ABSTRACT
The distinctive extra-virgin olive oil aroma consists of a complex mixture of volatile compounds. We comparatively studied the volatiles of eight autochthonous monovarietal extra-virgin olive oils from the north-east part of Algeria via headspace solid-phase microextraction GC-MS. We determined the effect of ripening of Chemlal olive fruit on aroma compounds. Twenty volatile analytes belonging to different chemical classes were identified and quantified. Both quantitative and qualitative differences were found among cultivars, indicating a close dependence of the composition of the volatile profile on the enzymatic pool, directly related to genetic characteristics. Moreover, differences in volatiles composition were observed for Chemlal oils during maturation.
Abbreviations: AAT, alcohol acetyl transferase; E, east; EVOO, extra-virgin olive oil; GC, gas chromatography; HS, headspace; LOX, lipoxygenase; MI, maturity index; MS, mass spectrometry; N, north; PCA, principal component analysis; SD, standard deviation; SPME, solid-phase microextraction technique; VOO, virgin olive oil.
Introduction
Virgin olive oil (VOO), one of the main foods used by people living in Mediterranean countries, is a fruit juice endowed with bioactive ingredients; the original characteristic aroma properties[Citation1] present in VOO are the most significant factors which can shape the quality of the product and affect the consumer’s behaviors toward it.[Citation2] In fact, the distinctive flavors of premium quality VOO are due to the presence of a complex mixture of volatile compounds, belonging to several chemical classes: aldehydes, alcohols, esters, ketones, hydrocarbons, acids, furans, terpenes, and probably other unidentified volatiles which are present at extremely low concentrations.[Citation3,Citation4] Many of these compounds are produced enzymatically by the so-called lipoxygenase (LOX) pathway, occurring in the disrupted cell structure during olive oil production, from free polyunsaturated fatty acids, particularly linoleic and α-linolenic acids.[Citation3,Citation5] More specifically, both C6 aldehydes, alcohols, and their corresponding esters, and minor amounts of C5 carbonyl compounds, alcohols, and pentene dimers are responsible for the positive odors and pleasant notes in olive oils. On the other hand, other volatile compounds have been reported to be responsible for the unpleasant aroma and odors resulting from olive oil; these compounds can be derived from different mechanisms such as: sugar fermentation, amino acid conversion, enzymatic activities of molds or anaerobic microorganisms, and other auto-oxidative processes.[Citation6]
The volatile compounds profile of VOO is influenced by all factors interfering in the entire production process, for example, pedoclimatic conditions, the cultivar, agronomic practices, technological features of the milling process, and olive oil storage conditions.[Citation7,Citation8] The cultivar is one of the main factors that can determine the content and profile of VOO volatile compounds.[Citation5,Citation8] Its effect was evidenced by the presence of different amounts of C6 compounds arising from oils produced under the same operating conditions of the extraction process of different fruit cultivars harvested at the same ripening stage.[Citation9,Citation10] It has been presumed that enzyme levels and enzyme’s activity, involved in the biogenesis of volatiles, are genetically determined in each olive cultivar.[Citation8,Citation9] Another important factor that influences VOO volatile compounds composition, is the ripening degree which is in turn strongly dependent on other factors; ripening is in fact directly related to both genetic characteristics, and environmental conditions.[Citation7,Citation11]
In Algeria, olive trees existed since the 12th millennium BC. The olive growing area is spread over an area of 330,000 hectares and concentrated mainly in the central region of the country, i.e., the Kabylie area. Several authochtonous olive cultivars are present; however, the Algerian olive orchards are dominated by the Chemlal cultivar, comprising 40% of the orchards, probably owing to its unique adaptation to various pedoclimatic conditions.[Citation12] With an average production of 61,600 tonnes per annum, which represent 2.1% of the world’s olive oil production, Algeria is ranked sixth in the world after the European Union, Tunisia, Turkey, Syria, and Morocco, according to data obtained from the International Olive Council.[Citation13] Support for the olive oil sector is one of the Algerian government’s priorities that seeks to increase both VOO production and exportation, based on similar models followed by Tunisia and Morocco, which both export 20–80% of their national production to the world’s markets.[Citation13] Nevertheless, to compete with other Mediterranean producers, Algeria faces a number of challenges, most notably by continuously improving the quality of its VOO. In depth studies on factors modulating the composition and sensorial characteristics of Algerian VOO are quite important in order to select the type of VOO to be produced in high quality.
In this context, this paper presents the first investigation which is aimed at the characterization of aroma profile of monovarietal Algerian extra-virgin olive oil (EVOO). To the best of our knowledge, only one recent work studying the semi-quantification of volatile compounds of VOO extracted from three varieties and collected from different regions in Algeria can be found in the literature.[Citation14] Thus, the present study is designed to determine the effect of eight cultivars on the concentration of volatile compounds in EVOO. Since sensorial properties of EVOO are deeply affected by the ripening degree at which olives are processed, the identification of the optimum harvesting date is crucial to ensure a high oil quality and to please the consumer. In addition, the present study was also aimed at comparing the EVOO volatile compounds contents, extracted from the most abundant olive cultivar in Algeria, Chemlal, collected at four different harvesting dates.
Materials and methods
Chemicals
(Z)-2-Penten-1-ol, hexanal, (E)-2-hexenal, (E)-2-hexen-1-ol, benzaldehyde, (E)-2-heptenal, 6-methyl-5-hepten-2-one, (Z)-3-hexen-1-yl acetate, hexyl acetate, p-cymene, limonene, nonanal, phenylethyl alcohol, (E,Z) alloocimene, methyl salicylate, α-farnesene, refined olive oil, and C7–C40 saturated alkanes standards were purchased from Sigma-Aldrich (St. Louis, USA). Ethanol, heptane, and chlorobenzene were provided by Carlo Erba (Val de Reuil, France). All standards were of analytical grade.
Experimental design
The study was carried out on monovarietal EVOO in accordance with other previously performed chemical analyses according to International Olive Oil Council standards quality (data not published) gathered from eight Algerian autochthonous cultivars (Aaleh, Abani, Bouricha, Chemlal, Ferkani, Limli, Mekki, and Rougette de Mitidja). All cultivars were obtained from a collection gathered by the Technical Institute of Fruit Arboriculture; the olive trees, located in the area of Soummam valley (36°34ʹ49.00″N, 4°40ʹ7.92″E), Sidi-Aïch (Bejaia, Algeria), were cultivated under identical agronomic and pedoclimatic conditions. The selected olive trees were mature, aged more than 60 years-old, spaced (10 × 10 m) and subjected to non-irrigated conditions throughout the year except for months of July and August, where trees were irrigated (50 L/m2 per tree) once per week.
Approximately 10–15 kg of olive fruits were handpicked from October 2014 to January 2015, corresponding to a maturation index (MI) ranging from 2.01 to 3.30. Chemlal olives were handpicked at four different ripening stages (MI = 3.00, 4.20, 5.50, and 6.20). MI of fruits was determined according to the guidelines of Estación de Olivicultura y Elaiotecnia, Jaén, Spain,[Citation15] which primarily relies on the assessment of olive fruit epidermis and their mesocarp color.
Only healthy olive fruits, possessing no infection or physical damage, were processed. Olive fruits of each cultivar were processed separately in an oleodoseur Siol 20240 (Ghisonaccia, France) within 24 h from harvesting. Fruits were crushed with a hammer mill and the olive paste was malaxed at 18°C, room temperature for 40 min, in an olive paste mixer. The olive oil was separated by centrifugation without the addition of warm water using a two phase decanter. Prior to and following the preparation of each olive oil sample, the extraction plant was cleaned. The obtained oils were stored in amber glass bottles without headspace (HS) at −20°C until further analysis.
Analysis of volatile compounds
HS-SPME
Analyses of the volatile compounds of Algerian EVOO were performed in triplicates, using a HS solid-phase microextraction (SPME) technique. Fibers were obtained from Supelco Company (Bellefonte, PA, USA). The fiber utilized for the volatile compounds extraction was divinylbenzene/carboxen/polydimethylsiloxane 50/30 µm. The fiber was conditioned before use, as recommended by the manufacturer. Olive oil samples (5 mL) were placed in a vial (8 mL) and spiked with 1.33 µg/mL chlorobenzene as an internal standard (10 µL of a 666 µg/mL solution in refined olive oil); the vials were then closed by PTFE/silicone septum. The extraction was carried out at 20 ± 0.1°C, and by stirring the fiber for 6 h in a vial HS with magnetic stirring.[Citation4]
Gas chromatography–mass spectrometry (GC–MS)
The volatile compounds were analyzed by GC–MS Hewlett Packard G1800C instrument (Palo Alto, CA), equipped with a Rxi™-5 ms capillary column (30 m × 0.25 mm, 0.25 μm) (Resteck, USA). The fiber was left in the injection port (equipped with a 0.75 mm i.d. inlet liner) for 4 min. The injector was set at 270°C and operated in the splitless mode for 1 min. The blank runs were performed during the study to reveal possible carryover. The carrier gas was helium, running at a constant flow of 1 mL/min; the oven temperature was held isothermal at 30°C for 15 min, and then ramped to 200°C at a rate of 10°C/min. Before sampling, the fiber was reconditioned for 5 min at 270°C. The detector temperature was set at 270°C. The transfer line temperature was set at 270°C; the ion source and the quadrupole were heated by conduction. The mass spectrometer was operated in electron impact mode within the range of 30–300 m/z.
Identification and quantification of volatile compounds
The identification of all constituents was based on the comparison of their retention times and mass spectra with those of pure standards under the same conditions, and by determining their linear retention indices relative to a series of n-alkanes, whereby the averaged values reported in the bibliography for chromatographic columns were similar to those used; computer matching against commercial (NIST 1998) libraries was also performed.
The external calibration curve using at least four points was created for the purpose of quantifying the amount volatile compounds. The peak area of each compound was normalized with respect to the area of the internal standard peak and further interpolated on the calibration curve. Working ranges[Citation16] and regression parameters () of each compound were constructed by preparing a solution containing a mixture of known amounts of analyte and the internal standard in the refined olive oil. Solutions containing various concentrations were then obtained by further diluting the refined olive oil. Simple linear regression procedure was used for modeling the calibration curves. Regression parameters and their standard deviations (SDs), the coefficient of determination, and the sum of squares of errors () were obtained using MacCurveFit 1.5.4, Copyright © 1991–2000, Kevin Raner Software. Limit of detection and limit of quantification () were evaluated according to Şengül[Citation17] and Evard et al.[Citation18] The refined olive oil was analyzed to evidence the absence of target volatiles in the refined olive oil. The detection and quantification of volatiles were performed in full scan mode. The quantitative analysis of ethanol, benzaldehyde, and (E)-2-heptenal was carried out using the selected ion monitoring mode, by analyzing the following ions: m/z 31, 77, and 41, respectively. The concentration of those compounds, for which the pure standard was not available (1-penten-3-one and 1-hexanol and β-ocimene), was calculated using the calibration curves of another compound with a similar chemical structure, according to the formula and chemical properties (6-methyl-5-hepten-2-one, (E)-2-hexen-1-ol and (E,Z) alloocimene), respectively.
Table 1. Regression parameters of volatile compounds analysis using SPME-GC-MS.
Statistical analysis
Values are expressed as mean ± SD of three measurements. Significant differences for each ripening stage were determined for each compound using one-way ANOVA statistical analysis. Tukey’s test was used to discriminate among the mean of the variables. Differences with p < 0.05 were considered significant. The volatiles’ data were analyzed by principal component analysis (PCA) method, using XLStat (version 2015.6.01.24027), and an add-in software package for Microsoft Excel (Addinsoft Corp., Paris, France), to evidence the differences among oil samples.
Results and discussion
Effect of cultivar on the volatile compounds in Algerian EVOO
The volatile compounds were analyzed in monovarietal EVOO extracted from Aaleh, Abani, Bouricha Chemlal, Ferkani, Limli, Mekki, and Rougette de Mitidja. Initially, tests were carried out to identify the volatile profile of Algerian EVOO. Out of 44 tested standards, 20 compounds, found to belong to the class of alcohols (5), ketones (2), aldehydes (5), esters (3), and terpenes (5), were identified and quantified by HS-SPME-GC-MS, using the regression parameters detailed in . The number of volatile compounds identified varied according to the type of olive cultivar, and was arranged in the following order: Bouricha (13) > Limli and Chemlal (10) > Rougette de Mitidja (9) > Abaniand Ferkani (8) > Mekki (7) > Aaleh(6). All studied samples showed both quantitative and qualitative differences in the profile of volatile compounds. shows the amount of total volatile compounds and their different chemical classes (in mg/L) for each studied variety. Moreover, a detailed overview of the content of volatile compounds is shown in . -supplementary material, shows the SD of the corresponding volatile compounds concentration. Algerian EVOO contained a wide range of concentrations of total volatile compounds (8.06–34.24 mg/L), according to the type of cultivar. Rougette de Mitidja is the variety with the highest amount of volatile compounds, followed closely by Limli (32.45 mg/L), Bouricha (26.86 mg/L), and Chemlal (25.61 mg/L), whereas Ferkani had the lowest amount of volatile compounds (). Hence, the amount of volatiles is strongly dependent on the type of olive cultivar, given that the agronomic, pedoclimatic, and extraction conditions were the same for all cultivars, and the MIs were very close. The results are in accordance with previously published data by Brkić Bubola et al.[Citation10] and Luna et al.,[Citation19] both found that the amount of volatiles compounds in VOO was dependent on the type of olive variety. Moreover, aldehydes were predominant in Bouricha (18.51 mg/L), Chemlal (14.92 mg/L), Limli (27.03 mg/L), and Mekki (13.49 mg/L), whereas ketones dominated the volatile profile of Aaleh (5.38 mg/L), Abani (4.97 mg/L), and Ferkani (5.23 mg/L) (). Rougette de Mitidja contained an important quantity of both aldehydes (19.71 mg/L) and ketones (13.91 mg/L) ().
Table 2. Volatile compounds concentration (mg/L, mean of three replicates) in eight Algerian monocultivar EVOOs.
Figure 1. Principal chemical group quantified (mg/L) from the volatile fraction of eight Algerian monocultivar EVOOs: Aaleh, Abani, Bouricha, Chemlal (MI = 3.00), Ferkani, Limli, Mekki, and Rougette de Mitidja.
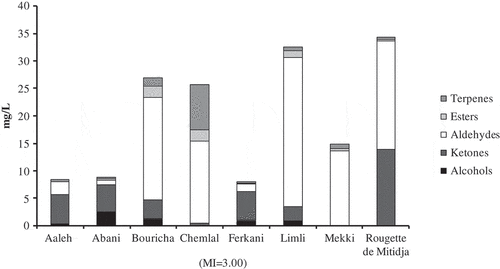
The accumulation of C6 volatiles, derived from the LOX pathway, also called “green volatiles,” is responsible for the high quality of EVOOs; the latter is strongly dependent on the genotype of the sample. Limli and Abani EVOOs were characterized by the highest (26.51 mg/L) and lowest (0.61 mg/L) C6 volatile contents, respectively (). Among these compounds, aldehydes are the most abundant C6 volatile compounds, as already reported.[Citation20,Citation21] In our study, aldehydes presented 88–100% () of the whole C6 fractions, depending on the olive cultivar. The differences in C6 aldehyde fractions could be due to the different enzymatic activities involved in their synthesis such as: (i) hydroperoxidelyase, which catalyzes the cleavage of fatty acid hydroperoxides to produce volatile aldehydes and (ii) acyl hydrolase which can influence the availability of free polyunsaturated fatty acids.[Citation22] Generally, C6 aldehydes are among the major contributors to the odor of olive oil because they have very low odor thresholds, and are usually of positive sensory characteristics, for example, green, fruity, and sweet.[Citation19] The most prevalent C6 aldehyde present among five olive varieties was (E)-2-hexenal, with concentrations ranging from 10.74 to 26.51 mg/L (), this result is in accordance with the literature data obtained from other monovarietal olive oils. In fact, (E)-2-hexenal is the most abundant volatile compound in European,[Citation23–Citation29] Tunisian,[Citation20,Citation30] and Moroccan[Citation21] olive oils. Surprisingly, EVOO obtained from the variety Ferkani did not contain (E)-2-hexenal (), whereas the latter was found at relatively low amounts in Aaleh and Abani varieties (2.26 and 0.38 mg/L, respectively). Nevertheless, Nigri et al.[Citation14] did not find (E)-2-hexenal in the VOO of Chemlal variety located in Tizi-Ouzou (Algeria). The absence or the presence of low amounts of (E)-2-hexenal could be the result of (i) poor availability of (E)-2-hexenal precursor (namely (Z)-3-hexenal) and consequently of linolenic acid and (ii) low or lack in (Z)-3:(E)-2-enal isomerase activity in the cultivars. It is well known that (E)-2-hexenal is produced by the enalisomerase which catalyzes the isomerization of (Z)-3-hexenal to the more stable (E)-2 form.[Citation5] Further investigations are needed to confirm this hypotheses.
The other C6 aldehyde identified was hexanal (0.23–3.88 mg/L) (). Hexanal is the oxidation product of linoleic acid and is derived from either LOX action or from chemical oxidation. The compound has been related to grassy, green sweet, and green-apple odors,[Citation3] while its presence in higher amounts is described as unpleasant sebaceous. In fact, Dierkes et al.[Citation31] showed that C6 aldehydes present in concentrations higher than 900 μg/kg lead to a negative effect on olive oil sensory quality. In the present study, hexanal was not found in Aaleh, Limli, and Mekki oil varieties. Similar results were also found by Šarolić et al.[Citation24] for Levantinka olive oil, a Croatian variety. This result could be explained by the low amount or lack of activity of the enzyme involved in the generation of hexanal in these varieties.
In terms of C6 alcohols, hexanol was the only compound identified at very low concentrations in the varieties Bouricha (0.28 mg/L), Limli (0.21 mg/L), and Rougette de Mitidja (0.01 mg/L) (), indicating the presence of a low alcohol dehydrogenase enzyme activity, which is genetically controlled in each cultivar.[Citation9] However, C6 alcohols in olive oil have less sensory significance than aldehydes owing to their higher odor threshold values; their sensory descriptions are associated with fruity, soft green, and aromatic sensory notes.[Citation19]
In addition, C6 esters, namely (Z)-3-hexen-1-yl acetate contributing to the green, banana pleasant notes,[Citation20] and hexyl acetate contributing to sweet, fruity notes,[Citation3] were present in low amounts, with values in the range of 0.08–1 mg/L and 0.09–1.54 mg/L, respectively (). Furthermore, two varieties (Aaleh and Abani) did not contain these esters. This fact could be explained by the low presence of alcohol acetyl transferase (AAT), which catalyzes their biosynthesis.[Citation32] Moreover, AAT has an activity optimal pH in the range neutral to basic, while regular pH value of olive paste during production is found in the acidic range.[Citation33]
In addition to C6 LOX compounds, we determined an additional branch of LOX pathway, the C5 LOX compounds. In contrast to other varieties, Chemlal did not contain this class of chemical compounds. Our findings do not agree with those of Nigri et al.,[Citation14] studying the same variety planted in a different region in Algeria. In the present study, C5 LOX compounds were composed of two compounds (1-pentene-3-one and 2-penten-1-ol). The 1-pentene-3-one was the most abundant one, with concentrations between 2.67 and 13.91 mg/L for Limli and Rougette de Mitidja olive cultivars, respectively (). The latter ketone is associated with leaf, bitter, and pungency tastes [Citation34] with a very low odor threshold; hence its contribution to the whole aroma is considered important.[Citation10,Citation20] Nevertheless, bitter and pungent sensory characteristics are mostly dependent on the fraction of phenolic compounds.[Citation35] Concerning 2-penten-1-ol, its concentration varied from 0.15 to 0.39 mg/L, according to the type of olive cultivar (). This alcohol has been associated with a banana aroma.[Citation19]
Other minor compounds such as terpenes (p-cymene, limonene, β-ocimene, and (E,Z) alloocimene), were also found in our EVOOs depending on the type of olive cultivar, although their influence on VOO sensory profile has not been much described in the literature. Limonene was present in all studied EVOOs and varied from 0.08 to 0.47 mg/L (). This compound plays a very important role in the fragrance of olive oil.[Citation25] Vichi et al.[Citation36] showed that the presence of monoterpenes could be used as a genetic or geographic markers of VOO. For instance, Zunin et al.[Citation37] observed that the presence of three terpenic compounds (α-copaene, α-muurolene, and α-farnesene) helped identifying EVOO produced in West Liguria from those produced in other Mediterranean regions.
The presence of 6-methyl-5-hepten-2-one, found only in the Chemlal cultivar (0.50 mg/L) () and mainly produced from the degradation of carotenoids, resulted in an active odor described as oily, herbaceous, and green.[Citation38] We also noted the presence of phenylethyl alcohol in Limli EVOO (0.72 mg/L) (). Both 6-methyl-5-hepten-2-one and phenylethyl alcohol are commonly present in olive oils.[Citation4,Citation30]
Furthermore, nonanal, a volatile compound associated with sensory defects, was found in our analyzed oils, but at relatively low concentrations (0.06–0.56 mg/L) (). The volatile originate from the oxidation of oleic acid which inevitably starts after the extraction of VOO, a process that it is also responsible for olive oil rancidity.[Citation25] Likewise, ethanol, a sugar fermentation marker, was present in three varieties (Abani, Bouricha, and Ferkani) at concentrations ranging from 0.81 to 2.22 mg/L (), although ethanol does not contribute to aroma owing to its high odor threshold. Two other volatiles potentially produced by microbial activity,[Citation39] were found in our EVOOs, namely benzaldehyde, found in Bouricha (0.66 mg/L) EVOO, and methyl salicylate, found in both Abani (0.35 mg/L) and Mekki (0.37 mg/L) EVOO (); the presence of those compounds in EVOO was previously reported by Kandylis et al.[Citation40]
Heptane was also found in Algerian EVOO samples, depending on the type of cultivar (data not shown). However, quantification tests of heptane showed a nonlinear trend in our calibration curve, indicating a competition phenomenon against other compounds during adsorption on SPME fiber. Indeed, Oliver-Pozo et al.[Citation41] highlighted anomalies in the volatiles’ markers quantification in highly odorant VOO due to competition phenomena between volatiles that are molecule-specific. Many literature authors semi-quantified heptane in Chemlal and Blanquette Algerian olive varieties,[Citation14] French VOO of the Cailletier variety[Citation26], and Tunisian VOO of Chemlali and Chétoui varieties.[Citation42] However, to the best of our knowledge, there is no literature precedent on the quantification of heptane in olive oil. Moreover, heptane is characterized by a very low odor threshold (0.67),[Citation42] so it is possible the compound contributes to whole aroma of olive oil. More work is therefore required to investigate the presence of this compound using SPME extraction method, in addition to its sensory contribution.
Effect of fruit ripening on volatile compounds of Chemlal EVOO
The effect of ripening stage on the volatile compounds of EVOO of Chemlal variety, handpicked at four different stages with MI = 3.00, 4.20, 5.50, and 6.20 was studied; the results are shown in and . In terms of the number of the identified volatile compounds, a total of 10 compounds were identified and quantified at the first maturity stage, whereas a total of 12 compounds were identified and quantified at the others maturity stages (). Furthermore, concentration of the total volatile fraction increased from 25.61 mg/L at the MI = 3.00 to reach a maximum value of 47.50 mg/L at the second maturity stage (MI = 4.20); beyond that point, the concentration decreased to 36.91 and 37.32 mg/L at MI = 5.50 and 6.20, respectively (). Likewise, the amount of C6 compounds and C6 aldehydes increased to reach a maximum concentration of 26.68 mg/L and 21.87 mg/L, respectively, at the second maturity stage (MI = 4.20) (, ). The latter stage corresponded to a fruit with a black skin and a white flesh. Our findings are different from those published by other researches, whereby the maximum concentration of C6 volatiles occurred at a time when the fruit’s skin color turned from yellow-green to purple[Citation5]; after this period, the amount of volatiles was inversely proportional to the degree of ripening due to the lower activity of enzymes involved in the production of this class of compounds[Citation5] and/or poor availability of the substrates.[Citation43] Additional studies will be required to confirm these hypotheses. Investigations with other agronomic conditions should be conducted.
Figure 2. Evolution of C6 aldehydes, C6 alcohols, and C6 esters (mg/L, mean of three replicates) in Chemlal EVOO at four stages of fruit maturity.
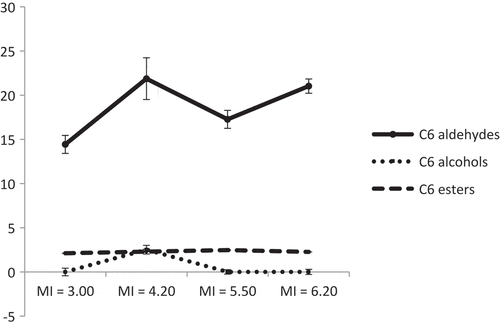
Within the same class of C6 aldehydes, the content of (E)-2-hexenal increased to 15.83 mg/L () at MI = 4.20; the amount of hexanal also increased during the ripening process. This trend was previously described by Gómez-Rico et al.[Citation27] for VOO obtained from Morisca, Picolimón, and Picudo cultivars. The authors described an increase in the amount of C6 volatiles, especially that of (E)-2-hexenal, at very early ripening stages (MI1 = 1.0–1.5 and MI2 = 2.3–3.0). However, VOO obtained from Arbequina, Picual, and Cornicabra cultivars, the amount of C6 aldehydes decreased at two different ripening stages (MI1 = 2.0–2.5 and MI2 = 3.5–4.0). Moreover, in other work by Gómez-Rico et al.[Citation44] for EVOO obtained from rainfed and irrigated olive groves of the Cornicabra variety, showed that the amounts of total volatiles, C6 aldehydes fraction, (E)-2-hexenal, and hexanal decreased throughout fruit maturation (MI between 2 and 5).
Analysis of C6 alcohols showed only the presence of (E)-2-hexen-1-ol at MI = 4.20 (). For C6 esters, the amounts of (Z)-3-hexen-1-yl acetate and hexyl acetate did not change that much throughout the different ripening stages (). Regardless of the ripening stage, C5 LOX pathway compounds were not detected in EVOO of this variety (). The content of terpenes differ at different olive ripening stages; the amounts of p-cymene and β-ocimene remained at similar levels during the different olive ripening stages while the concentration of limonene and (E,Z) alloocimene slightly increased with ripening degree (). α-Farnesene was detected only at latest ripening stages (MI = 5.50 and 6.20), a result which is similar to the one previously reported on Tunisian VOO.[Citation30] Moreover, Vichi et al.[Citation45] showed that the amount of acyclic sesquiterpenes with a farnesane skeleton increased as MI is increased, whereas the amount of bicyclic sesquiterpenes were decreased. The content of nonanal and (E)-2-heptenal increased over the course of MI (), a trend previously reported by Kandylis et al.[Citation40] studying olive oils derived from Cretan species.
Principal component analysis
PCA was performed in order to clarify the similarities and differences between samples. PCA was performed on 20 volatile compounds concentrations. The cumulative percentage variance described by PC1 and PC2 was 59.89%. Principal components 1 and 2 accounted for 44.95% and 14.94%, respectively. constituted the loading plot. Each quadrant contained at least one variable. The variables were more concentrated in the right part of the plane. A score plot map is shown in . Cultivars were clearly separated from each other; nevertheless, we were able to notice four groups. Hence, the first group was formed by Limli and Bouricha cultivar, characterized in this study by their presence in high numbers (≥10) and concentrations (≥26.86 mg/L) of volatile compounds, in addition to high levels of 1-hexanol and the exclusive presence of compounds like phenylethyl alcohol in Limli cultivar and benzaldehyde in Bouricha cultivar. The second one was formed by Aaleh, Abani, Mekki, and Ferkani, owing to their low numbers (≤8) and low concentrations (<15 mg/L) of volatile compounds. Chemlal was located in the fourth quadrant of PCA graph, clearly separated from the rest of cultivars. Chemlal cultivar is characterized by a high content of volatile compounds (25.61–47.50 mg/L) and absence of C5 LOX compounds. The cultivar Rougette de Mitidja is also separated from the other varieties; the latter is characterized by the presence of high concentration (34.24 mg/L) of volatile compounds and 1-penten-3-one compared to the remaining cultivars.
Figure 3. Principal component analysis of volatile compounds: (a) plot of component weights and (b) scatter plot of EVOOs samples: Aaleh, Abani, Bouricha, Chemlal, Ferkani, Limli, Mekki, and Rougette de Mitidja.
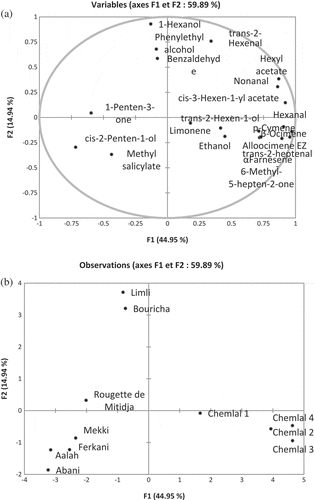
Our results are in agreement with previous works which reporting that the genetic characteristics are responsible for deciding the presence of different enzymes which are in turn responsible for the qualitative and quantitative composition of volatiles compound.[Citation9]
It is rewarding to see that samples of the Chemlal cultivar, collected at different ripening stages, are well grouped within the same region of the PCA graph ().
Contribution of volatile compounds to odor and flavor
No sensory panels were used in this study because the properties of many of the detected compounds are known from the literature. The influence of each volatile compound on odor and flavor depends on both concentration and odor threshold. It follows that small amounts of low odor threshold volatile compounds may have a greater sensory influence than huge amounts of high odor threshold compounds. Alcohols usually have high odor threshold. The aldehydes and ketones commonly have low odor and flavor thresholds.[Citation46] Consequently, carbonyl compounds have a much stronger influence on positive sensory properties, compared to alcohols. They contribute with fruit and vegetables notes with descriptions such as green/fruity/planty, and earthy; nonanal in particular has a fruity-like and plant-like aroma.[Citation46]
Conclusions
Our present study reports for the first time the composition of volatile compounds of Algerian monocultivar EVOOs, collected from Sidi-Aïch. The obtained results provided quantitative information of about 20 volatile compounds and demonstrated that the type of cultivar has a marked influence on the volatile profiles both quantitatively and qualitatively. Moreover, differences in volatile production where observed for Chemlal oils, handpicked at different harvesting dates. The amounts of volatile compounds and (E)-2-hexenal also increased, reaching a maximum concentration when olive fruits skin change color from purple to black. A better understanding on the key aroma compounds responsible for positive odor or defects is useful for improving the potential of autochthonous cultivars for the production of high quality monovarietal olive oil.
Supplemental Material
Download ()Acknowledgments
The authors wish to thank Prof. Margherita Bonanni for helpful support. We are grateful to the experimental installations of the Institut Technique d’Arboriculture Fruitière (ITAF) for plant material and oil extraction. We thank Dr. Sehailia Moussa for his advice.
Supplemental data
Supplemental data for this article can be access on the publisher’s website.
References
- Boskou, D. Epilogue. In Olive Oil: Minor Constituents and Health; Boskou, D., Ed.; CRC Press: Boca Raton, FL, 2009; 211–218.
- Kalua, C. M.; Bedgood, D. R., Jr; Bishop, A. G.; Prenzler, P. D. Flavour Quality Critical Production Steps from Fruit to Extra-Virgin Olive Oil at Consumption. Food Research International 2013, 54, 2095–2103. DOI:10.1016/j.foodres.2013.04.021.
- Kalua, C. M.; Allen, M. S.; Bedgood, D. R., Jr; Bishop, A. G.; Prenzler, P. D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: Acritical Review. Food Chemistry 2007, 100, 273–286. DOI:10.1016/j.foodchem.2005.09.059.
- Cecchi, T.; Alfei, B. Volatile Profiles of Italian Monovarietal Extra Virgin Olive Oils via HS-SPME–GC–MS: Newly Identified Compounds, Flavors Molecular Markers, and Terpenic Profile. Food Chemistry 2013, 141, 2025–2035. DOI:10.1016/j.foodchem.2013.05.090.
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. F. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. Journal of Chromatography 2004, 1054, 17–31. DOI:10.1016/S0021-9673(04)01298-1.
- Bendini, A.; Valli, E.; Barbieri, S.; Gallina Toschi, T. Sensory Analysis of Virgin Olive Oil. In Olive Oil Constituents, Quality, Health Properties and Bioconversions; Boskou, D., Ed.; InTech: Rijeka, Croatia, 2012; pp 109–130.
- D’Imperio, M.; Gobbino, M.; Picanza, A.; Costanzo, S.; Della Corte, A.; Mannina, L. Influence of Harvest Method and Period on Olive Oil Composition: An NMR and Statistical Study. Journal of Agricultural Food Chemistry 2010, 58, 11043–11051. DOI:10.1021/jf1026982.
- Fregapane, G.; Salvador, M. D. Production of Superior Quality Extra Virgin Olive Oil Modulating the Content and Profile of Its Minor Components. Food Research International 2013, 54, 1907–1914. DOI:10.1016/j.foodres.2013.04.022.
- Angerosa, F.; Bastin, C.; Vito, R. Virgin Olive Oil Volatile Compounds from Lipoxygenase Pathway and Characterization of Some Italian Cultivars. Journal of Agricultural and Food Chemistry 1999, 47, 836–839. DOI:10.1021/jf980911g.
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Lukić, I. Volatile Compounds and Sensory Profiles of Monovarietal Virgin Olive Oil from Buža, Črna and Rosinjola Cultivars in Istria (Croatia). Food Technology and Biotechnology 2012, 50, 192–198.
- Ben Mansour, A.; Flamini, G.; Ben Selma, Z.; Le Dréau, Y.; Artaud, J.; Abdelhedi, R.; Bouaziz, M. Olive Oil Quality is Strongly Affected by Cultivar, Maturity Index and Fruit Part: Chemometrical Analysis of Volatiles, Fatty Acids, Squalene and Quality Parameters from Whole Fruit, Pulp and Seed Oils of Two Tunisian Olive Cultivars. European Journal of Lipid Science and Technology 2015, 117, 976–987. DOI:10.1002/ejlt.201400159.
- Haddadi, M.; Yakoub-Bougdal, S. Olive Rootstock Production from Olea Europea Var. Chemlal Cultured In Vitro. Cahiers Agriculture 2010, 19, 288–291.
- International olive council (IOC). World Olive Oil Figures. http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures. Accessed 03 December 2016.
- Nigri, S.; Oumeddour, R.; Fernandez, X. Analysis of Some Algerian Virgin Olive Oils by Headspace Solid Phase Micro-Extraction Coupled to Gas Chromatography/Mass Spectrometry. La Ravista Italiana Delle Sostanze Grasse 2012, GrLXXXIX, 54–61.L54.
- Hermoso, M.; Uceda, M.; Frias, L.; Beltran, G. Maduración. In El Cultivo, del Olivo, Barranco, D., Fernandez-Escobar, R., Rallo, L., Eds.; MundiPrensa: Madrid, Spain, 1997; 137–153.
- Romero, I.; Garcia-Gonzalez, D. L.; Aparicoi-Ruiz, R.; Morales, M. T. Validation of SPME-GC-MS Method for the Analysis of Virgin Olive Oil Volatiles Responsible for Sensory Defects. Talanta 2015, 134, 394–401. DOI:10.1016/j.talanta.2014.11.032.
- Şengül, Ü.;. Comparing Determination Methods of Detection and Quantification Limits for Aflatoxin Analysis in Hazelnut. Journal of Food and Drug Analysis 2016, 24, 56–62. DOI:10.1016/j.jfda.2015.04.009.
- Evard, H.; Kruve, A.; Leito, I. Tutorial on Estimating the Limit of Detection Using LC-MS Analysis, Part I: Theoretical Review. Analytica Chimica Acta 2016, 942, 23–39. DOI:10.1016/j.aca.2016.08.043.
- Luna, G.; Morales, M. T.; Aparicio, R. Characterisation of 39 Varietal Virgin Olive Oils by their Volatile Compositions. Food Chemistry 2006, 98, 243–252. DOI:10.1016/j.foodchem.2005.05.069.
- Baccouri, O.; Guerfel, M.; Baccouri, B.; Cerretani, L.; Bendini, A.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Chemical Composition and Oxidative Stability of Tunisian Monovarietal Virgin Olive Oils with Regard to Fruit Ripening. Food Chemistry 2008, 109, 743–754. DOI:10.1016/j.foodchem.2008.01.034.
- Bajoub, A.; Sánchez-Ortiz, A.; Ajal, E. A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Beltrán, G.; Carrasco-Pancorbo, A. First Comprehensive Characterization of Volatile Profile of North Moroccan Olive Oils: A Geographic Discriminant Approach. Food Research International 2015, 76, 410–417. DOI:10.1016/j.foodres.2015.05.043.
- Sánchez-Ortiz, A.; Pérez, A. G.; Sanz, C. Cultivar Differences on Non Esterified Polyunsaturated Fatty Acid as a Limiting Factor for the Biogenesis of Virgin Olive Oil Aroma. Journal of Agricultural and Food Chemistry 2007, 55, 7869–7873. DOI:10.1021/jf071202i.
- Pouliarekou, E.; Badeka, A.; Tasioula-Margari, M.; Kontakos, S.; Longobardi, F.; Kontominas, M. G. Characterization and Classification of Western Greek Olive Oils according to Cultivar and Geographical Origin Based on Volatile Compounds. Journal of Chromatography 2011, 1218, 7534–7542. DOI:10.1016/j.chroma.2011.07.081.
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C. I. G.; Jerković, I. Phytochemicals and Other Characteristics of Croatian Monovarietal Extra Virgin Olive Oils from Oblica, Lastovka and Levantinka Varieties. Molecules 2015, 20, 4395–4409. DOI:10.3390/molecules20034395.
- Vichi, S.; Pizzale, L.; Conte, S. L.; Buxaderas, S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Modifications Induced by Oxidation and Suitable Markers of Oxidative Status. Journal of Agricultural and Food Chemistry 2003, 51, 6564–6571. DOI:10.1021/jf030268k.
- Cavalli, J. F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A. M. Characterization of Volatile Compounds of French and Spanish Virgin Olive Oils by HS-SPME: Identification of Quality-Freshness Markers. Food Chemistry 2004, 88, 151–157. DOI:10.1016/j.foodchem.2004.04.003.
- Gómez-Rico, A.; Fregapane, G.; Desamparados Salvador, M. Effect of Cultivar and Ripening on Minor Components in Spanish Olive Fruits and Their Corresponding Virgin Olive Oils. Food Research International 2008, 41, 433–440. DOI:10.1016/j.foodres.2008.02.003.
- Campus, M.; Sedda, P.; Delpiano, D.; Secci, S.; Damasco, G.; Zurru, R.; Bandino, G. Variability in Composition, Sensory Profiles and Volatile Compounds of Sardinian Monovarietal Virgin Olive Oils Grown in Different Area. Ravista Italiana Delle Sostanze Grasse 2013, xc, 237–248.
- Peres, F.; Jelen, H. H.; Majcher, M. M.; Arraias, M.; Martins, L. L.; Ferreira-Dias, S. Characterization of Aroma Compounds in Portuguese Extra Virgin Olive Oils from Galega Vulgar and Cobrançosa Cultivars Using GC-O and GC × GC-ToF MS. Food Research International 2013, 54, 1979–1986. DOI:10.1016/j.foodres.2013.06.015.
- Hachicha Hbaieb, R.; Kotti, F.; Gargouri, M.; Msallem, M.; Vichi, S. Ripening and Storage Conditions of Chétoui and Arbequina Olives: Part I. Effect on Olive Oils Volatiles Profile. Food Chemistry 2016, 203, 548–558. DOI:10.1016/j.foodchem.2016.01.089.
- Dierkes, G.; Bongartz, A.; Guth, H.; Hayen, H. Quality Evaluation of Olive Oil by Statistical Analysis of Multicomponent Stable Isotope Dilution Assay Data of Aroma Active Compounds. Journal of Agricultural and Food Chemistry 2011, 60, 394–401. DOI:10.1021/jf203406s.
- Angerosa, F.; D’Alessandro, N.; Basti, C.; Vito, R. Biogeneration of Volatile Compounds in Virgin Olive Oil: Their Evolution in Relation to Malaxation Time. Journal of Agricultural and Food Chemistry 1998, 46, 2940–2944. DOI:10.1021/jf970641m.
- Salas, J. J.;. Characterization of Alcohol Acyltransferase from Olive Fruit. Journal of Agricultural and Food Chemistry 2004, 52, 3155–3158. DOI:10.1021/jf035433a.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Improvements in the Malaxation Process to Enhance the Aroma Quality of Extra Virgin Olive Oils. Food Chemistry 2014, 158, 534–545. DOI:10.1016/j.foodchem.2014.02.140.
- Angerosa, F.; Mostallino, R.; Basti, C.; Raffaella, V. Virgin Olive Oil Odour Notes: Their Relationships with Volatile Compounds from the Lipoxygenase Pathway and Secoiridoid Compounds. Food Chemistry 2000, 68, 283–287. DOI:10.1016/S0308-8146(99)00189-2.
- Vichi, S.; Guadayol, J. M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Monoterpene and Sesquiterpene Hydrocarbons of Virgin Olive Oil by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography/Mass Spectrometry. Journal of Chromatography 2006, 1125, 117–123. DOI:10.1016/j.chroma.2006.05.029.
- Zunin, P.; Boggia, R.; Salvadeo, P.; Evangelisti, F. Geographical Traceability of West Liguria Extra Virgin Olive Oils by the Analysis of Volatile Terpenoid Hydrocarbons. Journal of Chromatography 2005, 1089, 243–249.O. DOI:10.1016/j.chroma.2005.07.005.
- Silva, L. R.; Azevedo, J.; Pereira, M. J.; Valentao, P.; Andrade, P. B. Chemical Assessment and Antioxidant Capacity of Pepper (Capsicum Annuum L.) Seeds. Food and Chemical Toxicology 2013, 53, 240–248. DOI:10.1016/j.fct.2012.11.036.
- Sinesio, F.; Moneta, E.; Raffo, A.; Lucchetti, S.; Peparaio, M.; D’Aloise, A.; Pastore, G. Effect of Extraction Conditions and Storage Time on the Sensory Profile of Monovarietal Extra Virgin Olive Oil (cv Carboncella) and Chemical Drivers of Sensory Changes. Food Science and Technology 2015, 63, 1–8.
- Kandylis, P.; Vekiari, A. S.; Kanellaki, M.; Grati Kamoun, N.; Msallem, M.; Kourkoutas, Y. Comparative Study of Extra Virgin Olive Oil Flavor Profile of Koroneiki Variety (Olea Europaea Var. Microcarpa Alba) Cultivated in Greece and Tunisia during One Period of Harvesting. Food Science and Technology 2011, 44, 1333–1341.
- Oliver-Pozo, C.; Aparicio-Ruiz, R.; Romero, I.; García-González, D. L. Analysis of Volatile Markers for Virgin Olive Oil Aroma Defects by SPME-GC/FID: Possible Sources of Incorrect Data. Journal of Agricultural and Food Chemistry 2015, 63, 10477–10483. DOI:10.1021/acs.jafc.5b03986.
- Tena, N.; Lazzez, A.; Aparicio-Ruiz, R.; Garcia-González, D. L. Volatile Compounds Characterizing Tunisian Chemlali and Chétoui Virgin Olive Oils. Journal of Agricultural and Food Chemistry 2007, 55, 7852–7858. DOI:10.1021/jf071030p.
- Salas, J. J.; Sánchez, J. The Decrease of Virgin Oil Flavour Produced by High Malaxation Temperature is due to Inactivation of Hydroperoxideslyase. Journal of Agricultural and Food Chemistry 1999, 47, 809–812. DOI:10.1021/jf981261j.
- Gómez-Rico, A.; Desamparados Salvador, M.; La Greca, M.; Fregapane, G. Phenolic and Volatile Compounds of Extra Virgin Olive Oil (Olea Europaea L. Cv. Cornicabra) with Regard to Fruit Ripening and Irrigation Management. Journal of Agricultural and Food Chemistry 2006, 54, 7130–7136. DOI:10.1021/jf060798r.
- Vichi, S.; Lazzez, A.; Grati Kamoun, N.; López-Tamames, E.; Buxaderas, S. Evolution of Sesquiterpene Hydrocarbons in Virgin Olive Oil during Fruit Ripening. Journal of Agricultural and Food Chemistry 2010, 58, 6972–6976. DOI:10.1021/jf100497c.
- Alasalvar, C.; Taylor, K. D. A.; Shaidi, F. Comparison of Volatiles of Cultured and Wild Seabream (Sparusaurata) during Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography–Mass Spectrometry. Journal of Agricultural and Food Chemistry 2005, 53, 2616–2622. DOI:10.1021/jf0483826.
