ABSTRACT
The aim of this study was to determine the chemical composition and the in vitro antimicrobial effects of seed essential oil of Ferulago angulata. The oil analyses by GC and GC/MS resulted in the identification of 39 compounds representing 91.07% of the oil. The major constituents were (Z)-β-ocimene (19.93%), α-pinene (15.50%), p-cymene (7.67%), sabinene (7.49%), β-phellandrene (5.5%), and α-phellandrene (4.95%). The oil was also screened for its antimicrobial properties against six bacteria (Erwinia amylovora, Xanthomonas oryzae, Pseudomonas syringae, Pectobacterium carotovorum, Ralstonia solanacearum, Bacillus thuringiensis) and six fungi (Alternaria alternata, Culvularia fallax, Macrophomina phaseolina, Fusarium oxysporum, Cytospora sacchari, Colletotrichum tricbellum). According to the results of antibacterial activity, B. thuringiensis (with 8 µL mL−1 minimal inhibitory concentration (MIC) and 15 µL mL−1 minimum bactericidal concentration (MBC)) was the most sensitive bacterium; P. carotovorum and R. solanacearum (with 20 µL mL−1 MIC and 30< MBC) were the most resistant bacteria. Additionally, a broad differentiation against all of the tested fungi showed that the most susceptible and resistant fungi after 6 days at the highest concentration (800 µL L−1) were F. oxysporum (100.0 ± 0.00%) and C. tricbellum (52.50 ± 1.67%) of growth inhibition, respectively.
Introduction
Apiaceae (Umbelliferae) family comprises 300 genera and 2500–3000 species distributed in most parts of the world. The genus Ferulago belongs to this family consisting of 35–40 species in the world which are centred in southwestern Asia, and 7 of them are endemic in Iran, including Ferulago contracta, Ferulago angulata, Ferulago macrocarpa, Ferulago galbanifera, Ferulago trachycarpa, Ferulago phialocarpa, and Ferulago carduchrom.[Citation1,Citation2] F. angulata (Schlecht.) Boiss. (referred to locally as ‘Chavir’ or ‘Chavil’ in Iran) is an endemic natural and native plant in western Iran.[Citation1] Traditionally, the aerial parts of the plant added to diary and edible oil by natives to prevent from the oils expiration besides adding a pleasant taste to it.[Citation3] Results of an ethnobotanical study[Citation4] indicated the aerial parts of F. angulata have been used as antiseptic, spice, and air fresher by the people of Abdanan and Dehloran districts, western Iran. Furthermore, it has been used in folk medicine as sedative, tonic, digestive, carminative, vermifuge, aphrodisiac, anti-oxidation, anti-diabetics, anti-parasitic, and for the treatment of chronic ulcers, snakebites, intestinal worms, haemorrhoids, headache, and diseases of the spleen.[Citation5,Citation6]
Previous phytochemical studies of some species of the genus Ferulago have led to isolation of volatile oils from aerial parts of these plants which contain a variety of components with different therapeutic effects such as digestion diseases, different pains,[Citation5–Citation8] and the subject of some earlier studies were the scant biological activity investigations.[Citation9,Citation10] Also, the essential oils of several species in the genus Ferulago have been studied from an antimicrobial point of view.[Citation2,Citation10]
The extraction of F. angulata essence has already been done through hydro-distillation and extraction by solvent. Its major chemical compounds have been identified and their percentages were different.[Citation2,Citation3,Citation7,Citation11] The F. angulata oils predominantly included ferulagone, β-hydroxy-13-epi-manoyl oxide, α-pinene, 2,5-dimethoxy-p-cymene, p-cymene, methyl carvacrol, trans-chrysanthenyl acetate, γ-terpinene, myrcene, (Z)-β-ocimene, terpinolene, 2,4,5-trimethyl- benzaldehyde, and α-phellandrene.[Citation7,Citation12,Citation13] The parts of the plant, its stage of development, and geographical origin can significantly influence the composition of the oils obtained from the species.[Citation14,Citation15]
The control of plant disease is a considerable problem in agriculture practice. Plant pathogens include fungi and bacteria and can cause diseases or damages in plants, also being responsible for significant losses of crop production.[Citation16,Citation17] For many years, a variety of different synthetic chemicals such as benzimidazoles, aromatic hydrocarbons, and sterol biosynthesis inhibitors have been used as antifungal agents to inhibit the growth of plant pathogenic fungi.[Citation18] Moreover, many phytopathogenic bacteria have acquired resistance to synthetic pesticides.[Citation19] Therefore, there is an increasing interest in the antimicrobial activities of some natural composition, especially those found in medicinal plants, which may play a role in preventing various diseases. Despite the high degree of activity shown by these plants against phytopathogens, antimicrobial investigation of aromatic plants has been limited.[Citation20] Among the plant products, essential oils have been widely investigated for their toxicities against various fungi, bacteria, and insects.[Citation21] Essential oils are natural mixtures of complex compounds formed by several volatile compounds, mainly monoterpenoids and sesquiterpenoids[Citation22], which are characterized by a strong odour and play an important role in the protection of the plants against bacteria, virus, fungi, insects, herbivores, and as attractant to pollinators. Moreover, they are used in agriculture and food industries as food preservers, additives, and natural remedies, owing to their notable antimicrobial and antioxidant properties.[Citation23] Owing to the increasing incidence of adverse side effects associated with conventional synthetic antimicrobial poisons, essential oils may therefore constitute effective organic alternatives to synthetic compounds of chemical composition in agro-industries.[Citation24,Citation25]
Considering the capability of plant essential oils for substituting synthetic antimicrobial products, the study of potential of F. angulata can be useful for industrial application of this plant as a natural product. Therefore, the objective of the present work was to characterize the chemical composition of the F. angulata seed oil, and investigate and compare the antimicrobial effects of the oil against some phytopathogenic bacteria and fungi for the first time.
Materials and methods
Plant material
F. angulata seeds were harvested from the plants grown in Kohgiluyeh va Boyer Ahmad province, southwestern of Iran. One herbarium sample of plant was collected and a voucher specimen of plant was deposited at the Herbarium of the Faculty of Sciences, Isfahan University, Isfahan, Iran.
Isolation of the essential oil
Essential oil was isolated from the dried seeds of the plants that were subjected to hydrodistillation for 4 h using a Clevenger-type apparatus. The essential oil was collected over water, separated, and dried over anhydrous sodium sulphate and stored in sealed vials at 4°C until chemical analysis and antimicrobial studies.
Analysis of essential oil
GC and GC-MS analysis
Composition of the essential oils was determined by GC and GC-MS. GC analysis was done on an Agilent Technologies 7890 GC equipped with FID and a HP-5MS 5% capillary column (30.00 m × 0.25 mm, 0.25 µm film thicknesses). The carrier gas was helium at a flow of 0.8 mL min−1. Initial column temperature was 60°C and programmed to increase at 4°C min−1 to 280°C. The split ratio was 40:1. The injector temperature was set at 300°C. The purity of helium gas was 99.999% and 0.1 µL samples were injected manually in the split mode. GC-MS analysis was done on the mentioned Agilent Technologies 5975 Mass system. Mass spectra were recorded at 70 eV. Mass range was from m/z 50–550.
Compounds identification
Constituents were identified by comparison of their Kovats index (KI) relative to C5–C24 n-alkanes obtained on a nonpolar HP-5 MS column by comparison of the KI, provided in the literature, by comparison of the mass spectra with those recorded by the NIST 08 (National Institute of Standards and Technology) and Willey (ChemStation data system). The individual components were identified by retention indices and compared with constituents known from the literature.[Citation26,Citation27] The percentage composition was computed from the GC peak areas without using any correction factors.
Antifungal activity
Fungal species
Six phytopathogenic fungi, including Alternaria alternata strain C1445, Culvularia fallax strain I21, Macrophomina phaseolina strain M21, Fusarium oxysporum strain 104, Cytospora sacchari strain 125, and Colletotrichum tricbellum strain 108, were obtained from the collection of Department of Plant Diseases, Tehran University, Iran.
Antifungal activity assays
Activities of oil were tested against six fungi pathogens above using a range of different concentration (100, 200, 400, and 800 µL L−1) with two different methods in four replicates.
Agar dilution method
The oil was dissolved in ethyl alcohol and 5% Tween 80, and added to the culture medium at a temperature of 40–45°C, then poured into Petri dishes (90 mm). The fungi were inoculated as soon as the medium had solidified. Discs (5 mm) of mycelia material, taken from the edge of 7-day-old fungal cultures, were placed at the centre of each Petri dish. The controls set were prepared similarly by inoculating fresh medium with ethyl alcohol + 5% Tween 20 and aqueous solutions were used as second controls. The Petri dishes with the inoculum were placed in incubator under controlled temperature condition of 25 ± 2°C. The efficacy of treatments was evaluated after 3 and 7 days. The percentage of inhibition of mycelia growth was calculated from the mean values of colony diameter of treated and control (ethyl alcohol + 5% Tween 80).
Disc diffusion method
Sterile filter paper discs (7 cm diameter) soaked in 100, 200, 400, or 800 µL L−1 pure essential oil were placed on the inner surface of the Petri dish lid. The dishes were sealed with parafilm and incubated upside-down at 25°C. Colony radius (in millimetre) was measured at third and sixth days. Control consists of sterile distilled water-soaked filter paper in the lid of Petri dish. The percentage of inhibition of mycelia growth was calculated from the mean values of colony diameter of treated and control.
Antibacterial activity assays
Microbial strains and growth conditions
Five Gram-negative bacteria (including Erwinia amylovora strain BPD, Xanthomonas oryzae strain IR42, Pseudomonas syringae strain 32, Pectobacterium carotovorum strain 863, Ralstonia solanacearum strain 145) and a Gram-positive bacterium (Bacillus thuringiensis strain KD2) were obtained from the collection of Department of Plant Diseases, Ferdowsi University, Iran. All strains were maintained as stock strains in 25% glycerol in Eppendorf microtubes and kept at −70°C until use. Bacteria strains were grown in nutrient broth at 28°C for 24 h and adjusted to approximately 1 × 106 CFU mL−1.
Disc diffusion assay
The antibacterial activity of F. angulata seed essential oil was tested against six phytopathogens by disc diffusion method. Whatman No.1 sterile filter paper discs (6 mm diameter) were impregnated with defined concentrations of essential oil (40 µL/disc), prepared in 1% DMSO. The discs were allowed to dry for 15 min and placed on the inoculated agar. The standard reference drug, ampicillin (40 µL/disc), was used as a positive control. Negative controls were prepared using the same solvents employed to dissolve the samples. The plates were incubated at 28°C for 24 h. The degree of the essential oil activity is revealed by the size of inhibition zone that is expressed by the diameter of the inhibition zone (in millimetre) and usually the diameter of the disc is included. Each assay in this experiment was replicated three times.[Citation28]
MIC and MBC assay
In order to determine the minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by microtitre plates, bacteria were cultured overnight at 28°C. The oils were dissolved in 1% DMSO. Dilutions were prepared in a 96-well microtitre plates to get final concentrations from 0 to 30 µL mL–1. Finally, 40 µL of inoculums (106–107 CFU mL−1) was inoculated onto the microplates and the tests were performed in a volume of 200 µL. Plates were incubated at 28°C for 24 h. The antibiotic, gentamicin/ampicillin, was used as a positive control for the tested plant pathogenic bacteria. The lowest concentrations of tested samples, which did not show any visual growth after macroscopic evaluation, were determined as MICs, which were expressed in µL mL−1. Using the results of the MIC assay, the concentrations showing complete absence of visual growth of bacteria were identified and 50 µL of each culture broth was transferred onto the agar plates and incubated for the specified time and temperature as mentioned above. The complete absence of growth on the agar surface in the lowest concentration of sample was defined as the MBC.[Citation29] For more accurate determination of MIC, all the bacterial cultures were co cultivated with various concentrations of oil in 5 mL of nutrient broth medium (in higher volume for less error). After the specified incubation period (28°C, for 24 h), 0.1 mL of cultures from all the test tubes were placed on nutrient agar medium to find out the MIC.[Citation29] All the experiments were done in triplicate.
Statistical analysis
In the disc sensitivity test, zones of inhibition were measured millimetres with a centimetre rule. Data on inhibitory effect of essential oils on mycelia growth were subjected to analysis of variance using SPSS 21. The means comparisons were made using Duncan’s multiple-range tests at p ≤ 0.05. All data are presented as mean values ± standard deviation (SD).
Results and discussion
Oil analysis
The essential oil from F. angulata seed was obtained in the yield of 0.7% (v/w). The chemical composition of the oil was examined by GC and GC/MS. The major components of the oil are presented in . In total, 39 compounds representing 91.07% of the oil have been identified. The oil consisted mainly of monoterpene hydrocarbons (77.04%), oxygenated monoterpenes (10.86%), sesquiterpene hydrocarbons (1.05%), oxygenated sesquiterpenes (0.71%), and other components (0.22%), respectively. The major constituents were (Z)-β-ocimene (19.93%), α-pinene (15.50%), p-cymene (7.67%), sabinene (7.49%), β-phellandrene (5.50%), and α-phellandrene (4.95%).
Table 1. Chemical compositions of the essential oil from Ferulago angulata seed.
Based on the literature surveys, the species of Ferulago genus are rich in essential oil. Earlier studies have been shown that aerial parts of F. angulata plant including stems, leaves, flowers, and seeds contain essential oil.[Citation9,Citation10,Citation30] Chemical compounds and biological activity of some species from the genus Ferulago have been the subject of preceding studies.[Citation8,Citation9,Citation14,Citation17] The reports on the chemical compositions of the oils isolated from the aerial parts F. angulata are abundant[Citation6,Citation7,Citation11,Citation13,Citation30–Citation32] as most of these investigations showed patterns of oil compositions similar to our present study. Although differences can be observed in the percentage distribution, in conformity with our findings (Z)-β-ocimene and α-pinene were dominant oil compounds in an earlier investigation.[Citation32]
Moreover, regarding the previously reported contents of the aerial parts, oil of the plant consists of monoterpenes as a major constituent[Citation9] which is in conformity with our results. On the other hand, our findings differ from those reported in some investigations, in which other components such as α-phellandrene and β-phellandrene[Citation3,Citation9], suberosin, spathulenol, trans-β-caryophyllene, arcurcumene, and bicyclogermacrene[Citation6], α-pinene, limonene, β-myrcene, and fenchyl acetate[Citation9] were the major compounds. A qualitative comparison of the oil constituents of F. angulata from different studies indicated significant differences in varying compositions, while some components that were not detected in our sample have been reported as the main compounds in others. This variation could have aroused from this fact that the plants used in each study were collected from different regions. Also, another important factor was the harvesting time. Previous investigations showed that the oil yield and its chemical composition were under various factors,[Citation6] including species and genotype, ecological conditions[Citation33], growth stage, and extraction methods.[Citation6]
Antifungal assay
The antifungal effect of F. angulata oil against six tested plant pathogenic fungi at different concentrations with two methods is shown in . Our findings indicated that all of the tested fungi could significantly inhibit fungal growth on all concentrations compared to the control and exhibited a moderate to high antifungal activity. According to the per cent of mycelia growth inhibition for each fungus, a difference between the resistance against the tested fungi in relation to the oil concentrations and times can be observed.
Table 2. Antifungal activity of the essential oil from seeds of Ferulago angulata by different methods.
Agar dilution method
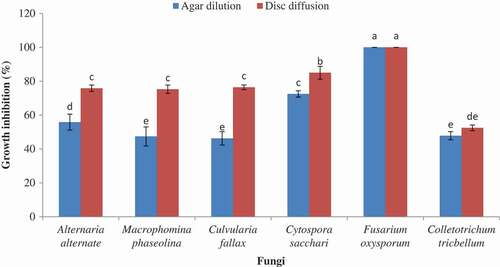
The inhibition of mycelia growth percentage by agar dilution method is shown in . As mentioned in our results, various concentrations had different effects on each fungus. In this method, all fungi were controlled by four concentrations of the oil. The oil exhibited antifungal activity at 100, 200, 400, and 800 µL L−1 concentrations against the tested fungi with various mycelia growth due to the concentrations and times from 17.6 ± 2.50 to 100 mean values. Our data indicated that F. angulata seed essential oil on all concentrations except at 100 µL L−1 could completely (100%) inhibit the F. oxysporum growth. Moreover, the weakest effect of the oil was observed against C. tricbellum. So, the most susceptible and the most resistant fungi at the highest concentration (800 µL L−1) after 6 days were F. oxysporum and C. tricbellum, respectively ().
Disc diffusion method
The per cent inhibition of mycelia growth by disc diffusion method is indicated in . A broad differentiation in the antifungal properties of essential oil against various fungi was observed. The oil exhibited antifungal activity at 100, 200, 400, and 800 (µL L−1) concentrations against the tested fungi with inhibition of mycelia growth percentage from 23.60 ± 2.10 to 100 in different concentrations and times. At the highest oil concentration (800 µL L−1), C. tricbellum showed a greater tolerance and F. oxysporum indicated the major susceptibility within the selected concentration range. So, in this concentration the most susceptible fungus was F. oxysporum and the most resistant fungus was C. tricbellum with 100.0 ± 0.00 and 52.5 ± 1.67% inhibition of mycelia growth, respectively. Furthermore, after 3 days the essential oil at 400 and 800 µL L−1 concentrations completely inhibited from C. sacchari growth. In this method completely fungistatic activities of the oil in all concentrations were observed only on F. oxysporum. According to the results, the various concentrations had different effects on each fungus. In contrast, in all cases with rise in the concentration of the oil, the zones of growth inhibition increased. Also about all fungi with passing the time the resistances were increased. Overall, the antifungal activity of the essential oil is related to the respective composition of the herbal essential oil, the structural configuration of the constituent components, and their functional groups and possible synergistic interactions between components.[Citation34]
The comparison between two methods at the highest concentration (800 µL L−1) of the oil indicated that disc diffusion was more effective to assay the percentage of growth inhibition of all of the tested fungi after 3 and 6 days ( and ). Therefore, all of the tested fungi were shown to be more resistant in agar dilution method. Although after 3 days there were no significant differences between two methods about C. sacchari and F. oxysporum, after 6 days C. sacchari showed lower percentage of growth inhibition by agar dilution method. So, C. sacchari was more resistant in this method. In total, according to the applied methods, the susceptibility and resistance of the tested fungi were different ( and ).
Figure 1. Effect of antifungal activity methods on per cent of growth inhibition different fungi after 3 days at the highest concentration (800 µL L−1).
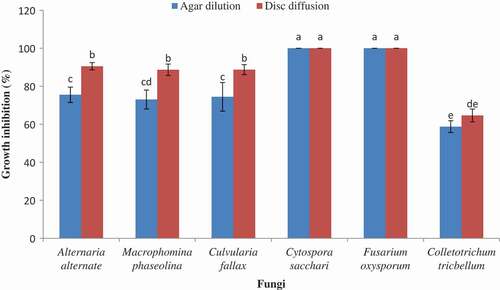
Figure 2. Effect of antifungal activity methods on per cent of growth inhibition different fungi after 6 days at the highest concentration (800 µL L−1).
Previous studies on the analysis and antifungal activity of essential oil of some species of different plants genera have shown that they have various degrees of growth inhibition effects against some phytopathogenic fungal species.[Citation35] Expression of different levels of antifungal activity may be due to the differences in the content of known antimicrobial compounds in each essential oil. In recent years, several researchers have reported that monoterpene and sesquiterpene hydrocarbons and their oxygenated derivatives are the major components of essential oils of plant origin, which have enormous potential to strongly inhibit microbial pathogens.[Citation2] In the preceding literature, the antifungal activity of p-cymene, α-pinene, linalool, terpinen-4-ol, 4-terpineol, α-terpineol, γ-terpinene, myrcene, β-caryophyllene, and β-pinene has been reported.[Citation36] This inhibition effect of the essential oil against the fungal species may be due to the presence of a relatively high proportion of oxygenated monoterpenes in the oil. It is also possible that the minor components might be involved in some type of synergism with the other active compounds.[Citation34]
In addition, some earlier papers on the analysis and antifungal properties of the essential oils of some species of various genera have shown that they have varying degrees of growth inhibition effects against some Fusarium, Botrytis, and Rhizoctonia species due to their different chemical compositions.[Citation35–Citation37] The strong antifungal activity of Cuminum cyminum oil against F. solani and F. oxysporum was reported earlier which was related to main components, such as α-pinene, 1,8-cineole, and linalool.[Citation38] It is necessary to mention that there is a direct relationship between inhibitory effects of essential oil on fungal growth and fusariotoxins production.[Citation39]
Antibacterial assay
In present work, it was aimed to examine the in vitro antibacterial activity of the F. angulata seed oil against six different phytopathogenic bacteria. It was evaluated against a Gram-positive (B. thuringiensis) and five Gram-negative (E. amylovora, X. oryzae, P. syringae, P. carotovorum, and R. solanacearum) phytopathogenic bacteria which were morphologically and physiologically different and their potency were assessed qualitatively and quantitatively by the presence inhibition zones, MIC, and MBC values ( and ).
Table 3. Minimal inhibitory concentration (MIC) and minimal bactericide concentration (MBC) (µL mL−1) of Ferulago angulata seed oil against microorganisms.
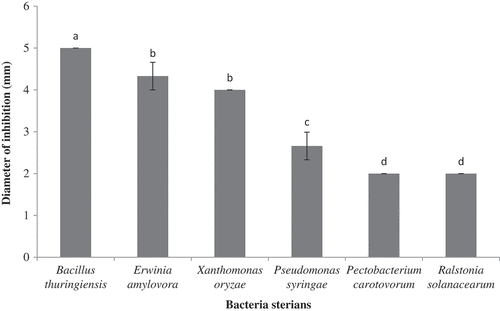
The oil exhibited antibacterial activity against all of the tested strains, but in variable degrees (). The inhibition zones for bacterial strains sensitive to the oil were in range of 2–5 mm. The data indicated that the highest activity of the oil was observed against B. thuringiensis with the strongest inhibition zones (5 mm). E. amylovora among the Gram-negative bacteria was the most sensitive strain tested. Also, P. carotovorum and R. solanacearum were the most resistant bacterium to the oil with a weak inhibition zones (). The resistance of the bacteria might be derived from the presence of hydrophilic properties of their impermeable outer membrane to lipophilic compounds such as oils. Essential oils are phytochemical complex blends of different aromatic components with different lipophilic and hydrophilic properties influencing the antimicrobial property in respect of its dilution in the tested medium.[Citation40]
Furthermore, in this investigation the bacteriostatic and bactericidal effectiveness of the oil estimated by MIC and MBC, respectively, are shown in . Although the oil showed activity on all of the tested bacteria, the results indicated a high variation of MICs and MBCs. The MIC and MBC values for bacterial strains sensitive to the plant products were in the range of 8–20 and 15 to >30 µL mL−1, respectively. The seed essential oil had the most bactericidal and bacteriostatic properties against the Gram-positive bacterium, B. thuringiensis. Moreover, the seed essential oil had the lowest MIC and MBC against E. amylovora and X. oryzae strains, among the Gram-negative bacteria. According to the results, B. thuringiensis was the most sensitive bacterium to the oil (8 µL mL−1 MIC). In addition, P. carotovorum and R. solanacearum were the most resistant bacteria ().
The antibacterial activities of several medicinal plants and their oils have been investigated in several studies and could be attributed to the major components. All of the Apiaceae family plants are aromatic that are known to possess antimicrobial properties, particularly due to their essential oils and can produce monoterpene, sesquiterpene, and phenyl components and related resins in their secretary ducts, roots, stem, leaf, flowers, and seeds and fruits.[Citation2] The antimicrobial efficacy of essential oils is the consequence of interaction between the minor and major components and conditioned by the activity of their components.[Citation34]
The existence of some antimicrobial constituents such as linalool[Citation23], α-terpinene, p-cymene[Citation14,Citation41], and terpinen-4-ol[Citation42] combined with other minor constitutes might be involved in improving the overall antimicrobial activity of essential oils. According to our results, the F. angulata seed oil contains more monoterpene hydrocarbons including (Z)-β-ocimene, α-pinene, p-cymene, and sabinene. In the previous literature, the antibacterial and antifungal effects of α-pinene, β-pinene, 4-terpineol, α-terpineol, and caryophyllene oxide have been reported.[Citation14] Referring to the literature, the antimicrobial activity of essential oil may be due to monoterpene hydrocarbons.[Citation2] The stronger antimicrobial activity of the essential oil is likely due to the presence of these mentioned components. Results of our study indicated that p-cymene might be related to a synergistic phenomenon. Our findings as well as literature data allow us to suggest that constituents such as linalool, terpinen-4-ol, and 1,8-cineole, alongside oxygenated sesquiterpenes, might contribute to antimicrobial activity agree with earlier reports.[Citation23,Citation42,Citation43–Citation44] Although these compounds are not abundant in essential oil, their activity is important. It is necessary to signal that other components can contribute to improve this activity.
A number of Ferulago species have previously been studied for their essential oil compositions, biological activity, and the antimicrobial activity.[Citation12] Some of these compounds have both antibacterial and antifungal activities.[Citation3,Citation13] Moreover, the antibacterial and antifungal activities have previously been investigated for some Ferulago species as Ferulago asparagifolia, F. galbanifera, Ferulago humilis, F. trachycarpa, Ferulago thyrsiflora, Ferulago sylvatica, Ferulago bernardii, Ferulago nodosa, Ferulago longistylis, and Ferulago angulata subsp. carduchorum and inhibitory effects for microorganisms have been observed.[Citation2,Citation10] The essential oil of F. angulata showed stronger antimicrobial activity than other species.[Citation45] In a study, the essential oil of F. bernardii exhibited a low level of the antibacterial activity against Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Candida albicans and no activity against Pseudomonas aeruginosa.[Citation2] Although in earlier research antimicrobial effects of essential oils obtained from aerial parts and seeds of F. angulata subsp. Carduchorum against tested bacteria and fungi have been reported[Citation10], in other study essential oils of F. angulata ssp. angulata exhibited no considerable biological activities.[Citation46,Citation47]
The major components or their derivatives may also contribute to antimicrobial activity, thus it is still worthwhile to test the individual major components also against other pathogens in future works. As far as the mechanism of the antimicrobial activity is concerned, the complex and variable chemical composition of essential oils, which can include many different molecules, prevents the understanding of the mechanism of action. This study indicated a potent antimicrobial activity of F. angulata seed essential oil against different bacteria and fungi is noteworthy, but needs further survey to evaluate the suitability of this remarkable antimicrobial property.
Conclusion
In recent years, interest has been generated in the development of safer antifungal agents such as plant-based essential oils to control phytopathogens in agricultural systems. Essential oils are promising natural antimicrobial agents with potential applications in agro-industries to control plant pathogenic microorganisms causing severe destruction in crops. Due to the interest in antimicrobial substances from plant sources, we performed a study on F. angulata oil which revealed significant antimicrobial activity against different plant pathogenic microorganisms causing severe diseases in plants. Our findings indicated that the oil of this plant is effective for inhibition or to control the tested bacteria and fungi. In this way, the oil has a good capacity as an alternative to synthetic products in various industries. The essential oil of F. angulata seed could be used as a natural antimicrobial agent. Finally, it would also be interesting to study the effects of essential oils of F. angulata against other important bacteria and fungi for developing new natural antibacterial and antifungal agents to control serious fungal diseases in plants. Further work is necessary to explore the efficacy and potential of suitable concentrations of this essential oil in foods and bearing industries to consider the possible interactions of the oil with food ingredients.
References
- Mozaffarian, V. A. A.;. Dictionary of Iranian Plant Names; Farhang Moaser Publischer: Tehran, Iran, Farhang Moaser Publischer 1996; pp 230.
- Khalighi-Sigaroodi, F.; Hadjiakhoondi, A.; Shahverdi, H. R.; Mozaffarian, V. A.; Shafiee, A. Chemical Composition and Antimicrobial Activity of the Essential Oil of Ferulago bernardii Tomk. And M. Pimen. DARU Journal of Pharmaceutical Science 2005, 13, 100–104.
- Rustaiyan, A.; Sedaghat, S.; Larijani, K.; Khossravi, M.; Masoudi, S. Composition of the Essential Oil of Ferulago angulata (Schlecht.) Boiss. Journal of Essential Oil Research 2002, 14, 447–448, DOI: 10.1080/10412905.2002.9699917.
- Ghasemi Pirbalouti, A.; Momeni, M.; Bahmani, M. Ethnobotanical Study of Medicinal Plants Used by Kurd Tribe in Dehloran and Abadan Districts, Ilam Province, Iran. African Journal of Traditional, Complementary and Alternative Medicines 2013, 10, 368–385.
- Amirghofran, Z.; Bahmani, M.; Azadmehr, A.; Javidnia, K.; Miri, R. Immunomodulatory Activities of Various Medicinal Plant Extracts: Effects on Human Lymphocytes Apoptosis. Iranian Journal of Science and Technology, Transactions A: Science 2009, 38, 181–192.
- Sodeifian, G.; Ansari, K.; Bamoniri, A.; Mirjalili, B. Study of Chemical Composition of the Essential Oil of Ferulago angulata (Schelcht) Boiss. From Iran Using Supercritical Fluid Extraction and Nanoscale Injection. Digest Journal of Nanomaterials and Biostructures 2011, 6, 161–168.
- Javidnia, K.; Miri, R.; Edraki, N.; Khoshneviszadeh, M.; Javidnia, A. Constituents of the Volatile Oil of Ferulago angulata (Schlecht.) Boiss. From Iran. Journal of Essential Oil Research 2006, 18, 548–550, DOI: 10.1080/10412905.2006.9699163.
- Kilic, C. S.; Ozkan, A. M. G.; Demirci, B.; Coskun, M.; Baser, K. H. C. Essential Oil Composition of Four Endemic Ferulago Species Growing in Turkey. Natural Product Communications 2010, 5, 1951–1954.
- Akhlaghi, H.;. Volatile Constituents from the Aerial Parts of Ferulago angulata (Schlecht.) Boiss. Growing Wild Northeast Iran. Analytical Chemistry Letters 2012, 2, 133–138, DOI: 10.1080/222979282000.10648261.
- Taran, M.; Ghasempour, H. R.; Shirinpour, E. Antimicrobial Activity of Essential Oils of Ferulago angulata Subsp. carduchorum. Jundishapur Journal of Microbiology 2010, 3, 10–14.
- Khanahmadi, M.; Janfeshan, K. Study on Antioxidation Property of Ferulago angulata Plant. Asian Journal of Plant Science 2006, 5, 521–526, DOI: 10.3923/ajps.2006.521.526.
- Baser, K. H. C.; Demirci, B.; Ozek, T.; Akalln, E.; Ozhatay, N. Volatile Compounds from Ferulago Species Growing in Western Turkey. Pharmaceutical Biology 2002, 40, 466–471, DOI: 10.1076/phbi.40.6.466.8439.
- Ghasempour, H. R.; Shirinpour, E.; Heidari, H. Analysis by Gas Chromatography-Mass Spectrometry of Essential Oil from Seeds and Aerial Parts of Ferulago angulata (Schlecht.) Boiss Gathered in Nevakoh and Shahoo, Zagross Mountain, West of Iran. Pakistan. Pakistan Journal of Biological Sciences 2007, 10(5), 814–817.
- Maggi, F.; Cecchini, C.; Cresci, A.; Coman, M. M.; Tirillini, B.; Sagratini, G.; Papa, F. Chemical Composition and Antimicrobial Activity of the Essential Oil from Ferula glaucaL. (F. communis L. Subsp. glauca) Growing in Marche (Central Italy). Fitoterapia 2009, 80, 68–72, DOI: 10.1016/j.fitote.2008.10.001.
- Maxia, A.; Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Goncalves, M. J.; Cavalerio, C.; Salquerio, L. Chemical Characterization and Biological Activity of Essential Oils from Daucus carota L. Subsp. carota Growing Wild on the Mediterranean Coast and on the Atlantic Coast. Fitoterapia 2009, 80, 57–61, DOI: 10.1016/j.fitote.2008.09.008.
- Montesinos, E.;. Development, Registration and Commercialization of Microbial Pesticides for Plant Protection. International Microbiology 2003, 6, 245–252, DOI: 10.1007/s10123-003-0144-x.
- Nölke, G.; Fischer, R.; Schillberg, S. Antibody-Based Pathogen Resistance in Plants. Journal of Plant Pathology 2004, 86, 5–17.
- Pavela, R.;. Possibilities of Botanical Insecticide Exploitation in Plant Protection. Pest Technology 2007, 1, 47–52.
- White, D. G.; Zhao, S.; Simjee, S.; Wagner, D. D.; McDermott, P. F. Antimicrobial Resistance of Foodborne Pathogens. Microbes and Infection 2002, 4, 405–412, DOI: 10.1016/S1286-4579(02)01554-X.
- Soylu, E. M.; Soylu, S.; Kurt, S. Antimicrobial Activities of the Essential Oils of Various Plants against Tomato Late Blight Disease Agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128, DOI: 10.1007/s11046-005-0206-z.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils–A Review. Food and Chemical Toxicology 2008, 46, 446–447, DOI: 10.1016/j.fct.2007.09.106.
- Başer, K. H. C.; Demirci, F. Chemistry of Essential Oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Ustainability, Berger, R. G., Ed.; Berlin: Springer, 2007; pp 43–86.
- Cavar, S.; Maksimovic, M.; Solic, M. E.; Jerkovic-Mujkic, A.; Besta, R. Chemical Composition and Antioxidant and Antimicrobial Activity of Two Satureja Essential Oils. Food Chemistry 2008, 111, 648–653, DOI: 10.1016/j.foodchem.2008.04.033.
- Zaidi, M. A.; Crow, S. A. Biologically Active Traditional Medicinal Herbs from Balochistan, Pakistan. Journal of Ethnopharmacology 2005, 96, 331–334, DOI: 10.1016/j.jep.2004.07.023.
- Ahmet, C.; Saban, K.; Hamdullah, K.; Ercan, K. Antifungal Properties of Essential Oil and Crude Extracts of Hypericum linarioides Bosse. Biochemical Systematics and Ecology 2005, 33, 245–256, DOI: 10.1016/j.bse.2004.08.006.
- Adams, R. P.;. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, Illinois, USA, 2007.
- McLafferty, F. W.;. Wiley Registry of Mass Spectral Data, 9th ed.; Wiley Inc.: Hoboken, N.J., USA, 2009.
- Kumar, A. S.; Venkatesalu, V.; Kannathasan, K.; Chandrasekaran, M. Chemical Constituents and Antibacterial Activity of the Leaf Essential Oil of Feronia limonia. Indian Journal of Microbiology 2010, 50, 70–73, DOI: 10.1007/s12088-010-0052-7.
- NCCLS, (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disc Susceptibility Test, Ninth International Supplement, M100-S9; National Committee for Clinical Laboratory Standards: Wayne, PA, 1999.
- Sefidkon, F.; Omidbaigi, R. Chemical Composition of the Essential Oil of Ferulago angulatafrom Iran. Journal of Essential Oil Bearing Plant 2004, 7, 60–63, DOI: 10.1080/0972-060X.2004.10643366.
- Asghari, J.; Khamoie Touli, C.; Mazaheritehrani, M.; Aghdasi, M. Comparison of the Microwave-Assisted Hydrodistillation with the Traditional Hydrodistillation Method in the Extraction of Essential Oils from Ferulago angulata. European Journal of Medicinal Plants 2012, 2, 324–334, DOI: 10.9734/EJMP/2012/1488.
- GhasemiPirbalouti, A.; Sedaghat, L.; Hamedi, B.; Tirgir, F. Chemical Composition and Antioxidant Activity of Essential Oils of Three Endemic Medicinal Plants of Iran. Bangladesh Journal of Botany 2013, 42, 327–332.
- GhasemiPirbalouti, A.; Moalem, E. Variation in Antibacterial Activity of Different Ecotypes of Satureja khuzestanica Jamzad, as an Iranian Endemic Plant. Indian Journal of Traditional Knowledge 2013, 12, 623–629.
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical Composition and Antimicrobial Activity of the Essential Oil of Scutellari abarbata. Phytochemistry 2004, 65, 881–884, DOI: 10.1016/j.phytochem.2004.02.005.
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the Chemical Composition and Antioxidant Activity of the Essential Oil of Artemisia dracunculus and the Antifungal and Antibacterial Activities of Turkish Artemisia absinthium, A. dracunculus, A. santonicum, and A. spicigera Essential Oils. Journal of Agricultural and Food Chemistry 2005, 53, 9452–9458, DOI: 10.1021/jf0516538.
- Deba, F.; Xuan, T. D.; Yasuda, M.; Tawata, S. Chemical Composition and Antioxidant, Antibacterial and Antifungal Activities of the Essential Oils from Bidens pilosa Linn. Var. Radiata. Food Control 2008, 19, 346–352, DOI: 10.1016/j.foodcont.2007.04.011.
- Bouchra, C.; Achouri, M.; Hassani, L. M. I.; Hmamouchi, M. Chemical Composition and Antifungal Activity of Essential Oils of Seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. Journal of Ethnopharmacology 2003, 89, 165–169, DOI: 10.1016/S0378-8741(03)00275-7.
- Salehi Surmaghi, H.;. Medicinal Plants and Phytotherapy. Donyaee Taghazie, nourishing world: Tehran, Iran 2006; pp 55–58.
- Dambolena, J. S.; Lopez, A. G.; Canepa, M. C.; Theumer, M. G.; Zygadlo, J. A.; Rubinstein, H. R. Inhibitory Effect of Cyclic Terpenes (Limonene, Menthol, Menthone and Thymol) on Fusarium verticillioides MRC 826 Growth and Fumonisin B1 Biosynthesis. Toxicon 2008, 51, 37–44, DOI: 10.1016/j.toxicon.2007.07.005.
- Sandri, I. G.; Zacaria, J.; Fracaro, F.; Delamare, A. P. L.; Echeverrigaray, S. Antimicrobial Activity of the Essential Oils of Brazilian Species of the Genus Cunila against Foodborne Pathogens and Spoiling Bacteria. Food Chemistry 2007, 103, 823–828, DOI: 10.1016/j.foodchem.2006.09.032.
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical Composition and Antimicrobial Properties of Essential Oils of Three Australian Eucalyptus Species. Food Chemistry 2010, 119, 731–737, DOI: 10.1016/j.foodchem.2009.07.021.
- Lopes-Lutz, D.; Alviano, D. S.; Alviano, C. S.; Kolodziejczyk, P. P. Screening of Chemical Composition, Antimicrobial and Antioxidant Activities of Artemisia Essential Oils. Phytochemistry 2008, 69, 1732–1738, DOI: 10.1016/j.phytochem.2008.02.014.
- Bajpai, V. K.; Al-Reza, S. M.; Choi, U. K.; Lee, J. H.; Kang, S. C. Chemical Composition, Antibacterial and Antioxidant Activities of Leaf Essential Oil and Extracts of Metasequioa glyptostroboides Miki Ex Hu. Food and Chemical Toxicology 2009, 47, 1876–1883, DOI: 10.1016/j.fct.2009.04.043.
- Arslan, I.; Ili, P. Genotoxicological Assessment of nebuloside-A a Triterpenoid Saponin Compound on Whole Blood DNA. International Journal of Food Properties 2015, 18, 2374–2379, DOI: 10.1080/10942912.2014.971185.
- Ozkan, A. M. G.; Demirci, B.; Demirci, F.; Baser, K. H. C. Composition and Antimicrobial Activity of Essential Oil of Ferulago Longistylis Boiss. Fruits. Journal of Essential Oil Research 2008, 20, 569–573, DOI: 10.1080/10412905.2008.9700090.
- Hosseini, N.; Akbari, M.; Ghafarzadegan, R.; ChangiziAshtiyani, S.; Shahmohammadi, R. Total Phenol, Antioxidant and Antibacterial Activity of the Essential Oil and Extracts of Ferulago angulata Ssp. Angulata. Journal of Medicinal Plants 2012, 3(43), 80–89.
- Celik, A.; Arslan, I.; Herken, E. N.; Ermis, A. Constituents, Oxidant-Antioxidant Profile, and Antimicrobial Capacity of the Essential Oil Obtained from Ferulago sandrasica Peşmen and Quézel. International. International Journal of Food Properties 2013, 16, 1655–1662, DOI: 10.1080/10942912.2011.618898.