ABSTRACT
Gynura bicolor DC. (G. bicolor) is a popular vegetable consumed in many Asian countries. In this study, chemical profile and antioxidant activity of G. bicolor ethanolic extract were studied. The extraction conditions were optimized and the total phenolic content of G. bicolor was determined as 10.38 mg of gallic acid equivalents per gram of dry matter. Twenty three compounds were identified in G. bicolor by HPLC-Q-TOF-MS analysis. Among them, 14 phenolic compounds, including ninepolyphenols and five flavonoids were found. 3-O- and 5-O-p-coumaroylquinic acid were the most abundant phenolic compounds in the vegetable. The ethanolic extract of G. bicolor showed appreciable antioxidant activity as analyzed by radical scavenging and reduce power tests.
Introduction
Epidemiological studies have proved the positive effects of consumption vegetables and fruits on human health. One possible reason is that vegetables and fruits contain high amount of antioxidants, which can scavenge excess reactive oxygen species (ROS) and prevent cell death and tissue damage.[Citation1] Hence, the studies on natural antioxidant, especially phenolic compounds, have gained increasing attention.[Citation2–Citation4]
Gynura bicolor DC. (G. bicolor) is a perennial plant belonging to the Asteraceae family. Its leaf part with characteristics of reddish purple color on the abaxial side is a popular vegetable consumed in many Asian countries (e.g. China, Japan, Thailand, etc). The cooked vegetable shows good flavor and is believed to confer many health-promoting effects, such as antihyperglycemic, antioxidative, etc. Scientific studies have showed that the extract of G. bicolor could decrease ROS formation, preserve glutathione content, and retain glutathione peroxide and catalase activities in high-glucose-treated HUVE cells.[Citation5] Teoh et al. have found that the ethyl acetate extract of G. bicolor possessed cytotoxicity and induced apoptotic and necrotic cell death in human colon carcinoma cells).[Citation6] Hsieh et al. have showed that injection of water extract of G. bicolor could maintain physiological homeostasis and enhance immunity against V. alginolyticus infection in white shrimp.[Citation7] Similar results were also obtained in white shrimp through dietary administration of G. bicolor extract for 7–28 days.[Citation8]
Phenolic compounds, such as phenolic acids, flavonoids and anthocyanins are thought major related to the physiological effects of G. bicolor. However, researches on the phytochemical profile of G. bicolor are rare. Shimizu et al. have isolated three anthocyanins from the leaves of G. bicolor.[Citation9] Chao et al. have determined some common phenolic acids and flavonoids in G. bicolor by HPLC using limited external standards.[Citation5] Two terpenes, four megastigmane-type norisoprenoids and two glycosides were isolated from the ethanolic extract of G. bicolor.[Citation10] GC–MS analysis showed that (E)-caryophyllene, α-humulene, and bicyclogermacrene are the major components of the volatiles of field-grown G. bicolor.[Citation11] However, the definite phytochemical profile of this plant remain unclear. In the present study, the chemical profile of G. bicolor ethanolic extract was analyzed by HPLC-Q-TOF-MS. Besides, the total phenolic content was determined and its antioxidant activity was compared with vitamin C.
Material and methods
Plant material
Fresh leaves of G. bicolor was harvested from experimental field of Jiangxi Agricultural University in April 2017. The leaves was dried at 60°C in oven. The dry material was smashed by high speed pulverizer in the lab (Qijian Q-400B, Shanghai, China), and then filtered through 40 mesh sieve.
Chemicals
1,1-diphenyl-2-picryl-hydrazil (DPPH), 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), vitamin C, formic acid (HPLC grade), ammonium thiocyanate, potassium persulfate, ferrous chloride, and ferric chloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Milli-Q water was prepared by Millipore apparatus (Millipore, MA, USA). HPLC grade methanol was purchased from RCI Labscan Ltd. (Bangkok, Thailand). All other chemicals were of analytical grade.
Sample extraction
One-half gram of G. bicolor powder was immersed with 25.0 mL of 40% ethanol and then sonicated for 30 min at 40°C using a bath sonicator (100 W, 45 kHz, Kunshan, China). The mixture was centrifuged at 4000 rpm for 5 min. The supernatant was filtered by 0.22 mm pore size filter and then used for total phenolic content determination and HPLC-Q-TOF-MS analysis.
Five gram of G. bicolor powder was immersed with 250 mL of 40% ethanol and then sonicated for 30 min at 40°C. The mixture was centrifuged at 4000 rpm for 5 min. The supernatant was concentrated to 100 mL by vacuum rotary evaporation at 60°C to remove ethanol. The concentrated solution was used for antioxidant study after proper dilution. Its total phenolic content was determined by Folin-Ciocalteu method.
Determination of total phenolic content
The total phenolic content in the extract was determined by Folin-Ciocalteu method.[Citation12] Briefly, in a 10 mL flask, a 0.5 mL aliquot of the extract and Folin-Ciocalteu reagent was added, followed by addition of 1 mL of sodium carbonate solution (15%, w/v). The mixture was diluted to the marker and measured at 765 nm after staying 30 min. A blank sample consisting of water and reagents was used as a reference. The total phenolic content was expressed as gallic acid equivalents utilizing a calibration curve of Y = 94.526X + 0.0388, R2 = 0.9954, where Y is the absorbance and X is the concentration of gallic acid (ranged from 1.5 to 15 μg/mL).
Antioxidant activity test
Scavenging of DPPH radical[Citation12]: Briefly, a 1.0 mL aliquot of 0.1 mM DPPH radical solution dissolved in ethanol was mixed with 0.2 mL of G. bicolor extract at various concentrations. Vitamin C was used as positive control. After staying 10 min at the room temperature, the absorbance was measured at 517 nm on an Analytik Jena-Specord 200 spectrophotometer (Germany). The DPPH radical-scavenging activity (%) was calculated as:
Scavenging activity = (1 – A1/A0) × 100, where A1 and A0 are the absorbance in the presence of extract and water, respectively .
Scavenging of ABTS+ radical[Citation12]: Briefly, ABTS+ was produced by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate (final concentration). The mixture was stood in dark at room temperature for 12–16 h before use. Prior to assay, the solution was 70 times diluted in water to give an absorbance of 0.75 at 734 nm in a 1 cm cuvette. A 1.0 mL aliquot of the diluted ABTS+ solution was mixed with 0.2 mL of G. bicolor extract at various concentrations or positive control (vitamin C). After staying 10 min at the room temperature, the mixture was monitor at 734 nm. The radical scavenging activity was calculated as the same as DPPH test.
Reducing power[Citation12]: Briefly, a 1.0 mL aliquot of extract at various concentrations was mixed with 1 mL of phosphate buffer (pH 6.6, 0.2 M) and potassium ferricyanide [K3Fe(CN)6](1% in water, w/v). The mixture was incubated at 50°C for 20 min and the reaction was stopped by addition of 1 mL of trichloroacetic acid (10% in water, w/v), followed by centrifugation at 5000 rpm for 10 min. A 1 mL aliquot of the upper layer of solution was mixed with 0.5 mL of FeCl3 (0.1% in water, w/v), and the absorbance was measured at 700 nm against blanks that contained all reagents except the extract.
HPLC-Q-TOF-MS analysis
The mass spectrometry analysis was performed on a Q-TOF 5600-plus mass spectrometer equipped with Turbo V sources and a Turbolonspray interface (AB Sciex Corporation, Foster City, CA, USA) coupled to a Shimadzu LC-30A UHPLC-DAD system (Shimadzu Corporation, Kyoto, Japan). Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm, Waters) was used. The flow rate was 0.3 mL/min, injection volume was 3 μL, and column temperature was 40°C. The mobile phase was methanol (A) and 0.1% formic acid aqueous solution (B) using a linear gradient program of 1–90% (A) in 0–30 min. The mass spectrometer was operated in the negative ion mode. Ultrapure nitrogen was used as ion source gas 1 (50 psi), ion source gas 2 (50 psi), and curtain gas (40 psi). The Turbo Ion Spray voltage and temperature were set at −4500 V and 500°C, respectively. Declustering potential, collision energy, and collision energy spread were set at 100 V, −40 V, and 10 V, respectively. Data acquisition was performed with Analyst 1.6 software (AB Sciex).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) of triplicates. Statistical analysis, plotting, and curve fitting were performed by Origin 7.0 (Origin Lab Co., Northampton, MA, USA). One-way ANOVA was used for statistical analysis. Differences were considered significant when P < 0.05.
Results and discussion
Extraction optimization and total phenolic content determination
To determine the total phenolic content in G. bicolor, the sonication assisted extraction conditions were optimized first. Ultrasound treatment has been widely used for the extraction of bioactive compounds from plant material. The propagation of ultrasound pressure waves results cavitation phenomena, which can enhance extraction efficiency through accelerating the eddy diffusion and internal diffusion.[Citation13] The combination of specimen particle size, extraction solvent, sonication temperature, and sonication time have significant effects on extraction efficiency. As shown in ), when water was used as the extraction solvent, the extraction yield of total phenolics was 5.2 mg of gallic acid equivalents per g of G. bicolor. However, the extraction yield quickly increased with the addition of ethanol to a final concentration of 40%. Further increasing the ethanol concentration decreased the extraction yield. The optimal extraction solvent was 40% ethanol. Sonication time also showed significant effects, the extraction yield of total phenolics quickly increased from 10 to 30 min, and then slightly decreased with further increasing of sonication time. The highest extraction yield was obtained at 30 min (). ) showed the effects of liquid-to-solid ratio. The extraction yield of total phenolics stably increased with the rise of liquid-to-solid ratio first and then became stable. It’s easy to understand that to a certain extent, the more extractant, the better mass transfer. As shown in ), the optimal extraction temperature was selected at 40°C.
Figure 1. Total phenolics extraction optimization, effects of extraction solvent (a), sonication time (b), liquid-to-solid ratio, (c) and sonication temperature (d). Different letter in graph means significant difference (ANOVA, p < 0.05).
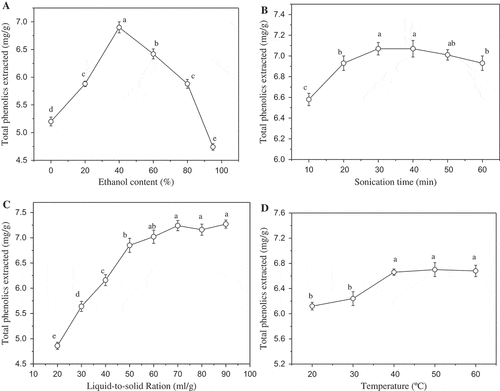
With the criterion of higher extraction yield of total phenolics, the optimal conditions of extraction solvent, sonicaiton time, temperature, and liquid-to-solid ratio were selected as 40% ethanol, 30 min, 40°C, and 50:1, respectively. G. bicolor sample was extracted under these conditions for three times. The total phenolic content determined in each times were 7.52 ± 0.27, 1.61 ± 0.11, and 1.25 ± 0.13 mg of gallic acid equivalents per gram of dry matter, respectively. Thus, the total phenolic content in G. bicolor sample was 10.38 mg of gallic acid equivalents per gram of dry matter.
Chemical profile of G. bicolor ethanolic extract
HPLC-Q-TOF-MS/MS was used to analyze the chemical profile of G. bicolor ethanolic extract. The compounds identification was accomplished by the information of molecular mass and its fragmentation patterns obtained by collision induced dissociation of specified [M-H]–. ) was the base peak chromatogram of G. bicolor extract. As shown, 28 main peaks were found in the chromatogram. Among them, 23 compounds were identified. The detailed information of each peak was summarized in .
Table 1. Characterization of phenolic components in G. bicolor extract by HPLC-Q-TOF-MS.
Table 2. The EC50 values of G. bicolor extract and vitamin C with different antioxidant tests (μg/mL).
Figure 2. Base peak chromatogram (a) and absorbance chromatogram recorded at 327 nm (b) of G. bicolor total phenolic extract.
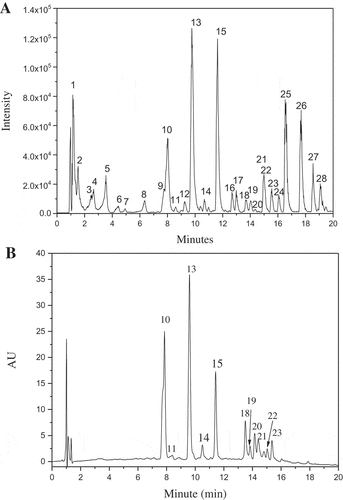
Seven hydroxycinnamic acid derivatives were identified in G. bicolor, including 5-O-caffeoylquinic acid, caffeoyl glucose, 3-O-p-coumaroylquinic acid, 3-O-feruloylquinic acid, 5-O-p-coumaroylquinic acid, 3,5-Di-O-caffeoylquinic acid, and 4,5-Di-O-caffeoylquinic acid. The six hydroxycinnamoylquinic acids yielded a product ions at m/z 191, which is quinic acid moiety and represent the base peak of the fragments. The linkage position of acyl substituent groups on the quinic acid molecule can be deduced based on the fragmentation patterns. As summarized by Gouveia et al.[Citation25] and Weisz et al.[Citation26], when acyl groups connected to the 4-OH position of quinic acid, [quinic acid–H–H2O]− ion at m/z 173 is the base peak. When the acyl group is attached to the 3-OH or 5-OH position, the [quinic acid–H]− ion, at m/z 191, appears as the base peak and the [caffeic acid–H]− ion at m/z 179 or [coumaroylquinicacid–H]− ion at m/z 163 is more significant for 3-OH compounds. Based on these experiences, the isomeric substances of peaks 13 and 15, peaks 18 and 22, were differentiated.
Five flavonoids, quercetin-3-O-rutinoside (rutin), quercetin-3-O-galactoside, quercetin-3-acetylhexose, kaempferol-3-O-glucoside, and kaempferol-3-O-caffeoylate, were identified in G. bicolor. Two other phenolic compounds, protocatechuate-O-glucoside and isobavachalcone were also found. Besides, citric acid and malic acid, the two organic acid commonly found in plants, and one coumarin substance, dihydro-phellopterin, were identified.
) showed the absorbance chromatogram of G. bicolor extract recorded at 327 nm by DAD detector. Hydroxycinnamic acid derivatives show characteristic maximum absorbance at 300–330 nm. Many flavonoids also have absorbance at these wavelength. As shown in , 11 compounds could found corresponding peak in the chromatogram. According to the peak area in ) and (), it can be deduced that hydroxycinnamoylquinic acids are the main phenolic constitutes of G. bicolor. Hydroxycinnamic acid derivatives are important class of polyphenolic compounds originated from the Mavolanate-Shikimate biosynthesis pathways in plants. Studies have showed that these phenolic compounds possess potent antioxidant and anti-inflammatory properties, and have potential therapeutic benefit in experimental diabetes and hyperlipidemia.[Citation27] Our findings may provide some supported evidences for the health-promoting functions of G. bicolor.
In summary, 14 phenolic compounds, including 9 polyphenols and 5 flavonoids were identified in G. bicolor. 3-O-and 5-O-p-coumaroylquinic acid were the most abundant phenolic compounds in G. bicolor. Compared with the HPLC analysis performed by Chao et al.,[Citation5] most of these compounds were identified and reported for the first time in G. bicolor.
Antioxidant activity of G. bicolor ethanolic extract
The accumulation of ROS causes high oxidative stress in human body, which may contribute to the development of many diseases, such as cardiovascular disease, neurodegenerative disorders and even cancers.[Citation28,Citation29] Although human have endogenous antioxidant defenses against ROS, consumption of dietary antioxidants is also very important. There are many methods for testing antioxidant activity of plant extract. In the present study, three commonly used in vitro methods, DPPH, ABTS radical scavenging, and reduce power, were used for evaluating the antioxidant activity of G. bicolor ethanolic extract, and the results were compared with vitamin C as the positive control. The data may provide a useful indication of antioxidant activity of G. bicolor although it is difficult to be applied to biological systems.
As shown in ), the DPPH radical scavenging ability of G. bicolor extract steady increased with the rise of total phenolic content. The scavenging rate reached 90% under the concentration of 140 μg/mL. Similar results were found in ABTS radical scavenging test. The antioxidant activity of G. bicolor extract was weaker than that of positive control, vitamin C. However, they were comparable with difference in the same order of magnitude. As listed in , the EC50 values (the concentration at which the scavenging ratio reaches 50%) of G. bicolor extract to DPPH and ABTS radicals were 44.35 μg/mL and 36.36 μg/mL, respectively. Correspondingly, the EC50 values of vitamin C were 15.51 μg/mL and 5.83 μg/mL, respectively.
Figure 3. Antioxidant activity of G. bicolor total phenolic extract. (a) DPPH radical-scavenging assay; (b) ABTS radical-scavenging assay; (c) reducing power assay.
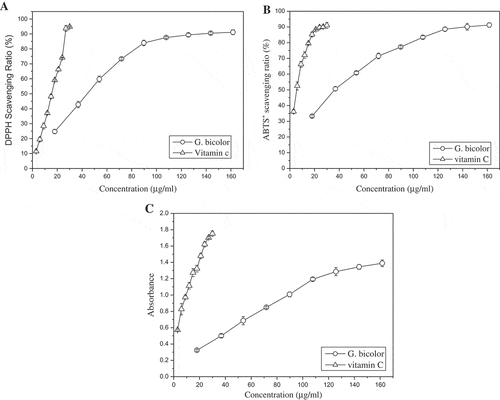
The reducing power is determined on the basis of the ability to reduce ferric (III) iron to ferrous (II) iron. Compounds with reducing power indicate that they are electron donors and can reduce the oxidized intermediates of lipid peroxidation processes, which is strongly correlated with its antioxidant properties. Increased absorbance of the reaction mixture indicates greater reduction capability. showed that G. bicolor extract possesses the reducing powder, and the reduction capability steady increased with the rise of concentation. The EC50 values (the concentration at which the absorbance reaches 0.5) of G. bicolor extract was 36.92 μg/mL, while vitamin C less than 3 μg/mL. Similar to DPPH and ABTS radicals scavenging test, the reducing powder of G. bicolor extract was weaker than that of vitamin C.
Conclusion
The optimized solvent for phenolic compounds extraction from G. bicolor was 40% ethanol and the total phenolic content was determined as 10.38 mg of gallic acid equivalents per g of dry matter. The ethanolic extract of G. bicolor showed appreciable antioxidant activity. Twenty three compounds were identified in G. bicolor by HPLC-Q-TOF-MS analysis. Among them, 14 phenolic compounds, including 9 polyphenols and 5 flavonoids were found. Compared with published literatures, most of these compounds were identified and reported for the first time in G. bicolor. Hydroxycinnamic acid derivatives, particularly 3-O- and 5-O-p-coumaroylquinic acid, were the main phenolic constituents of G. bicolor. Because hydroxycinnamic acid derivatives possess many health-promoting bioactivities both in vitro and in vivo, our present study provided some evidences that daily consumption of G. bicolor, the vegetable popular in many Asian countries, may bring some positive effects on human health.
Additional information
Funding
References
- Tsao, R.; Deng, Z. Separation Procedures for Naturally Occurring Antioxidant Phytochemicals. Journal of Chromatography B 2004, 812, 85–99. DOI: 10.1016/S1570-0232(04)00764-0.
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sciences 2004, 74, 2157–2184. DOI: 10.1016/j.lfs.2003.09.047.
- De Beer, D.; Joubert, E.; Gelderblom, W. C. A.; Manley, M. Phenolic Compounds: A Review of Their Possible Role as in Vivo Antioxidants of Wine. South African Journal of Enology and Viticulture 2017, 23, 48–61. DOI: 10.21548/23-2-2155.
- Martins, N.; Barros, L.; Ferreira, I. C. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends in Food Science & Technology 2016, 48, 1–12. DOI: 10.1016/j.tifs.2015.11.008.
- Chao, C. Y.; Liu, W. H.; Wu, J. J.; Yin, M. C. Phytochemical Profile, Antioxidative and Anti-Inflammatory Potentials of Gynura Bicolor DC. Journal of the Science of Food and Agriculture 2015, 95, 1088–1093. DOI: 10.1002/jsfa.6902.
- Teoh, W. Y.; Sim, K. S.; Moses Richardson, J. S.; Abdul Wahab, N.; Hoe, S. Z. Antioxidant Capacity, Cytotoxicity, and Acute Oral Toxicity of Gynura Bicolor. Evidence-based Complementary and Alternative Medicine 2013. DOI: 10.1155/2013/958407.
- Hsieh, S. L.; Wu, C. C.; Liu, C. H.; Lian, J. L. Effects of the Water Extract of Gynura Bicolor (Roxb. & Willd.) DC on Physiological and Immune Responses to Vibrio Alginolyticus Infection in White Shrimp (Litopenaeus vannamei). Fish and Shellfish Immunology 2013, 35, 18–25. DOI: 10.1016/j.fsi.2013.03.368.
- Jiang, C. M.; Hsieh, S. L. Dietary Administration of Gynura Bicolor (Roxb. Willd.) DC Water Extract Enhances Immune Response and Survival Rate against Vibrio Alginolyticus and White Spot Syndrome Virus in White Shrimp Litopeneaus Vannamei. Fish & shellfish immunology 2015, 42, 25–33. DOI: 10.1016/j.fsi.2014.10.016.
- Shimizu, Y.; Imada, T.; Zhang, H.; Tanaka, R.; Ohno, T.; Shimomura, K. Identification of Novel Poly-Acylatedanthocyanins from Gynura Bicolor Leaves and Their Antioxidative Activity. Food Science and Technology Research 2010, 16, 479–486. DOI: 10.3136/fstr.16.479.
- Chen, J.; Mangelinckx, S.; Adams, A. N.; Li, W. L.; Wang, Z. T.; De Kimpe, N. Chemical Constituents from the Aerial Parts of Gynura Bicolor. Natural product communications 2012, 7, 1563–1564.
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. Volatiles from Leaves of Field‐Grown Plants and Shoot Cultures of Gynura Bicolor DC. Flavour and Fragrance Journal 2009, 24, 251–258. DOI: 10.1002/ffj.v24:5.
- Zhang, Q. F.; Zhang, Z. R.; Cheung, H. Y. Antioxidant Activity of Rhizoma Smilacis Glabrae Extracts and Its Key Constituent-Astilbin. Food Chemistry 2009, 115, 297–303. DOI: 10.1016/j.foodchem.2008.11.053.
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and Opportunities for Ultrasound Assisted Extraction in the Food Industry-A Review. Innovative Food Science & Emerging Technologies 2008, 9, 161–169. DOI: 10.1016/j.ifset.2007.04.014.
- Bystrom, L. M.; Lewis, B. A.; Brown, D. L.; Rodriguez, E.; Obendorf, R. L. Characterisation of Phenolics by LC–UV/Vis, LC–MS/MS and Sugars by GC in Melicoccus bijugatus Jacq.‘Montgomery’ Fruits. Food Chemistry 2008, 111, 1017–1024. DOI: 10.1016/j.foodchem.2008.04.058.
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Comparative Characterization of Phenolic and Other Polar Compounds in Spanish Melon Cultivars by Using High-Performance Liquid Chromatography Coupled to Electrospray Ionization Quadrupole-Time of Flight Mass Spectrometry. Food Research International 2013, 54, 1519–1527. DOI: 10.1016/j.foodres.2013.09.011.
- Guo, M.; Zhang, L.; Liu, H.; Qin, L.; Zhang, Z.; Bai, X.; Gao, X. A Metabolomic Strategy to Screen the Prototype Components and Metabolites of Qingkailing Injection in Rat Urine by High-Performance Liquid Chromatography with Tandem Mass Spectrometry. Journal of Separation Science 2014, 37, 2844–2850. DOI: 10.1002/jssc.v37.20.
- Kim, D. W.; Curtis-Long, M. J.; Yuk, H. J.; Wang, Y.; Song, Y. H.; Jeong, S. H.; Park, K. H. Quantitative Analysis of Phenolic Metabolites from Different Parts of Angelica Keiskei by HPLC-ESI MS/MS and Their Xanthine Oxidase Inhibition. Food Chemistry 2014, 153, 20–27. DOI: 10.1016/j.foodchem.2013.12.026.
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The Modulatory Effect of Infusions of Green Tea, Oolong Tea, and Black Tea on Gut Microbiota in High-Fat-Induced Obese Mice. Food & Function 2016, 7, 4869–4879. DOI: 10.1039/C6FO01439A.
- Ma, S.; Chen, L.; Luo, G.; Ren, K.; Wu, J.; Wang, Y. Off-Line Comprehensive Two-Dimensional High-Performance Liquid Chromatography System with Size Exclusion Column and Reverse Phase Column for Separation of Complex Traditional Chinese Medicine Qingkailing Injection. Journal of Chromatography 2006, 1127, 207–213. DOI: 10.1016/j.chroma.2006.06.021.
- Mullen, W.; Yokota, T.; Lean, M. E.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. DOI: 10.1016/S0031-9422(03)00281-4.
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of Isorhamnetin Glycosides in Extracts of Apples (Malus domestica cv.“Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochemical 2002, 13, 87–94. DOI: 10.1002/pca.630.
- Geng, P.; Zhang, R.; Aisa, H. A.; He, J.; Qu, K.; Zhu, H.; Abliz, Z. Fast Profiling of the Integral Metabolism of Flavonols in the Active Fraction of Gossypium herbaceam L. Using Liquid Chromatography/Multi-Stage Tandem Mass Spectrometry. Rapid Communications in Mass Spectrometry 2007, 21, 1877–1888.
- Downey, M. O.; Rochfort, S. Simultaneous Separation by Reversed-Phase High-Performance Liquid Chromatography and Mass Spectral Identification of Anthocyanins and Flavonols in Shiraz Grape Skin. Journal of Chromatography 2008, 1201, 43–47. DOI: 10.1016/j.chroma.2008.06.002.
- Park, A. Y.; Park, S. Y.; Lee, J.; Jung, M.; Kim, J.; Kang, S. S.; Youm, J. R.; Han, S. B. Simultaneous Determination of Five Coumarins in Angelicae dahuricae Radix by HPLC/UV and LC-ESI-MS/MS. Biomedical Chromatography 2009, 23, 1034–1043. DOI: 10.1002/bmc.1219.
- Gouveia, S.; Castilho, P. C. Antioxidant Potential of Artemisia Argentea L’Hér Alcoholic Extract and Its Relation with the Phenolic Composition. Food Research International 2011, 44, 1620–1631. DOI: 10.1016/j.foodres.2011.04.040.
- Weisz, G. M.; Kammerer, D. R.; Carle, R. Identification and Quantification of Phenolic Compounds from Sunflower (Helianthus Annuus L.) Kernels and Shells by HPLC-DAD/ESI-MSn. Food Chemistry 2009, 115, 758–765. DOI: 10.1016/j.foodchem.2008.12.074.
- Alam, M. A.; Subhan, N.; Hossain, H.; Hossai, M.; Reza, H. M.; Rahman, M. M.; Ullah, M. O. Hydroxycinnamic Acid Derivatives: A Potential Class of Natural Compounds for the Management of Lipid Metabolism and Obesity. Nutrition & metabolism 2016, 13, 27. DOI: 10.1186/s12986-016-0080-3.
- Zhu, Y. Z.; Huang, S. H.; Tan, B. K. H.; Sun, J.; Whiteman, M.; Zhu, Y. C. Antioxidants in Chinese Herbal Medicines: A Biochemical Perspective. Natural Product Reports 2004, 21, 478–489. DOI: 10.1039/b304821g.
- Antolovich, M.; Prenzler, P. D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–198. DOI: 10.1039/b009171p.
