?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The male flower of Juglans regia L., were investigated for its in vitro antioxidant activity, antimicrobial activity, and chemical constituents. The antioxidant activity showed that the methanol extract of J. regia male flower (MEJR) had highest scavenging potential than the other solvents (ethanolic = EEJR and aqueous = AEJR). The antimicrobial activity showed that Staphylococcus aureus and Escherichia coli were the most sensitive organisms and significant activity was also recorded against both the fungal strains tested, with highest activity against Candida albicans. Totally, 26 constituents were identified by high-resolution-liquid chromatography-mass spectrometry analyses from which seven compounds were identified first time from the extract.
Introduction
The Juglandaceae family includes several genera among which Juglans genus is the important representative, with 7–45 species. Among these Juglans regia L. is one of the premium tree traditionally cultivated for its valuable wood and fruits. The seed is a nut of high economic interest to the food industry and is globally popular and valued for its nutritional, health, and sensory attributes.[Citation1] The vast biodiversity of Himalaya provides this royal species mostly in the Kashmir region, growing up to 25–35 m.
Juglans regia is considered to treat a variety of health complaints traditionally, including Cancer, Inflammation, Diabetes, Antiradicalar, Hyperhidrosis, Antidiarriec, Prostate, Antiradicalar, and Cardiovascular disorders.[Citation2–Citation5] However, researchers investigated that almost all parts of the plant are important against different health disorders as well as for preservation of food grains.[Citation6] The extracts from J. regia nut inhibited oxidative damages,[Citation7–Citation10] inflammation,[Citation11,Citation12] tumor growth,[Citation8,Citation13] antiwrinkle, and photoageing.[Citation14] Kernels as a dietary food, against diabetes, hypoxia, some skin diseases, and inflammation[Citation15,Citation16]; leaves as antidiarrheals, anthelmintic, depurative[Citation17] and also mixed with stored-grains as an insecticide and fungicide.[Citation17,Citation18] Stem bark as an astringent, anthelmintic, depurative, bactericide, diuretic, digestive, laxative, stimulant, detergent, and insecticidal.[Citation19] Juglans regia L. shell is reported for polishing gun-casings, jewelry, and metal material and is used as media to separate water and crude oil.[Citation20]
Juglans regia L. is a good source of flavonoids, Polyphenols, flavonols, carbohydrates, fatty acids, cardiac glycosides, steroids, minerals, tannins, protein, dietary fiber, melatonin, plant sterols, α‐tocopherol, folate, tannins, vitamin A and C, and vitamin E family compound.[Citation1,Citation21–Citation23] Several studies demonstrated the antimicrobial activity of phenolic extracts,[Citation20–Citation25] making them as best substitute to antibiotics and food preservatives. Juglans regia L. is a natural product of high economic interest to the food industry and is very popular and largely consumed as royal food globally and valued for its nutritional, health, and sensory attributes.[Citation1] There is an extended interest in using natural antimicrobial compounds, due to the increasing resistance to antibiotics.[Citation24] Although all parts of this valuable plant has been investigated for different biological properties, no study regarding the phytochemical analysis, antioxidant and antimicrobial activity has been reported yet for the male flower of J. regia L. from Himalayan region. The objective of this study is thus to evaluate the phytochemical analysis, antioxidant, and antimicrobial activity of male flower of J. regia L. and its further utilization in food products.
Materials and methods
Reagents and instruments
2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), α,α-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′- azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ascorbic acid (ABA), sodium nitrite, aluminum chloride, gallic acid, and quercitin were purchased from Sigma-Aldrich Chemical Co.(St. Louis, Mo., USA). All organic solvents were of analytical grade (Merck, Darmstadt, Germany), a Multimode reader (Tecan, Austria) were used for the determination of antioxidant and antimicrobial activities. Spectrum RX1 FT-IR spectrophotometer (Perkin Elmer, USA) was used for FT-IR spectra recording and liquid chromatography/mass spectrometry (UHPLC-PDA-Detector Mass spectrometer (HR-LCMS 1290 Infinity UHPLC System, 1260 infinity Nano HPLC with Chipcube, 6550 iFunnel QTOFs), Agilent Technologies, USA) were used for polyphenolics analysis and identification.
Collection and preparation of plant extracts
The male flower part of J. regia L. (locally referred to as Doon Fidin) were collected from the Indian Himalayan region of Jammu and Kashmir during the flowering stage (April 2014), at an altitude 33.7167°N 74.8333°E. The plant materials were identified by Dr. D. Kumarasamy, the designated plant taxonomist of the Department of Botany, Annamalai University, with herbarium voucher no. ABH-2023. The plant material was shade dried and powdered by using electric grinder. Plant material was soaked into solvents (95% methanol and ethanol and water) in a ratio of 1: 6 (w/v) for 48 h at 20°C with vigorous shaking. The extract was subjected to evaporation under vacuumed pressure and residual material was considered as source of crude extract and was stored at 4ºC for further analysis. The extraction yield has been calculated by fallowing the method of[Citation25] directly by using the formula
Antioxidant assays
ABTS radical scavenging assay
ABTS method measures the capacity of different compounds to scavenge the 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid radical cation (ABTS•+).[Citation26] The antioxidant activity was measured in a reaction mixture containing 0.5 mL of 15 µM H2O2, 0.5 mL of 7 mM ABTS, and 50 mM sodium phosphate buffer, pH 7.5. The absorbances were recorded by spectrophotometer at 734 nm and were compared with standard ascorbic acid. IC50 value is the concentration of sample required to inhibit 50% of ABTS•+ production. The percentage of ABTS radical scavenging was calculated as given below:
Where A0 was the absorbance of the control and A1 was the absorbance in the presence of the sample of ascorbic acid (ABA).
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was performed using a TPTZ solution.[Citation27] The working solutions were prepared by mixing 25 mL of acetate buffer (pH 3.6, 300 mM), 2.5 mL of TPTZ solution (10 mM), and 2.5 mL of FeCl 3 · 6H2O solution (20 mM), and were kept at 37°C. The sample solutions with different concentrations (mg/mL) were then mixed with the working solutions and were incubated under darkness at 37°C for 30 min. The absorbance was measured at 650 nm. The antioxidant activities were expressed as µmol/L FeSO4 equivalent/mg extracts, called FRAP values. A higher FRAP value corresponds to a greater antioxidant activity.
DPPH radical scavenging capacity
Free radical scavenging property of different extracts of J. regia male flower against DPPH• (2, 2-diphenyl-1-picrylhydrazyl) was determined spectrophotometrically by the method of.[Citation28] DPPH• is a stable free radical, becomes a stable diamagnetic molecule when gets reduced. DPPH• donate hydrogen when reacts with an antioxidant and gets reduced, this change in color was measured (from deep violet to light yellow) which is directly proportional to the amount and nature of radical scavenger present.
Antimicrobial activity
Microorganisms
The antimicrobial activity of male flower of J. regia was tested against two Gram positive bacterial strains viz. Staphylococcus aureus and Bacillus subtilis, two Gram negative bacterial strains viz. Escherichia coli and Proteus vulgaris, and against two fungal strains viz. Candida albicans and Candida glaborata. The microorganisms were obtained from Rajah Muthiah Medical College and Hospital, Annamalai University, India. The stock cultures were maintained on Muller–Hinton Agar (for bacteria) and Sabouraud dextrose agar medium (for fungi) at 4°C.
Disc diffusion assay and determination of minimum inhibitory concentration (MIC)
Antimicrobial activity of different extracts of male flower of J. regia was determined by using disc diffusion method of[Citation29] with little modifications. The susceptibility tests were performed on Muller–Hinton Agar (bacteria) and Sabouraud Dextrose Agar (fungi). 50, 25, and 12.5 mg (diluted with mg/mL 5% dimethyl sulfoxide (DMSO)) of all extracts were impregnated on the filter paper discs (6 mm) and used for the study. Ciprofloxacin (5 mg/disc) for bacteria and Amphotericin B (20 mg/disc) for fungi were used as positive reference standards to determine the sensitivity of the tested strains and 5% DMSO was used as blind control. Finally, the inoculated plates were incubated at 37°C for 24 h(for bacteria), 28°C for 48 h (for Candida) and the inhibition zones were observed including the diameter of the disc (6 mm).
MIC of the male flower of J. regia was tested by the twofold serial dilution method of.[Citation30] The extracts were dissolved in 5% DMSO to obtain 100 mg/mL stock solution. 0.5 mL of stock solution was incorporated into 0.5 mL of Mueller Hinton broth (bacteria) to get the concentration of 100 mg/mL and serially diluted to achieve 50, 25, and 12.5 mg/mL. Fifty microliters of standardized suspension of the test organism was transferred on to each tube. The control tube contained only organisms, without extracts and 5%DMSO was used as blind control. The culture tubes were incubated at 37°C for 24 h. The lowest concentrations, which did not show any growth of tested organisms after macroscopic evaluation was determined as MIC.
Phytochemical investigation
Total phenolic content (TPC)
TPC of MEJR, EEJR, and AEJR were analyzed by using Folin–Ciocalteu method fallowed by[Citation31] with little modifications. 0.5 mL of extracts (0.5 mg/mL) were diluted with 6 mL of double-distilled water, then mixed with 0.5 mL of 1N Folin–Ciocalteu’s reagent and were allowed to settle for 5 min. consequently 2 mL of 20% Na2CO3 solution were supplemented. Absorbance was measured at 750 nm by using UV–VIS spectrophotometer (Tecan, Austria), after the incubation of 1 h at RT. Gallic acid has been used as standard. Total content of phenolics were expressed as mg gallic acid equivalents/100g DW.
Total flavonoids content (TFC)
TFC of MEJR, EEJR, and AEJR were determined on the basis of formation of flavonoid-aluminum complex by using spectrophotometric method of[Citation32] with little modifications. 0.5 mL of 2% AlCl3 (aluminum chloride) with methanol were added to 0.5 mL of extract. Absorbances were measured at 430 nm after the incubation of 15 min at room temperature. Quercitin has been used as standard. The total content of flavonoids was expressed as mg quercitin equivalents/100 g DW. The values were presented as means of triplicate analyses.
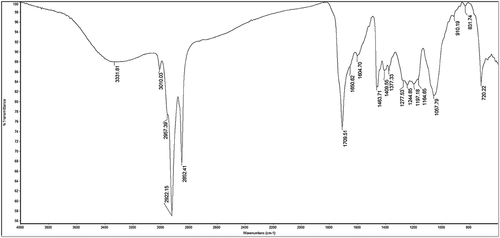
Fourier transform infrared (FT-IR) spectroscopy analysis
FT-IR spectra were recorded by using Spectrum RX1 FT-IR spectrophotometer (Perkin Elmer, USA) working in 4000–400 cm−1 region, Outfitted with a KBr beam splitter, DTGS detector and Nichrome source. Total of 100 scans were acquired for Final spectrum with 4 cm−1 resolution. For the preparation of translucent sample discs, the dried powdered extract was encapsulated in KBr. pellet.
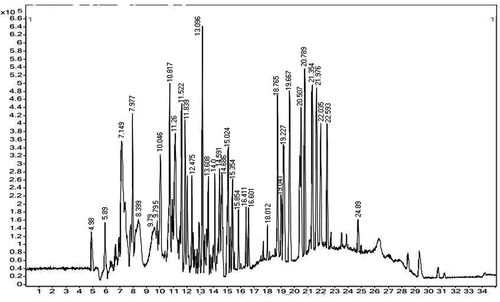
High resolution-liquid chromatography-mass spectrometry analysis (HR-LCMS)
The HR-LCMS analysis of MEJR was analyzed by with some modifications, by using UHPLC-PDA-Detector Mass spectrometer (HR-LCMS 1290 Infinity UHPLC System, 1260 Infinity Nano HPLC with Chipcube, 6550 iFunnel QTOFs), Agilent Technologies, USA. For chromatographic separation, an Agilent 1200 Series HPLC system (Agilent Technologies, USA) equipped with a binary gradient solvent pump, HiP Sampler, column oven and MS Q-TOF with Dual AJS ES Ion Source. Samples were separated on SB-C18 column (2.1 × 50 mm, 1.8-particle size; Agilent Technologies, USA) maintained at 25°C. The solvents used were: water containing 0.1% HCOOH and methanol containing 0.1% HCOOH. The following gradient elution program at a flow rate of 0.4 mL min−1 was applied. MS detection was performed in MS Q-TOF Mass spectrometer (Agilent Technologies). The identified constituents were quantified on the basis of their peak areas and comparison with a calibration curve obtained with the corresponding standards. Linearity ranges for calibration curves were specified.
Statistical analysis
All of the examinations were executed in triplicate, and the values were expressed as the mean ± standard deviation. The results were evaluated through analysis of variance (ANOVA) with Duncan’s multiple range tests (p < 0.05) using SPSS.
Results and discussion
Antioxidant activity of JR
To evaluate the antioxidant activities of three different extracts of male flower of J. regia L., DPPH, ABTS, and FRAP assay were analyzed as shown in . The DPPH, ABTS, and FRAP assays are based on electron transfer between sample and the reagent radical and are measured by evaluating their color changes spectrophotometrically. All three extracts of J. regia showed the significant free radical scavenging activity against DPPH, BTS, and FRAP assay, comparing with the high antioxidant effect of ascorbic acid. However, MEJR showed the highest antioxidant activity having IC50 value of 66.80 ± 2.13 for DPPH, 53.95 ± 6.46 for ABTS, and also FRAP value of 43.60 ± 4.83 µmol/L FeSO4/mg extract as compared with EEJR having IC50 value of 75.17 ± 4.43for DPPH, 63.40 ± 5.73 for ABTS and FRAP value of 54.35 ± 3.12 µmol/L FeSO4/mg extract, followed by AEJR having IC50 value of 76.76 ± 3.75 for DPPH, 64.30 ± 7.23 for ABTS and FRAP value of 52.70 ± 2.90 µmol/L FeSO4/mg extract. The free radical scavenging activity of different extracts are comparable with previously reported studies on leaves, bark, stem, and nuts of J. regia by using DPPH, ABTS, and FRAP assays.[Citation8,Citation22,Citation33–Citation36]
Table 1. Antioxidant activity of different extracts of male flower of J. regia L.
The results of the total phenolic content evaluated using Folin-Ciocalteu method, are shown in to support the antioxidant activity of the male flower of J. regia. MEJR showed the higher TPC (129.76 ± 3.11 mg/g DM) as compared to EEJR (124.12 ± 2.45 mg/g DM) and AEJR (122.1 ± 2.80 mg/g DM), which is similar with previously reported TPC (116.39 ± 5.63 and 92 ± 1.40 mg GAE/g) of J. regia nuts in methanolic and petroleum ether extracts, respectively.[Citation8]
Flavonoids are secondary metabolites, with several health benefits such as antioxidant, anti-inflammatory, and antimicrobial activities.[Citation37] Rutin, quercetin, Gallic acid, and kaempferol are major flavonoids reported previously in J. regia.[Citation38,Citation39] The TFC values in the present study were highest in MEJR (144.62 ± 2.40 mg/g) followed by EEJR (137.81 ± 3.28 mg/g) and AEJR (131.79 ± 4.69 mg/g), which is significant than previously reported TFC in the methanolic and ethyl acetate extract of Shell of J. regia where TFC was 48.90 ± 0.7 and 80.40 ± 0.55 mg QEs/g extract, respectively.[Citation40]
Antimicrobial activity
The antimicrobial potential of male flower of J. regia against two Gram positive and Gram negative bacteria and two fungi were evaluated by using disc diffusion method and determination of minimum inhibitory concentration and the results are presented in . The results revealed that the male flower of J. regia showed significant antimicrobial activity against all the bacterial and fungal strains tested and Gram positive bacteria were more susceptible than Gram negative bacteria and fungi. The mean zone of inhibition produced by all the extracts ranged from 9.28 ± 0.5 to 46.13 ± 4.1 mm and the MIC value were between 2.6 and 4.7 mg/mL. The MEJR showed highest antimicrobial activity with the highest mean zone of inhibition (46.13 ± 4.1 mm) and lowest MIC (2.6 mg/mL) values against S. aureus followed by EEJR against E. coli (39.19 ± 4.3 mm; MIC = 3.9) and AEJR against B. subtilis (39.15 ± 2.9 mm; MIC = 3.0). MEJR also showed the highest mean zone of inhibition against E. coli (44.7 ± 3.2 mm; MIC = 3.4;) and P. vilgaris (43.12 ± 1.8 mm; MIC = 3.0 µg/mL). The highest antifungal activity was also observed in MEJR against C. albicans (40.57 ± 3.7 mm) and C. globarata (39.4 ± 2.5) followed by EEJR against C. albicans (33.73 ± 3.52 mm) and AJR against (36.2 ± 1.8).
Table 2. Antimicrobial activity of different extracts of male flower of J. regia L.
Recently, Farooqui et al.[Citation41] found the bark of J. regia against 15 bacteria’s (viz, Staphylococcus aureus, Streptococcus pyogenes, Enterobacter cloacae, Citrobacter freuendii, etc.) with MIC ranging from 0.31 to >5 (mg/mL). Zakavi et al. (2013)[Citation42] reported that the ethanolic and aqueous extracts of J. regia bark against some oral Bacteria (Staphylococcus aureus, Streptococcus sanguis, Streptococcus salivarius, and Streptococcus mutans), having MIC up to 5 mg/mL. Cruz-Vega et al. (2008)[Citation43] also found leaves and bark as the active aerial part of J. regia against microbes with MICs ranging from 100 to 125 μg/mL. Noumi et al. (2010)[Citation44] reported the antibacterial effect of bark of J. regia L. with MIC ratio ranging from 0.006 to 3.125 (mg/mL). The differences registered between our results and previously reported data could be attributed to the extraction procedure, the plant origin, the tested microorganisms, and the size of the inoculums. The results of antifungal activity of different extracts of J. regia L., is comparable with the results of Pereira et al., 2007,[Citation45] Oliveira et al., 2007,[Citation46] and Sytykiewicz et al., 2015[Citation47] screened leaves and green husks of J. regia from Portugal and Poland, against Candida spp. with significant activity.
FT-IR spectral analysis
FT-IR spectral analysis of MEJR revealed the occurrence of various functional groups in it. Spectral data confirmed the existence of bioactive functional groups like alkanes (C–H stretch, C–H rock), alkenes (–C = C– stretch, = C–H bend), alkynes (–C ≡ C–H: C–H stretch), 1°, 2° amines and amides (N–H stretch), α, β–unsaturated aldehydes and ketones (C = O stretch), 1° amines (N–H bend), aromatics (C–C in–ring), aliphatic amines (C-N stretch), and alkyl halides (C–Cl stretch). IR absorption frequencies and the representative spectra are shown in and , respectively. The analyzed functional groups give the probable identification of compounds present in the extract and support the data analyzed by HR-LCMS.
Table 3. Major bands observed in the FT-IR spectra of MEJR.
Phytochemical composition of MEJR
The methanolic extract of J. regia male flower (MEJR) were selected for phytochemical analysis by using HR-LCMS, on the basis of significant antioxidant, extraction yield (%), TPC and TFC content, and antimicrobial activity. With the retention times, absorbance spectra and MS data, chemical composition of MEJR possess 26 bioactive compounds . The chemical formulae, retention time, and mass of the compounds are listed in . Some of the above compounds have already been identified in different parts of J. regia L. from the different ecological conditions like Arginine in nuts of J. regia from New Zealand,[Citation38] Docosahexaynoic acid in nuts from six J. regia Cultivars grown in Portugal,[Citation48] 11-amino-undecanoic acid (ursolic acid) were reported in green husks of J. regia in China,[Citation49] 2,2-Dimethyl-3-Oxo-Butyric Acid 2-Trimethylsilanyl; Quercetin-3-O-glucuronide; Hexanoic acid, trimethylsilyl ester; Oleic acid; n-Hexadecanoic acid and 1,2–Benzene dicarboxylic acid, bis-(2-methyl propyl) ester were reported in leaves, green husk, stem bark, and nuts of J. regia.[Citation50,Citation51,Citation10,Citation52] Chemical constituents like, Elephantopin, Gamma-L-Glutamyl-cysteine, Artemisinin, Madecassic acid, Dihydromyricetin, Swietenine, Securinine, and Sphinganine were reported first time from the J. regia as per our best knowledge. The differences in the phytochemistry may be varying by season, habitat, or the ecological conditions of plants.
Table 4. Identification of phenolic acids and flavonoids in the male flower of J. regia L. by high-resolution liquid-chromatography mass spectrometry (HR-LCMS) analysis.
Conclusion
The results obtained from the study showed that male flower of J. regia possess significant antioxidant and antimicrobial activity and can be used as an easily accessible source of natural bioactive compounds. However, tested strains were more susceptible to MEJR as compared to EEJR and AEJR and significant free radical scavenging activity was also observed in MEJR. Seven new bioactive compounds were also identified from the MEJR along with other known compounds. Thus, the study explored the fact that J. regia L. have great reservoir of new antioxidant and antimicrobial agents and hence demonstrated that J. regia L. is a potential source of bioactive compounds can be used as functional food supplements to deter the need of antioxidants as well as microbes related to digestive and gastrointestinal tract, which are further needed to be explored in more details. Therefore, further studies are in progress to isolate the antioxidant and antimicrobial agents from MEJR using different spectroscopic techniques.
References
- Martinez, M. L.; Labuckas, D. O.; Lamarque, A. L.; Maestri, D. M. Walnut (Juglans Regia L.): Genetic Resources, Chemistry, By-Products. Journal of the Science of Food and Agriculture 2010, 12, 1959–1967.
- Girzu, M.; Carnat, A.; Privat, A. M.; Fialip, J.; Carnat, A. P.; Lamaison, J. L. Sedative Effect of Walnut Leaf Extract and Juglone, an Isolated Constituents. Pharm. Biol 1998, 36, 280–286. DOI: 10.1076/phbi.36.4.280.4580.
- Mouhajir, F.; Hudson, J. B.; Rejdali, M.; Towers, N. Multiple Antiviral Activities of Endemic Medicinal Plants Used by Berber People of Morocco. Pharm. Biol 2001, 39, 364–374. DOI: 10.1076/phbi.39.5.364.5892.
- Vaidyaratnam, P. S. V.;. Indian Medicinal Plants a Compendium of 500 Species; Orient Longman Private Limited: Chennai, 2005; Vol. 3, pp. 264–265.
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Naghdi, N.; Rafieian-Kopaei, M. An Ethno-Medicinal Study of Medicinal Plants Used for the Treatment of Diabetes. J Nephropathol 2016, 5, 44–50. DOI: 10.15171/jnp.2016.08.
- Shah, T. I.; Sharma, E.; Ahmad, G. Juglans Regia Linn: A Phytopharmacological Review. World J Pharm Sci 2014, 2(4), 363–373.
- Isabel, F. A.; Fernandes, E.; Lima, L. F. C.; Costa, P. C.; Bahia, M. F. Walnut (Juglans Regia) Leaf Extracts are Strong Scavengers of Pro-Oxidant Reactive Species. Food Chemistry 2008, 106, 1014–1020. DOI: 10.1016/j.foodchem.2007.07.017.
- Carvalho, M.; Ferreira, P. J.; Mendes, V. S.; Silva, R.; Pereira, J. A.; Jenimo, C.; Silva, B. M. Human Cancer Cell Antiproliferative and Antioxidant Activities of Juglans Regia L. Food Chem. Toxicol 2010, 48, 441–447. DOI: 10.1016/j.fct.2009.10.043.
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In Vitro Antibacterial and Free Radical Scavenging Activity of Green Hull of Juglans Regia. Journal of Pharmaceutical Analysis 2013, 3, 298–302. DOI: 10.1016/j.jpha.2013.01.006.
- Zhao, M. H.; Jiang, Z. T.; Liu, T.; Li, R. Flavonoids in Juglans Regia L. Leaves and Evaluation of in Vitro Antioxidant Activity via Intracellular and Chemical Methods. Scientific World Journal 2014, 303–878.
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L. A.; Moutsatsou, P. Walnut Extract (Juglans Regia L.) and Its Component Ellagic Acid Exhibit Anti-Inflammatory Activity in Human Aorta Endothelial Cells and Osteoblastic Activity in the Cell Line KS483. Br J Nutr 2008, 99, 715–722. DOI: 10.1017/S0007114507837421.
- Hosseinzadeh, H.; Zarei, H.; Taghiabadi, E. Antinociceptive, Anti-Inflammatory and Acute Toxicity Effects of Juglans Regia L. Leaves in Mice. Iran Red Crescent Med J 2011, 13(1), 27–33.
- Negi, A. S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M. P. Antiproliferative and Antioxidant Activities of Juglans Regia Fruit Extracts. Pharm Biol 2011, 49, 669–673. DOI: 10.3109/13880209.2010.537666.
- Joshan, D. S.; Singh, S. K. Investigational Study of Juglans Regia Extract and Quercetin against Photoaging. Biomedicine & Aging Pathology 2013, 3, 193–200. DOI: 10.1016/j.biomag.2013.08.005.
- Tsao, R.;. Chemistry and Biochemistry of Dietary Polyphenols. Nutrient 2010, 2, 1231–1246. DOI: 10.3390/nu2121231.
- Ram, S.; Verma, R. C. P.; Chauhan, A.; Sanjog, T. Phytochemical Analysis of the Leaf Volatile Oil of Walnut Tree (Juglans Regia L.) From Western Himalaya. Industrial Crops and Products 2013, 42, 195–201. DOI: 10.1016/j.indcrop.2012.05.032.
- Cosmulescu, S.; Trandafir, I. Seasonal Variation of Total Phenols in Leaves of Walnut (Juglans Regia L.). Journal of Medicinal Plants Research 2011, 5(19), 4938–4942.
- Negi, K. S.; Kanwal, K. S. Plants Used as Fish Toxins in Garhwal Region of Uttarakhand Himalaya. Indian Journal of Traditional Knowledge 2009, 8(4), 535–538.
- Espin, J. C.; Soler-Rivas, C.; Wichers, H. J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2,2-Diphenyl-1-Picrylhydrazyl Radical. J Agric Food Chem 2000, 48(3), 648–656. DOI: 10.1021/jf9908188.
- Srinivasan, A.; Viraraghavan, T. Removal of Oil by Walnut Shell Media. Bioresource Technology 2008, 99, 8217–8220. DOI: 10.1016/j.biortech.2008.03.072.
- Caglarirmak, N.;. Biochemical and Physical Properties of Some Walnut Genotypes (Juglans Regia L). Nahrung Food 2003, 47, 28–32. DOI: 10.1002/food.200390004.
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Study of the Main Constituents of Some Authentic Walnut Oils. J Agric Food Chem 2005 15, 53(12), 4853–4860. DOI: 10.1021/jf0478354.
- Muradoglu, F. H.; Oguz, H. I.; Yildiz, K.; Yilmaz, H. Some Chemical Composition of Walnut (Juglans Regia L.) Selections from Eastern Turkey. Afr. J. Agric. Res 2010, 5, 2379–2385.
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara Scolymus L.) and Their Antimicrobial Activities. J. Agric. Food Chem 2004, 52, 7272–7278. DOI: 10.1021/jf0490192.
- Proestos, C.; Chorianopoulos, N.; Nychas, G. J. E.; Komaitis, M. RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. J. Agric. Food Chem 2005, 53, 1190–1195. DOI: 10.1021/jf040083t.
- Arnao, M. B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to the Total Antioxidant Activity. Food Chem 2001, 73, 239–244. DOI: 10.1016/S0308-8146(00)00324-1.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239(1), 70–76. DOI: 10.1006/abio.1996.0292.
- Orhan, I.; Aslan, M. Appraisal of Scopolamine-Induced Anti-Amnesic Effect in Mice and in Vitro Antiacetylcholinesterase and Antioxidant Activities of Some Traditionally Used Lamiaceae Plants. J Ethnopharmacol. 2009, 122, 327–332. DOI: 10.1016/j.jep.2008.12.026.
- Bauer, A. W.; Kirby, W. M. M.; Scherris, J. C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol 1966, 45, 493–496. DOI: 10.1093/ajcp/45.4_ts.493.
- Ericsson, H. M.; Sherris, J. C. Antibiotic Sensitivity Testing: Report of an International Collaborative Study. Acta Pathologica et Microbiologica Scandinavica–Sec B. Microbiol. Immunol 1971, 217, 1–90.
- Vuong, Q.; Goldsmith, C.; Dang, T.; Nguyen, V.; Bhuyan, D.; Sadeqzadeh, E.; Scarlett, C.; Bowyer, M. Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Capacity from Euphorbia Tirucalli Using Response Surface Methodology. Antioxidants 2014, 3, 604–617. DOI: 10.3390/antiox3030604.
- Lamaison, J. L.; Carnat, A.; Petitjean-Freytet, C. Main Flavonoid Contents of Commercial Samples of Filipendula Ulmaria (L.). Maxim. Plant. Med. Phytother 1991, 25, 1–5.
- Fukuda, T.; Ito, H.; Yoshida, Y. Antioxidative Polyphenols from Walnuts (Juglans Regia L.). Phytochem 2003, 63, 795–801. DOI: 10.1016/S0031-9422(03)00333-9.
- Almeida, I. F.; Fernandes, E.; Lima, J. L. F. C.; Costa, P. C.; Bahia, M. F. Walnut (Juglans Regia) Leaf Extracts are Strong Scavengers of Prooxidant Reactive Species. Food Chem 2008, 106, 1014–1020. DOI: 10.1016/j.foodchem.2007.07.017.
- Zhang, Z.; Liao, L.; Moore, J.; Wua, T.; Wang, Z. Antioxidant Phenolic Compounds from Walnut Kernels (Juglans Regia L.). Food Chem 2009b, 113, 160–165. DOI: 10.1016/j.foodchem.2008.07.061.
- Qamar, W.; Sultana, S. Polyphenols from Juglans Regia L. (Walnut) Kernel Modulate Cigarette Smoke Extract Induced Acute Inflammation, Oxidative Stress and Lung Injury in Wistar Rats. Hum. Exp. Toxicol 2011, 30, 499–506. DOI: 10.1177/0960327110374204.
- Kumar, S.; Pandey, A. K. Chemistry and Biological Activities of Flavonoids: An Overview. Scientific World Journal 2013, Article ID 162750, 16. DOI: 10.1155/2013/162750.
- Savage, G. P.;. Chemical Composition of Walnuts (Juglans Regia L.) Grown in New Zealand. Plant Foods Hum. Nutr 2001, 56, 75–82. DOI: 10.1023/A:1008175606698.
- Pereira, J. A.; Oliveira, I.; Sousa, A.; Ferreira, I. C. F. R.; Bento, A.; Estevinho, L. Bioactive Properties and Chemical Composition of Six Walnut (Juglans Regia L.) Cultivars. Food Chem. Toxicol 2008, 46, 2103–2111. DOI: 10.1016/j.fct.2008.02.002.
- Yang, J.; Chaoyin, C.; Shenglan, Z.; Feng, G.; Diqiu, L. The Inhibitory Effect of Different Solvents Extracts from Walnut Shell (Juglans Regia L.) On Pancreatic Lipase and Adipogenesis of 3T3-L1 Preadipocytes. Journal of Food and Nutrition Research 2014, 10, 664–670. DOI: 10.12691/jfnr-2-10-2.
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S. U.; Rubino, S. Synergistic Antimicrobial Activity of Camellia Sinensis and Juglans Regia against Multidrug-Resistant Bacteria. PLOS ONE 2015, 10(2), e0118431. DOI: 10.1371/journal.pone.0118431.
- Zakavi, F.; Golpasand, H. L.; Daraeighadikolaei, A.; Farajzadeh, S. A.; Daraeighadikolaei, A.; Leilavi, S. Z. Antibacterial Effect of Juglans Regia Bark against Oral Pathologic Bacteria. International Journal of Dentistry 2013, 854765.
- Cruz-Vega, D. E.; Verde-Star, M. J.; Salinas-González, N.; Rosales-Hernández, B.; Estrada-García, I.; Mendez-Aragón, P.; Carranza-Rosales, P.; González-Garza, M. T.; Castro-Garza, J. Antimycobacterial Activity of Juglans Regia, Juglans Mollis, Carya Illinoensis and Bocconia Frutescens. Phytother Res 2008, 22(4), 557–559. DOI: 10.1002/(ISSN)1099-1573.
- Noumi, E. M.; Snoussi, H.; Hajlaoui, E.; Bakhrouf, A. Antifungal Properties of Salvadora Persica and Juglans Regia L. Extracts against Oral Candida Strains. European Journal of Clinical Microbiology and Infectious Diseases 2010, 29, 81–88. DOI: 10.1007/s10096-009-0824-3.
- Pereira, A. P.; Ferreira, I. C. F. R.; Marcelino, F.; Valentão, P.; Andrade, F.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J. A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea Europaea L. Cv. Cobranc Osa) Leaves. Molecules 2007, 12, 1153–1162. DOI: 10.3390/12051153.
- Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.; Ferreira, I. C. F. R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L.; Pereira, J. A. Hazel (Corylus Avellana L.) Leaves as Source of Antimicrobial and Antioxidative Compounds. Food Chem 2007, 105, 1018–1025. DOI: 10.1016/j.foodchem.2007.04.059.
- Sytykiewicz, H.; Grzegorz, C.; Paweł, C.; Bogumił, L.; Iwona, S.; Robert, K.; Henryk, M. Antifungal Activity of Juglans Regia (L.) Leaf Extracts against Candida Albicans Asolates. Pol. J. Environ. Stud 2015, 24, 1339–1348. DOI: 10.15244/pjoes/34671.
- Amaral, J. S.; Casal, S.; Pereira, J. A.; Seabra, R. M.; Oliveira, B. P. Determination of Sterol and Fatty Acid Compositions, Oxidative Stability, and Nutritional Value of Six Walnut (Juglans Regia L.) Cultivars Grown in Portugal. J Agric Food Chem 2003 17, 51(26), 7698–7702. DOI: 10.1021/jf030451d.
- Zhou, Y.; Yang, B.; Liu, Z.; Jiang, Y.; Liu, Y.; Fu, L.; Wang, X.; Kuang, H. Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans Mandshurica Maxim in HepG-2 Cancer Cells. Molecules 2015, 20(10), 19252–19262. DOI: 10.3390/molecules201019252.
- Nabavi, S. F.; Mohammad, A. E.; Seyed, M. N.; Mitra, M.; Shabnam, K. R. Biological Activities of Juglans Regia Flowers. Revista Brasileira de Farmacognosia Brazilian Journal of Pharmacognosy 2011, 21(3), 465–470. DOI: 10.1590/S0102-695X2011005000092.
- Kale, A. A.; Tushar, V. G.; Swati, M. D.; Nirmala, R. D.; Jyoti, P. S. GC-MS Study of Stem Bark Extract of Juglans Regia L. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2012, 3, 740.
- Anjum, S.; Adil, G.; Mudasir, A.; Asima, S.; Yasir, S.; Asir, G. Antioxidant and Antiproliferative Activity of Walnut Extract (Juglans Regia L.) Processed by Different Methods and Identification of Compounds Using GC/MS and LC/MS Technique. Journal of Food Processing and Preservation 2016. DOI: 10.1111/jfpp.12756.