Frying Cooling: II: Solution of the Mathematical Model, Model Testing and Simulations. Food and Bioproducts Processing 2005, 83(4), 261–272.
- Yilmaz, N. Temperature-Dependent Viscosity Correlations of Vegetable Oils and Biofuel–Diesel Mixtures. Biomass and Bioenergy 2011, 35(7), 2936–2938.
- Noureddini, H.; Teoh, B. C.; Davis Clements, L. Densities of Vegetable Oils and Fatty Acids. Journal of the American Oil Chemists Society 1992, 69(12), 1184–1188.
- Esteban, B.; Riba, J.-R.; Baquero, G.; Rius, A.; Puig, R. Temperature Dependence of Density and Viscosity of Vegetable Oils. Biomass and Bioenergy 2012, 42, 164–171.
- White, J. L. Liquid Densitometry. In Physicochemical Measurements at High Temperatures; Bockris, J. O. M., White, J. L., Mackenzie, J. D.; Editors; Academic Press, Inc.: New York, NY, 1959; 193–213.
- Noureddini, H.; Teoh, B. C.; Davis Clements, L. Viscosities of Vegetable Oils and Fatty Acids. Journal of the American Oil Chemists Society 1992, 69(12), 1189–1191.
- Kalogianni, E. P.; Karapantsios, T. D.; Miller, R. Effect of Repeated Frying on the Viscosity, Density and Dynamic Interfacial Tension of Palm and Olive Oil. Journal of Food Engineering 2011, 105(1), 169–179.
- Moreno, M. C.; Bouchon, P. A Different Perspective to Study the Effect of Freeze, Air, and Osmotic Drying on Oil Absorption during Potato Frying. Journal of Food Science 2008, 73(3), E122–E128.
- Adamson, A. W.; Gast, A. P. Physical Chemistry of Surfaces; Wiley-Interscience, New York, NY. 1967.
- Flock, D. L.; Le, T. H.; Gibeau, J. P. The Effect of Temperature on the Interfacial Tension of Heavy Crude Oils Using the Pendent Drop Apparatus; J. Can. Pet. Technol 1986, 25, 72–77.
- Huygens, R. J. M.; Boersma, D. M.; Ronde, H.; Hagoort, J. Interfacial Tension Measurement of Oil-Water-Steam Systems Using Image Processing Techniques; SPE Advanced Technology Series 1995. 3, 129–138. 10.2118/24169-PA.
- Miller, K. S.; Singh, R. P.; Farkas, B. E. Viscosity and Heat Transfer Coefficients for Canola,Corn, Palm, and Soybean Oil. Journal of Food Processing and Preservation 1994, 18(6), 461–472.
- Stevenson, S. G.; Vaisey-Genser, M.; Eskin, N. A. M. Quality Control in the Use of Deep Frying Oils. Journal of the American Oil Chemists Society 1984, 61(6), 1102–1108.
- Katragadda, H. R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, Á. A. Emissions of Volatile Aldehydes from Heated Cooking Oils. Food Chemistry 2010, 120(1), 59–65.
- Spencer, C. F.; Danner, R. P. Improved Equation for Prediction of Saturated Liquid Density. Journal of Chemical & Engineering Data 1972, 17(2), 236–241.
- Halvorsen, J. D.; Mammel, W. C., Jr.; Clements, L. D. Density Estimation for Fatty Acids and Vegetable Oils Based on Their Fatty Acid Composition. Journal of the American Oil Chemists’ Society 1993, 70(9), 875–880.
- Palit, S. R. Thermodynamic Interpretation of the Eötvös Constant. Nature 1956, 177(4521), 1180–1180.
- Xu, T.; Rodriguez-Martinez, V.; Sahasrabudhe, S. N.; Farkas, B. E.; Dungan, S. R. Effects of Temperature, Time and Composition on Food Oil Surface Tension. Food Biophysics 2017, 12(1), 88–96.
- Yamada, T.; Gunn, R. D. Saturated Liquid Molar Volumes. Rackett Equation. Journal of Chemical & Engineering Data 1973, 18(2), 234–236.
- Restolho, J.; Mata, J. L.; Saramago, B. On the Interfacial Behavior of Ionic Liquids: Surface Tensions and Contact Angles. Journal of Colloid and Interface Science 2009, 340(1), 82–86.