?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the antioxidant, anticholinesterase, and anti-tyrosinase properties of (hexane, acetone, methanol, and water) extracts of Ferula elaeochytris and Sideritis stricta were determined with the total phenolic and flavonoid contents. The phenolic profile of the methanol and water extracts was analysed using HPLC-DAD. Protocatechuic acid was found as the major phenolic compound in the methanol (116.3 ± 3.1 µg/g) and water extracts (69.4 ± 1.3 µg/g) of F. elaeochytris. Coumarins (253.9 ± 4.1 µg/g) and catechin hydrate (175.2 ± 2.9 µg/g) were the most abundant phenolic compounds in the methanol and water extracts of S. stricta. β-carotene–linoleic acid, DPPH•, ABTS•+, CUPRAC, and metal-chelating assays were used to evaluate antioxidant properties of the extracts. The methanol and water extracts of F. elaeochytris and the acetone and methanol extracts of S. stricta containing the highest amount of total phenolic and flavonoid contents showed the highest antioxidant activities in β-carotene–linoleic acid, DPPH•, ABTS•+, and CUPRAC assays. The enzyme inhibitory potential of extracts was investigated against key enzymes involved in neurodegenerative (acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)) and skin (tyrosinase) disorders. In the cholinesterase inhibitory assays, the hexane extracts of two species exhibited the best activity against AChE, while the hexane extract of F. elaeochytris and the methanol extract of S. stricta observed to be the most active against BChE. As for anti-tyrosinase activity results of extracts, the only acetone and methanol extracts showed mild inhibitory activity for both species.
Introduction
The Apiaceae family (formerly Umbelliferae) comprises of flowering and aromatic plants mostly growing in temperate areas. The Ferula genus belongs to the family of Apiaceae, consists of 170 species which spreads from Central Asia to Mediterranean region. In Turkey, the Ferula genus is one of the most important genera and represented by 25 taxa. Ferula species are known as ‘Çakşır’, ‘Çakşır otu’ or ‘Çaşır’ in Turkey.[Citation1] The herbal parts of Ferula species are used as animal feed in winter months, while the root parts are used as an aphrodisiac in Eastern Turkey. The Ferula genus was reported as a rich source of gum resin. Therefore, this genus is used in the treatment of many diseases such as digestive disorders, rheumatism, headache, arthritis, toothache, and diabetes in folk medicine. Some species are used as traditional foods and a flavouring agent as well as a traditional medicine for many diseases in many parts of world.[Citation2] Ferula species is a rich source of biologically active compounds, such as coumarins, sesquiterpenes, sesquiterpene coumarins, sesquiterpene lactones, and daucane esters.[Citation3] As a result of many phytochemical studies, it has been reported that these seconder metabolites and the extracts from Ferula species showing biological properties including anti-diabetic, anti-fertility, antifungal, anti-inflammatory, antispasmodic, antitumor, antiviral, antiulcerogenic, cancer chemopreventive, digestive enzyme inhibition, hypotensive, and molluscicidal mutagenic.[Citation4] According to our knowledge, there are limited investigations about Ferula elaeochytris in the literature. To date, some sesquiterpene compounds were isolated from this species and evaluated their cytotoxic properties by Miski et al.[Citation5] and Alkhatib et al.[Citation6] In a different study, the chemical composition of essential oil of F. elaeochytris was reported.[Citation7]
Lamiaceae is a large plant family consisting of 200 genera and 7000 species worldwide. Salvia, Mentha, and Sideritis are the main species belonging to Lamiaceae family.[Citation8] The genus Sideritis, one of the most important members of the family Lamiaceae, involves more than 150 species widely distributed in Mediterranean area. This genus is represented by 46 species with >78.2% endemism rate in Turkey.[Citation9] The aerial parts of Sideritis species are mostly consumed as a tea and locally known as mountain tea in Anatolia. In the folk medicine, Sideritis species are used as medicine for the treatment of gastrointestinal diseases and common colds such as fever, flu, and bronchitis.[Citation10] In many previous studies, terpenes, flavonoids, essential oils, extracts, iridoids, coumarins, lignans, and sterol compounds with biological properties such as antioxidant, anti-inflammatory, antifeedant, antimicrobial, antiviral, antinociceptive, and anti-ulcer activities have been isolated from Sideritis species.[Citation11,Citation12] Sideritis stricta, an endemic species to Turkey, is used as a herbal tea and popularly known as ‘Dağ çayı’ and ‘Tosbağa çayı’ in Southern Anatolia. This species is considered to be herbal medicine due to their carminative and appetizing properties.[Citation13] In previous reports, phenolic, flavonoid, and diterpenoid compounds have been isolated from S. stricta and examined their human monoamine oxidase inhibitory, anti-inflammatory, antinociceptive, antibacterial, and antifungal activities.[Citation14–Citation16] Also, Kirimer et al.[Citation17] identified the main volatile compounds of essential oil of S. stricta.
Oxidative stress is well known to cause oxidation of biomolecules such as enzymes, lipids, and DNA, leading to cellular damage and death. Therefore, oxidative stress has an important role in the occurrence of various diseases including cardiovascular, neurodegenerative, and inflammatory diseases, cancer, and diabetes, as well as aging processes.[Citation18] It was reported that oxidative stress is associated with the pathogenesis of neurodegenerative disorder Alzheimer’s disease (AD). The risk of AD can be reduced by high consumption of antioxidant in daily life. Additionally, another approach in the treatment of AD is the inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.[Citation19] In many reports, antioxidant activity has been found to be associated with anticholinesterase and anti-tyrosinase activities.[Citation20,Citation21] For this reason, in our study, the antioxidant activity has been investigated with cholinesterase and tyrosinase enzyme inhibitory activities.
This study was performed to assess (i) phenolic profiles of methanol and water extracts and (ii) antioxidant and (iii) cholinesterase and tyrosinase enzyme inhibitory activities of the hexane, acetone, methanol, and water extracts with total phenolic and flavonoid contents of F. elaeochytris and S. stricta. The objective of this study was to evaluate phenolic profiles, antioxidant, anticholinesterase, and anti-tyrosinase activities of various extracts of two Turkish plant species. The phenolic profiles and bioactive properties of the extracts of F. elaeochytris and S. stricta were investigated in details for the first time in this study.
Materials and methods
Plant materials
The plant materials were identified by Dr Hasan Yildirim at Ege University, Izmir, Turkey. The voucher specimen has been deposited at the herbarium of Natural Products Laboratory of Muğla Sıtkı Koçman University. Collection areas and dates of collection of F. elaeochytris and S. stricta are as following: (i) F. elaeochytris: Aydıntepe county, Gümüşdamla village (1650 m altitude), Bayburt, Turkey; 2016 (Voucher no: MUMED1051). (ii) S. stricta: Fethiye-Antalya road, Karabel plateau (1300 m altitude), Muğla, Turkey; 2016 (Voucher no: MUMED1121).
Extraction
The aerial parts of F. elaeochytris (120 g) and S. stricta (140 g) were extracted separately with different solvents (2.5 L) according to their increasing polarity: hexane, acetone, and methanol at room temperature for 24 h and four times. Solvents were evaporated on a rotary evaporator to obtain hexane, acetone, and methanol extracts. The remaining plant part was allowed to stand for one day with water at 80°C. The water extract was obtained by lyophilization using a freeze-drier. All extracts were stored at +4°C until analysis.
Total phenolic and flavonoid contents
The phenolic content of extracts was stated as microgram of pyrocatechol equivalents (PEs).[Citation22] The phenolic contents were calculated according to the following equation that was obtained from standard pyrocatechol graph:
Measurement of flavonoid content of the extracts was based on the aluminium nitrate method, and results were expressed as microgram of quercetin equivalents (QEs).[Citation23] The flavonoid contents were calculated according to the following equation that was obtained from the standard quercetin graph:
Analysis of phenolic compounds
The phenolic compound analysis was performed by the method of Barros et al.[Citation24] with slight modification. The methanol and water extracts were dissolved in methanol and water and filtered through a 0.20-µm disposable LC filter disk for HPLC-DAD. Separation was achieved on an Intertsil ODS-3 reverse-phase C18 column (5 µm, 250 mm × 4.6 mm i.d.) thermostatted at 40°C. The solvent flow rate was 1.5 mL/min. The sample volume injection was 20 µL. The mobile phases used were: (A) 0.5% acetic acid in water and (B) 0.5% acetic acid in methanol. The elution gradient was as follows: 0−20% B (0−0.01 min); 20−60% B (0.01−2 min); 60−80% B (2−15 min); 100% B (15−30 min); 100–10% B (30–35 min); and 10–0% B (35–40 min). Detection was carried out photodiode array detector using 280 nm as the preferred wavelength. All analytical conditions were identical to those described in the recent publication.[Citation25] The phenolic compounds were characterized according to their retention times, and UV data were compared with commercial standards. Three parallel analyses were performed. For the quantitative analysis of phenolic compounds, calibration curves were obtained via the injection of known concentrations (0.0, 0.00782, 0.01563, 0.03125, 0.0625, 0.125, 0.25, 0.5, and 1.0 ppm) of different standard compounds, i.e., gallic acid, fumaric acid, protocatechuic acid, catechin hydrate, p-hydroxybenzoic acid, 6,7-dihydroxy coumarin, caffeic acid, vanillin, 2,4-dihydroxy benzoic acid, p-coumaric acid, ferulic acid, coumarins, trans-2-hydroxy cinnamic acid, ellagic acid, rosmarinic acid, and trans-cinnamic acid. The results were expressed as microgram per gram of dry weight (dw).
Antioxidant activity
β-carotene/linoleic acid assay
The total antioxidant activity was determined by β-carotene–linoleic acid method based on the measurement of the inhibition of conjugated dien hydroperoxides resulting from linoleic acid oxidation with slight modifications.[Citation26] A β-carotene–linoleic acid mixture was prepared as following: 0.5 mg β-carotene in 1 mL of chloroform was added to 25 μL linoleic acid and 200 mg Tween 40 emulsifier mixture. After evaporation of chloroform under vacuum, 100 mL of distilled water saturated with oxygen was added by vigorous shaking. One hundred and sixty microliters of this mixture were transferred into 40 μL of the samples at different concentrations. As soon as the emulsion was added into each tube, the zero time absorbance was measured at 470 nm using a 96-well microplate reader. Absorbance of the emulsion was read again at the same wavelength after incubation of the plate for 2 h at 50°C. Measurement of absorbance was continued until the colour of β-carotene disappeared. BHA and α-tocopherol were used as antioxidant standards for comparison of the activity. The same procedure was repeated with used antioxidant standards and a blank. The bleaching rate (R) of β-carotene was calculated according to Eq. (1).
where ln = natural log, a = absorbance at time zero, and b = absorbance at time t (120 min). Antioxidant activity was calculated in terms of percentage inhibition relative to the control, using Eq. (2).
DPPH free radical scavenging assay
The free radical scavenging activity was determined spectrophotometrically by the DPPH assay described by Blois[Citation27] with slight modification.[Citation26] In its radical form, DPPH absorbs at 517 nm, but upon reduction by an antioxidant or a radical species, its absorption decreases. Briefly, 40 μL sample solutions at different concentrations were added to 160 μL 0.4 mM 40 μL methanol solution of DPPH. Thirty minutes later, absorbance was measured at 517 nm by using a 96-well microplate reader. The capability of scavenging the inhibition activity (I) was calculated using Eq. (3).
ABTS cation radical scavenging assay
The spectrophotometric analysis of ABTS•+ scavenging activity was determined according to the method of Re et al.[Citation28] with slight modifications.[Citation26] Briefly, ABTS•+ was produced by the reaction between 7 mM ABTS in H2O and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12 h. The radical cation was stable in this form for more than 2 days when stored in the dark at room temperature. Before usage, the ABTS•+ solution was diluted to get an absorbance of 0.708 ± 0.025 at 734 nm with ethanol. Then, 160 µL of ABTS•+ solution was added to 40 µL of sample solution in ethanol at different concentrations. After 10 min, by using a 96-well microplate reader, the percentage inhibition at 734 nm was calculated for each concentration relative to a blank absorbance (ethanol). The scavenging capability of ABTS•+ was calculated using Eq. (3).
Cupric-reducing antioxidant capacity (CUPRAC) assay
The cupric-reducing antioxidant capacity was determined according to the method of Apak et al.[Citation29] with slight modifications.[Citation26] To each well, in a 96-well plate, 50 µL 10 mM Cu (II), 50 µL 7.5 mM neocuproine, and 60 µL NH4Ac buffer (1 M, pH 7.0) solutions were added. Forty microliters of extract at different concentrations were added to the initial mixture so as to make the final volume to 200 µL. After 1 h, the absorbance at 450 nm was recorded against a reagent blank by using a 96-well microplate reader.
Metal chelating assay
The chelating activity of the extracts on Fe2+ was measured as reported by Decker and Welch[Citation30] with slight modifications.[Citation31] About 80 µL extract solution dissolved in ethanol in different concentrations was added to 40 µL 0.2 mM FeCl2. The reaction was initiated by the addition of 80 µL 0.5 mM ferene. The mixture was shaken vigorously and left standing at room temperature for 10 min. After the mixture reached equilibrium, the absorbance was measured at 593 nm. The metal chelating activity was calculated according to Eq. (3).
Enzyme inhibitory activity
Cholinesterase inhibition
Acetylcholinesterase and butyrylcholinesterase inhibitory activity was measured by the spectrophotometric method developed by Ellman[Citation32] as previously reported with slight modification.[Citation26] AChE from electric eel and BChE from horse serum were used, while acetylthiocholine iodide and butyrylthiocholine chloride were employed as substrates of the reaction. DTNB (5,5′-Dithio-bis(2-nitrobenzoic)acid) was used for measurement of the cholinesterase activity. Briefly, 130 µL of 100 mM sodium phosphate buffer (pH 8.0), 10 µL of sample solution dissolved in ethanol at different concentrations, and 20 µL AChE or BChE solution in buffer were mixed and incubated for 15 min at 25°C, and 20 µL of 0.5 mM DTNB was added. The reaction was then initiated by addition of 0.71 mM, 20 µL of acetylthiocholine iodide, or 0.2 mM, 20 µL of butyrylthiocholine chloride. The hydrolysis of these substrates was monitored spectrophotometrically by the formation of yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine iodide or butyrylthiocholine chloride, respectively, at a wavelength of 412 nm utilizing a 96-well microplate reader.
Tyrosinase inhibition
Tyrosinase enzyme inhibitory activity was measured by the spectrophotometric method as described by Masuda et al.[Citation33] with slight modification. Mushroom tyrosinase was used, while L-DOPA was employed as substrates of the reaction. Briefly, 150 µL of 100 mM sodium phosphate buffer (pH 6.8), 10 µL of sample solution dissolved in ethanol at different concentrations, and 20 µL tyrosinase enzyme solution in buffer were mixed and incubated for 10 min at 37°C, and 20 µL L-DOPA was added. The sample and blank absorbances were read at 475 nm after 10 min incubation at 37°C in a 96-well microplate.
Statistical analysis
All data on antioxidant, anticholinesterase, and anti-tyrosinase activity tests were the average of three parallel sample measurements. Data were recorded as mean ± S.E.M. Significant differences between means were determined by Student’s test, and p values <0.05 were regarded as significant.
Results and discussion
Total phenolic and total flavonoid contents
The total phenolic and flavonoid contents of the extracts of F. elaeochytris and S. stricta were determined as pyrocatechol and QEs, respectively, and the results are presented in . The concentration of phenolic compounds in the extracts ranged from 1.77 ± 0.18 to 141.05 ± 0.11 µg PEs/mg. The highest level of phenolic compounds was measured in the acetone extract (141.05 ± 0.11 µg PEs/mg) and followed by the methanol extract (65.40 ± 0.32 µg PEs/mg) of S. stricta. The acetone extract (21.62 ± 0.73 µg PEs/mg) of F. elaeochytris exhibited the highest concentration of phenolic compounds, followed by the water extract (19.23 ± 0.21 µg PEs/mg). The total flavonoid contents in the examined extracts ranged from 0.62 ± 0.32 to 50.52 ± 0.59 µg QEs/mg. The highest level of flavonoid contents was measured in the acetone extract (50.52 ± 0.59 µg QEs/mg) and followed by the methanol extract (26.21 ± 0.39 µg QEs/mg) of S. stricta. The acetone extract (10.33 ± 0.23 µg QEs/mg) of F. elaeochytris showed the highest concentration of flavonoid compounds, followed by the methanol extract (9.93 ± 0.56 µg QEs/mg) ().
Table 1. Total phenolic and total flavonoid contents of the extracts of F. elaeochytris and S. stricta.a
Phenolic profiles
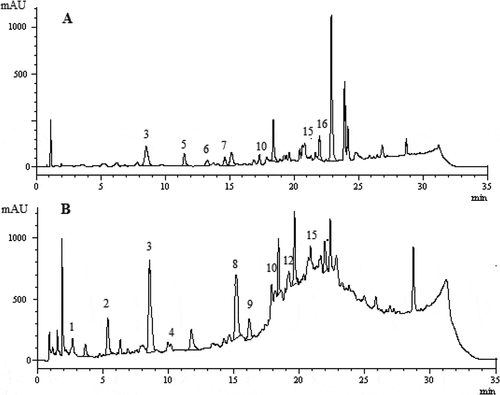
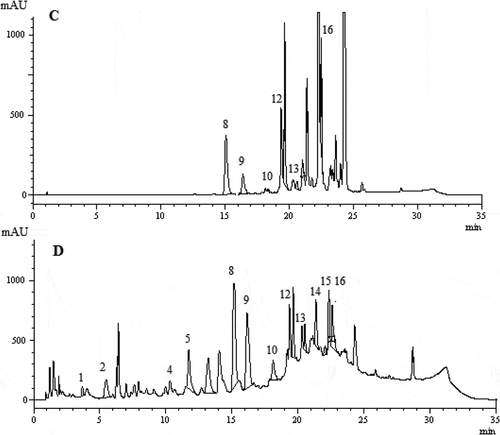
Phenolic and organic acid compounds of the methanol and water extracts of F. elaeochytris and S. stricta were determined by HPLC-DAD, and results are expressed as µg/g extract in . Totally 16 phenolic and organic acid compounds namely fumaric acid, gallic acid, protocatechuic acid, p-hydroxybenzoic acid, catechin hydrate, 6,7-dihydroxy coumarin, 2,4-dihydroxybenzoic acid, caffeic acid, vanillin, p-coumaric acid, ferulic acid, coumarins, trans-2-hydroxycinnamic acid, ellagic acid, rosmarinic acid, and trans-cinnamic acid were identified in the extracts. Protocatechuic acid was identified as a dominant phenolic compound in both extracts of F. elaeochytris (). Protocatechuic acid has antioxidant and anticancer activities, so high antioxidant activities of the methanol and water extracts are associated with high amount of protocatechuic acid.[Citation34] Also, the second major phenolic compound was catechin hydrate (105.5 ± 2.3 µg/g) in the methanol extract of F. elaeochytris and fumaric acid (52.5 ± 1.0 µg/g) in the water extract of F. elaeochytris. In the methanol extract of S. stricta, coumarins (253.9 ± 4.1 µg/g) were found as the major phenolic compound, while catechin hydrate (175.2 ± 2.9 µg/g) was found to be the major phenolic compound in the water extract. Caffeic acid was identified as the second major phenolic compound in both extracts (). It was reported that coumarins, catechin hydrate, and caffeic acid possess higher antioxidant activity when compared to BHA and α-tocopherol.[Citation35,Citation36] This may cause two extracts to exhibit higher antioxidant activity than standards. Consequently, both studied species were rich in phenolic compounds.
Table 2. Phenolic profile of the methanol and water extracts of F. elaeochytris and S. stricta by HPLC-DAD.
Antioxidant activity
Five types of antioxidant capacity measurements, namely β-carotene–linoleic acid, DPPH free radical scavenging, ABTS cation radical scavenging, cupric-reducing antioxidant capacity (CUPRAC), and metal chelating activity, were used to test antioxidant properties of the hexane, acetone, methanol, and water extracts of F. elaeochytris and S. stricta species. All of the extracts exhibited antioxidant activities in a dose-dependent manner. shows the IC50 values of the extracts and standard compounds (α-tocopherol, BHA, and EDTA).
Table 3. Antioxidant activities of the extracts of F. elaeochytris and S. stricta by β-carotene–linoleic acid, DPPH•, ABTS•+, CUPRAC, and metal chelating assays.a
In β-carotene–linoleic acid, DPPH•, ABTS•+, and CUPRAC assays, the methanol extract of F. elaeochytris showed the highest activity with IC50 values of 9.63 ± 0.10, 191.33 ± 0.53, 19.15 ± 0.13, and 121.62 ± 0.57 µg/mL, respectively. Also, ABTS•+ radical scavenging activity of the methanol extract (IC50: 19.15 ± 0.13 µg/mL) of F. elaeochytris was found higher than α-tocopherol (IC50: 38.51 ± 0.54 µg/mL). The highest ability to chelate ferrous ion was shown in the water extract of F. elaeochytris with 88.42 ± 0.18% inhibition at 200 µg/mL concentration and followed by the hexane extract (33.96 ± 0.46%). The hexane extract showed low activity in all test methods except for metal chelating activity method ().
Up to this time, antioxidant properties of various extracts (hexane, ethyl acetate, butanol, methanol, and ethanol) obtained from Ferula species (F. assafoetida, F. szowitsiana, F. microcolea, F. lutea, and F. hermonis) were investigated using different methods such as DPPH free and ABTS cation radical scavenging and metal chelating assays.[Citation37–Citation41] According to literature, generally the methanol extracts showed the highest antioxidant activity. The results are comparable to those of earlier studies.
The methanol extract of S. stricta showed the highest lipid peroxidation inhibition (IC50: 6.66 ± 0.14 µg/mL) and ABTS•+ radical scavenging activity (IC50: 13.66 ± 0.61 µg/mL) when the acetone extract indicated the highest DPPH• radical scavenging (IC50: 16.89 ± 0.21 µg/mL) and reducing activity (A0.05: 23.55 ± 0.06 µg/mL). Among the extracts, the methanol and acetone extracts of S. stricta indicated higher antioxidant activity than BHA and α-tocopherol in DPPH• and CUPRAC assays. In ABTS•+ assay, the methanol (IC50: 13.66 ± 0.62 µg/mL), acetone (IC50: 14.20 ± 0.28 µg/mL), and water (IC50: 37.20 ± 0.83 µg/mL) extracts of S. stricta showed higher radical scavenging activities than α-tocopherol (IC50: 38.51 ± 0.54 µg/mL). Moreover, the water extract (88.20 ± 0.08%) showed close metal chelating activity compared to EDTA (94.7 ± 0.60%) used as a standard (). Radical scavenging activities of the acetone and methanol extracts of S. stricta were found to be very high when compared to standards, as it was previously observed in other Sideritis species.
Earlier studies have shown that the acetone and methanol extracts of S. brevibracteata have high activity in DPPH• assay and moderate activity in β-carotene–linoleic acid and CUPRAC assay[Citation42]; the methanol extracts of S. ozturkii, S. caesarea, and S. montana have high activity in DPPH• assay.[Citation43,Citation44] In the study of Zengin et al.,[Citation45] antioxidant properties of the petroleum ether, ethyl acetate, methanol, and water extracts of S. galactica were tested using free radical scavenging (DPPH, ABTS, and NO), reducing power (FRAP and CUPRAC), total antioxidant capacity, and chelating assays. It was determined that the methanol and water extracts had high radical scavenging and reducing power activities. The findings we obtained are completely in line with previous studies.
Phenolic compounds are the most important compound groups of plants that are capable of scavenging ability on free radicals by hydroxyl groups. Flavonoid compounds are important phenolic compounds showing antioxidant properties.[Citation46] The methanol and water extracts were found to be rich in protocatechuic acid, coumaric acid, catechin hydrate, and caffeic acid which were previously confirmed as good antioxidant agents.[Citation34–Citation36] Furthermore, the highest antioxidant activities of the acetone, methanol, and water extracts of F. elaeochytris and S. stricta can be explained by the highest amount of total phenolic and flavonoid compounds.
Anticholinesterase activity
There are many drugs used in the treatment of AD, and these drugs have some side effects. Therefore, it is necessary to obtain new inhibitor for AD which is less toxic. Today, there are a limited number of inhibitors (galantamine, tacrine, and physostigmine) that are derived from natural plant sources and reduce the effects of AD.[Citation47,Citation48] Scientists’ interest in the finding of new natural sources of medicines for the treatment of AD is growing recently. summarizes the results of cholinesterase enzyme inhibitory activities of the extracts of F. elaeochytris and S. stricta. The inhibitory activities of the extracts on AChE and BChE were reported as potent (>50%), moderate (30–50%), inactive, or low (<30%) activity.[Citation49]
Table 4. Anticholinesterase and anti-tyrosinase activities of the extracts of F. elaeochytris and S. stricta.a
According to this classification, the hexane extract of F. elaeochytris (70.66 ± 0.93%) showed potent inhibitory activity against AChE. The inhibitory activities of the acetone and methanol extracts of F. elaeochytris on AChE were found to be low, and the water extract exhibited no inhibition (). The BChE inhibitory activities of the extracts were higher than the AChE inhibitory activities. The hexane extract of F. elaeochytris showed higher BChE inhibitory activity with IC50 values of 49.80 ± 0.07 µg/mL when compared to galantamine (IC50: 50.80 ± 0.93 µg/mL) (). In previous reports, anti-AChE activities of the ethanolic extract of F. hermonis, the methanol extract of F. assafoetida, and the ethyl acetate and n-butanol extracts of F. lutea were studied, and all extracts showed low ability for inhibiting acetylcholinesterase.[Citation37,Citation41,Citation50] There is no study about anti-BChE activity of the extracts from Ferula species, so this the first study about anti-BChE activity.
The hexane extract of S. stricta (58.59 ± 0.25%) showed potent inhibitory activity against AChE. When the acetone and methanol extracts of S. stricta showed low AChE inhibitory activity, water extract exhibited no AChE inhibitory activity. Against BChE, the hexane and acetone extracts of S. stricta were found as potent inhibitors. (). There are only two studies in the literature about cholinesterase inhibition activity of Sideritis extracts. According to the results obtained by Miguel et al.[Citation51] the ethanolic extract of S. arborescens had no ability for inhibiting acetylcholinesterase. Zengin et al.[Citation45] reported that cholinesterase enzyme inhibitory activities of the extracts of S. galactica were decreased in order of ethyl acetate > petroleum ether > methanol > water extracts.
When studies on the extracts of Ferula and Sideritis species are examined, it is seen that nonpolar extracts have higher anticholinesterase activity than polar extracts. As it seen in , polar extracts of both species showed lower activity, so our results are consistent with the literature data. The highest cholinesterase inhibitory activities of the hexane extracts in both species studied can be explained by their non-phenolic composition.
Anti-tyrosinase activity
Over the past years, tyrosinase inhibitors are gaining importance due to the impact of tyrosinase on the human melanogenesis and plant and fungi browning. In the method used, tyrosinase enzyme inhibitory activity is based on the measurement of dopachrome formed in the presence of tyrosinase and L-DOPA as the enzyme substrate.[Citation52] As it seen in , the methanol (24.63 ± 0.31%) and acetone extracts (7.14 ± 0.08%) of F. elaeochytris displayed low tyrosinase enzyme inhibition activity, while the hexane and water extracts showed no activity. The methanol extracts of 13 plant species belonging to Umbelliferae family were screened for their tyrosinase inhibitory effects. According to Orhan et al.,[Citation53] all methanol extracts showed low tyrosinase inhibitory activity.
The methanol (23.29 ± 0.56%) and acetone (15.66 ± 0.11%) extracts of S. stricta were found to be low active against tyrosinase, while the hexane and water extracts were found to be inactive (). Previously, tyrosinase inhibitory activities of plants that are members of Lamiaceae family were investigated. Tundis et al.[Citation54] screened tyrosinase inhibitory activities of the hexane, dichloromethane, methanol, and ethanol extracts of Stachys lavandulifolia aerial parts. The result of this study is that all extracts have low tyrosinase inhibitory activity. Alimpic et al.[Citation55] reported IC50 values of tyrosinase inhibition of the ethanol extracts of Mentha species ranged between 108 ± 20 and 286 ± 45 μg/mL. The obtained data support the results of the literature. Tyrosinase inhibitory activities of the hexane, acetone, methanol, and water extracts of F. elaeochytris and S. stricta species were evaluated for the first time in this study.
Conclusion
In the present study, antioxidant, anticholinesterase, and tyrosinase inhibitory properties of various extracts of F. elaeochytris and S. stricta were determined with the total phenolic and flavonoid contents. The methanol and water extracts of F. elaeochytris and the acetone and methanol extracts of S. stricta containing the highest amount of total phenolic and flavonoid contents showed the highest antioxidant activities in β-carotene–linoleic acid, DPPH•, ABTS•+, and CUPRAC assays. The hexane extracts of both plant species with the lowest amount of total phenolic and flavonoid contents were found as potent inhibitors in cholinesterase enzyme inhibitory activity assay. Totally, 15 phenolic compounds were identified in the methanol and water extracts by using HPLC-DAD. Protocatechuic acid, coumarins, and catechin hydrate were found to be major phenolic compounds. This study represents the first report on phytochemical composition and biological activities of F. elaeochytris and S. stricta. Our results showed that Ferula and Sideritis species have promising activities and high phytochemical contents. They could be used as a potential source of natural antioxidant, anticholinesterase, and anti-tyrosinase agent in food, cosmetic, and pharmaceutical industries. Thus, further studies are needed to isolate and identify the antioxidant components from these species.
Acknowledgement
The authors would like to thank Dr Hasan Yildirim from Ege University, Izmir, Turkey, for taxonomic identification of the plant materials.
References
- Sahebkar, A.; Iranshahi, M. Biological Activities of Essential Oils from the Genus Ferula (Apiaceae). Asian Biomedicine 2010, 4, 835–847.
- Mohammad, S.; Aftab, A.; Sarwat, S. Asafoetida Inhibits Early Events of Carcinogenesis. A Chemopreventive Study. Life Sciences 2001, 68, 1913–1921.
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent Advances on Bioactive Constituents in Ferula. Drug Development Research 2017, 78, 321–331.
- Iranshahy, M.; Iranshahi, M. Traditional Uses, Phytochemistry and Pharmacology of Asafoetida (Ferula assafoetida Oleo-Gumresin)-A Review. Journal of Ethnopharmacology 2011, 134, 1–10.
- Miski, M.; Ulubelen, A.; Mabry, T. J. Six Sesquiterpene Alcohol Esters from Ferula elaeochytris. Phytochemistry 1983, 22, 2231–2233.
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaza, S.; Bailleul, F. Activity of Elaeochytrin a from Ferula elaeochytris on Leukemia Cell Lines. Phytochemistry 2008, 69, 2979–2983.
- Başer, K. H. C.; Özek, T.; Demirci, B.; Kürkçüoğlu, M.; Aytaç, Z.; Duman, H. Composition of the Essential Oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour and Fragrance Journal 2000, 15, 371–372.
- Romanucci, V.; Di Fabio, G.; D’Alonzo, D.; Guaragna, A.; Scapagninib, G.; Zarrellia, A. Traditional Uses, Chemical Composition and Biological Activities of Sideritis raeseri Boiss. & Heldr. Journal of the Science of Food and Agriculture 2017, 97, 373–383.
- Davis, P. H. Flora of Turkey and the East Aegean Islands; University Press: Edinburgh, 1998; Vol. 1, pp 1965–1985.
- Zengin, G.; Sarıkürkçü, C.; Aktümsek, A.; Ceylan, R. Antioxidant Potential and Inhibition of Key Enzymes Linked to Alzheimer’s Diseases and Diabetes Mellitus by Monoterpene-Rich Essential Oil from Sideritis galatica Born endemic to Turkey. Records of Natural Products 2016, 10, 195–206.
- Topcu, G.; Gören, A. C. Biological Activity of Diterpenoids Isolated from Anatolian Lamiaceae Plants. Records of Natural Products 2007, 1, 1–16.
- Gonzalez-Burgos, E.; Carretero, M. E.; Gomez-Serranillos, M. P. Sideritis spp.: Uses, Chemical Composition and Pharmacological Activities-A Review. Journal of Ethnopharmacology 2011, 135,209–225. DOI: 10.1016/j.jep.2011.03.014.
- Şahin, F. P.; Ezer, N.; Calis, I. Terpenic and Phenolic Compounds from Sideritis stricta. Turkish Journal of Chemistry 2006, 30, 495–504.
- Kilic, T. Isolation and Biological Activity of New and Known Diterpenoids from Sideritis stricta Boiss & Heldr. Molecules 2006, 11,257–262. DOI: 10.3390/11040257.
- Küpeli, E.; Sahin, F. P.; Yesilada, E.; Calıs, İ.; Ezer, N. In vivo Anti-Inflammatory and Antinociceptive Activity Evaluation of Phenolic Compounds from Sideritis stricta. Zeitschrift fur Naturforschung 2007, 62, 519–525.
- Turkmenoglu, F. P.; Baysal, I.; Ciftci-Yabanoglu, S.; Yelekci, K.; Temel, H.; Paşa, S.; Ezer, N.; Çalış, I.; Ucar, G. Flavonoids from Sideritis Species: Human Monoamine Oxidase (Hmao) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol. Molecules 2015, 20,7454–7473. DOI: 10.3390/molecules20057454.
- Kirimer, N.; Baser, K. H. C.; Demirci, B.; Duman, H. Essential Oils of Sideritis Species of Turkey Belonging to the Section Empedoclia. Chemistry of Natural Compounds 2004, 40, 19–23.
- Ratnam, D. V.; Ankola, D. D.; Bhardwaj, V.; Sahana, D. K.; Kumar, M. N. V. R. Role of Antioxidants in Prophylaxis and Therapy: A Pharmaceutical Perspective. Journal of Controlled Release 2006, 113, 189–207.
- Şenol, F. S.; Orhan, I.; Yilmaz, G.; Çicek, M.; Şener, B. Acetylcholinesterase, Butyrylcholinesterase, and Tyrosinase Inhibition Studies and Antioxidant Activities of 33 Scutellaria L. Taxa from Turkey. Food and Chemical Toxicology 2010, 48, 781–788.
- Ferreira, A.; Proenca, C.; Serralheiro, M. L. M.; Araujo, M. E. M. The in vitro Screening for Acetylcholinesterase Inhibition and Antioxidant Activity of Medicinal Plants from Portugal. Journal of Ethnopharmacology 2006, 108, 31–37.
- Fatiha, B.; Didier, H.; Naimaa, G.; Khodira, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic Composition, In vitro Antioxidant Effects and Tyrosinase Inhibitory Activity of Three Algerian Mentha Species: M. spicata (L.), M. pulegium (L.) And M. rotundifolia (L.) Huds (Lamiaceae). Industrial Crops and Products 2015, 74, 722–730.
- Slinkard, K.; Singleton, V. L. Total Phenol Analyses: Automation and Comparison with Manual Methods. The American Journal of Enology and Viticulture 1977, 28, 49–55.
- Park, Y. K.; Koo, M. H.; Ikegaki, M.; Contado, J. L. Comparison of the Flavonoid Aglycone Contents of Apis mellifera Propolis from Various Regions of Brazil. The Brazilian Archives of Biology and Technology 1997, 40, 97–106.
- Barros, L.; Duenas, M.; Ferreira, I. C. F. R.; Baptista, P.; Santos-Buelga, C. Phenolic Acids Determination by HPLC–DAD–ESI/MS in Sixteen Different Portuguese Wild Mushrooms Species. Food and Chemical Toxicology 2009, 47, 1076–1079.
- Tel-Çayan, G.; Öztürk, M.; Duru, M. E.; Rehman, M.; Adhikari, A.; Türkoglu, A.; Choudhary, M. I. Phytochemical Investigation, Antioxidant and Anticholinesterase Activities of Ganoderma adspersum. Industrial Crops and Products 2015, 76, 749–775.
- Tel, G.; Apaydın, M.; Duru, M. E.; Öztürk, M. Antioxidant and Cholinesterase Inhibition Activities of Three Tricholoma Species with Total Phenolic and Flavonoid Contents: The Edible Mushrooms from Anatolia. Food Analytical Methods. 2012, 5, 495–504.
- Blois, M. S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S. E.; Ercag, E. The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas. International Journal of Food Science and Nutrition 2006, 57, 292–304.
- Decker, E. A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. Journal of Agricultural and Food Chemistry 1990, 38, 674–677.
- Çayan, F.; Tel, G.; Duru, M. E.; Öztürk, M.; Türkoğlu, A.; Harmandar, M. Application of GC, GC-MSD, ICP-MS and Spectrophotometric Methods for the Determination of Chemical Composition and In vitro Bioactivities of Chroogomphus rutilus: The Edible Mushroom Species. Food Analytical Methods 2014, 7, 449–458.
- Ellman, G. L.; Courtney, K. D.; Andres, V.; Featherston, R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochemical Pharmacology 1961, 7, 88–95.
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among Extracts of Seashore Plants and Identification of Potent Inhibitors from Garcinia subelliptica. Bioscience, Biotechnology, and Biochemistry 2005, 69, 197–201.
- Khan, A. K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological Activities of Protocatechuic Acid. Acta Poloniae Pharmaceutica 2015, 72, 643–650.
- Magnani, C.; Isaac, V. L. B.; Correa, M. A.; Salgado, H. R. N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Analytical Methods 2014, 6, 3203–3210.
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A. A.; Mohamad, A. B. Antioxidant Activity of Coumarins. Systematic Reviews in Pharmacy 2017, 8, 24–30.
- Ghareeb, D. A.; ElAhwany, A. M. D.; El-Mallawany, S. M.; Saif, A. A. In vitro Screening for Anti-Acetyl Choliesterase, Anti-Oxidant, Anti-Glucosidase, Anti-Inflammatory and Anti-Bacterial Effect of Three Traditional Medicinal Plants. Biotechnology & Biotechnological Equipment 2014, 28(6), 1155–1164.
- Dehpour, A. A.; Ebrahimzadeh, M. A.; Fazel, N. S.; Mohammad, N. S. Antioxidant Activity of the Methanol Extract of Ferula assafoetida and Its Essential Oil Composition. Grasas Y Aceites 2009, 60(4), 405–412.
- Dehghan, G.; Shafiee, A.; Ghahremani, M. H.; Ardestani, S. K.; Abdollahi, M. Antioxidant Potential of Various Extracts from Ferula szovitsiana in Relation to Their Phenolic Content. Pharmaceutical Biology 2007, 45(9), 691–699.
- Amiri, H. Chemical Composition and Antioxidant Activity of Essential Oil and Methanolic Extracts of Ferula microcolea (Boiss.) Boiss (Apiaceae). International Journal of Food Properties 2014, 17, 722–730.
- Znati, M.; Jannet, H. B.; Cazaux, S.; Bouajila, J. Chemical Composition, Biological and Cytotoxic Activities of Plant Extracts and Compounds Isolated from Ferula lutea. Molecules 2014, 19, 2733–2747.
- Sagir, Z. O.; Carikci, S.; Kilic, T.; Goren, A. C. Metabolic Profile and Biological Activity of Sideritis brevibracteata P.H. Davis Endemic to Turkey. International Journal of Food Properties 2017, 20, 2994–3005.
- Radojevic, I. D.; Stankovic, M. S.; Stefanovic, O. D.; Topuzovic, M. D.; Comic, L. R. Antioxidative and Antimicrobial Properties of Different Extracts from Sideritis montana L. Romanian Biotechnology Letters 2012, 17, 7014–7022.
- Sagdic, O.; Aksoy, A.; Ozkan, G.; Ekici, L.; Albayrak, S. Biological Activities of the Extracts of Two Endemic Sideritis Species in Turkey. Innovative Food Science & Emerging Technologies 2008, 9, 80–84.
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A Source of Multifunctional Agents for the Management of Oxidative Damage, Alzheimer’s and Diabetes Mellitus. Journal of Functional Foods 2014, 11, 538–547.
- Stanković, M. S. Total Phenolic Content, Flavonoid Concentration and Antioxidant Activity of Marrubium peregrinum L. Extracts. Kragujevac Journal of Science 2011, 33, 63–72.
- Perry, N. S. L.; Bollen, C.; Perry, E. K.; Ballard, C. Salvia for Dementia Therapy: Review of Pharmacological Activity and Pilot Tolerability Clinical Trial. Pharmacology, Biochemistry and Behavior 2003, 75, 651–659.
- Salah, S. M.; Jäger, A. K. Screening of Traditionally Used Lebanese Herbs for Neurological Activities. Journal of Ethnopharmacology 2005, 97, 145–149.
- Vinutha, B.; Prashanth, D.; Salmab, K.; Sreeja, S. L.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of Selected Indian Medicinal Plants for Acetylcholinesterase Inhibitory Activity. Journal of Ethnopharmacology 2007, 109, 359–363.
- Ali, S. K.; Hamed, A. R.; Soltan, M. M.; Hegazy, U. M.; Lgorashi, E. E.; El-Garf, I. A.; Hussein, A. A. In-vitro Evaluation of Selected Egyptian Traditional Herbal Medicines for Treatment of Alzheimer Disease. BMC Complementary and Alternative Medicine 2013, 13, 121.
- Miguel, M.; Bouchmaaa, N.; Aazza, S.; Gaamoussi, F.; Lyoussi, B. Antioxidant, Anti-Inflammatory and Anti-Acetylcholinesterase Activities of Eleven Extracts of Moroccan Plants. Fresenius Environment bulletin 2014, 23(6), 1–14.
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. International Journal of Molecular Sciences 2009, 10, 2440–2475.
- Orhan, I. E.; Tosun, F.; Skalicka-Woźniak, K. Cholinesterase and Tyrosinase Inhibitory, and Antioxidant Potential of Randomly Selected Umbelliferous Plant Species and the Chromatographic Profile of Heracleum platytaenium Boiss. and Angelica sylvestris L. Var. Sylvestris. Journal of the Serbian Chemical Society 2016, 81(4), 357–368.
- Tundis, R.; Bonesi, M.; Pugliese, A.; Nadjafi, F.; Menichini, F.; Loizzo, M. R. Tyrosinase, Acetyl- and Butyryl-Cholinesterase Inhibitory Activity of Stachys lavandulifolia Vahl (Lamiaceae) and Its Major Constituents. Records of Natural Products 2015, 9(1), 81–93.
- Alimpića, A.; Knežević, A.; Milutinović, M.; Stević, T.; Šavikin, K.; Stajić, M.; Marković, S.; Marin, P. D.; Matevski, V.; Duletić-Laušević, S. Biological Activities and Chemical Composition of Salvia amplexicaulis Lam. Extracts. Industrial Crops and Products 2017, 105, 1–9.