ABSTRACT
Mints (Mentha species) are widely used as food, medicine, spice, and flavoring agents. At the present work, phenolics profile of infusion and ethanol extract of Mentha longifolia was determined using an RP–HPLC–DAD system. Total bioactive contents, radical scavenging, reducing power, metal chelating, and enzyme inhibitory activities relevant to Alzheimer’s disease, diabetes mellitus, and skin disorders were evaluated. Sixteen phenolic compounds (ten phenolic acids and six flavonoids) were identified in the extracts in which sinapic acid (7132 µg/g extract) and rosmarinic acid (6260 µg/g extract) were the most abundant compounds. Strong antioxidant effects were observed in 1,1-diphenyl-2-picrylhydrazyl radical, 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), cupric ion reducing activity, ferric reducing antioxidant power, phosphomolybdenum, and metal chelating assays. Results indicated selective acetylcholinesterase inhibitory activity and high α-amylase and α-glucosidase inhibitory potential. Findings showed that M. longifolia has promising health benefits due to its high concentration of useful phenolic compounds and has great potential for possible applications in the preparation of functional ingredients.
Introduction
The genus Mentha L. (Mentheae, Nepetoideae) belonging to the Lamiaceae family is consisted of mostly perennial aromatic herbs with invasive creeping rhizomes frequently living in wet habitations.[Citation1,Citation2] According to the plant list database (http://www.theplantlist.org), this genus contains around 56 accepted taxa including 42 species distributed worldwide especially in temperate and subtemperate regions. Mentha represents in Iran by seven species including Mentha longifolia.[Citation3] These plants, because of their multiple utilities, are very popular for humankind since antiquity. Mentha species are of big economic value today and largely consumed in pharmaceutical, beverage, confectionary, culinary, perfume, food, cosmetic, and tobacco industries.[Citation4–Citation6]
Mints have been using traditionally for centuries as flavoring, condiment, herbal tea, fresh vegetable, infusion, decoction, and distillate.[Citation7,Citation8] They are also used as breath freshener, carminative, choleretic, anti-infective, anti-inflammatory, antiemetic, diaphoretic, antispasmodic, analgesic, stimulant, emmenagogue, anticatarrhal, diuretic, antiallergic, stomach tonic, and insecticidal agents.[Citation9,Citation10] Accordingly, fresh, dried, and processed mints are empirically used in herbal medicine for treatment of several health problems and discomforts like gastric acidities, aerophagia, nausea, flatulence, vomiting, ulcerative colitis, stomach aches, dyspepsia, diarrhea, jaundice, liver complaints, anorexia, cramps, bronchitis, throat infections, and viral hepatitis.[Citation11,Citation12]
The genus Mentha is globally a phenomenal source of essential oil production. Beside the widely growing populations, Mentha piperita, Mentha spicata, and Mentha arvensis are vastly cultivated worldwide, mainly for their essential oil yield.[Citation13,Citation14] Mentha species are also rich in phenolic compounds, especially in phenolic acids and flavonoids.[Citation15,Citation16] Generally, biologic activities of mint species mostly originate from their phenolics and essential oil components.[Citation17,Citation18]
M. longifolia known as hors or wild mint is a sharply scented widespread species, breeding multi-flowered spike-shaped inflorescences. M. longifolia is a well-known overconsumed medicinal plant.[Citation3] It is used to provide household spices, infusion, additive, food, decoction, and distillate.[Citation19] Its pungent scent could repel mosquito and also keep the rodents away from the stored grains. Several bioactivities such as antibacterial, antifungal, antioxidant, anticandidal, pesticidal, insecticidal, antimutagenic, anticancer, calcium channel blocking, cyclooxygenase, and HIV 1 inhibitory properties have been reported for this species. Also, some remedial effects namely sedative, spasmolytic, diaphoretic, antipruritic, carminative, antiseptic, choleretic, central nervous system stimulant, antihistamine, diuretic, and hepatoprotective traits of M. longifolia have been verified.[Citation20–Citation25] Chemically variable M. longifolia, based on the morphologically discriminating characteristics, is subdivided into nine varieties in Iran, including var. calliantha (Stapf) Briq. The later taxon of wild mint is nominated in the Flora of Iran as an endemic plant, but it occurs in eastern Anatolia too.
To the best of our knowledge, there is not any information about the phytoconstituents and biological activities of M. longifolia var. calliantha (Stapf) Briq. Accordingly, at the present work, the phenolics profile of infusion and ethanolic extract of this plant are examined to find its bioactive metabolites. In addition, to explore its health benefits, several biological activities of M. longifolia such as radical scavenging, reducing power, metal chelating, and its enzyme inhibitory properties relevant to diabetes mellitus (DM), skin disorders, and Alzheimer’s disease (AD) are investigated.
Materials and methods
Plant material
The aerial plant parts including flowers, leaves, and juvenile stems were collected during flowering stage in early spring 2015 from Urmia, West Azerbaijan province of Iran, and authenticated by Mr. Shahram Bahadori (taxonomist) as M. longifolia var. calliantha (Stapf) Briq. In addition, a voucher specimen was deposited in Herbarium of Urmia Pharmacy School (HUPS-177), Urmia, Iran.
Preparation of extracts
The ethanol extract of the aerial parts of M. longifolia was obtained using maceration method. An amount of 50 g crushed dried material was extracted using 500 mL of EtOH. The extraction was performed by shaking at room temperature during 72 h. Finally, the extract was passed through a paper filter and the filtrated solution was concentrated by a rotary evaporator at 40°C. For preparation of the infusion, 50 g of the plant material was suspended in 500 mL of hot distilled water. The mixture was shaken for 10 min and the extract was centrifuged and filtered using paper filter. The obtained solution was lyophilized and stored in −18°C until analysis.
Profiling of phenolic compounds by RP–HPLC–DAD
Phenolic metabolites were analyzed using RP–HPLC–DAD (Shimadzu Scientific Instruments, Kyoto, Japan). Separation procedure was carried out at 30°C on Eclipse XDB C-18 reversed-phase column (250 mm × 4.6 mm length, 5 µm particle size, Agilent, Santa Clara, CA, USA). The eluates were detected at 278 nm. The phenolic compounds of the ethanolic and water extracts were determined using a previously modified method.[Citation26] Twenty-three standard phenolic compounds were used for the analysis including gallic acid, protocatechuic acid, catechin, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, epicatechin, syringic acid, vanillin, p-coumaric acid, ferulic acid, sinapic acid, benzoic acid, o-coumaric acid, rutin, hesperidin, rosmarinic acid, eriodictyol, cinnamic acid, quercetin, luteolin, kaempferol, and apigenin. Retention times, UV spectra, and comparison with commercial standard compounds were used for the characterization and quantitative analysis of phenolic compounds. The results were expressed as µg/g of dry extracts using external calibration curves.
Total phenolic content and total flavonoid content
The total phenolic content (TPC) of the extracts was determined using Folin–Ciocalteu method with slight modification [Citation27] and expressed as gallic acid equivalents (GAEs/g sample). Briefly, 20 μL of sample solution in methanol (2 mg/mL) was mixed with 100 μL of Folin–Ciocalteu reagent (1:10). After 6 min, 80 μL sodium carbonate 7.5% is added into the reaction mixture. The absorbance is measured at 740 nm after 2 h of incubation in the dark at 25°C. The TPC of the extracts is expressed as mg of gallic acid equivalents per g of samples (mg GAE/g) through the calibration curve with gallic acid. Total flavonoid content is determined using AlCl3 method with some modifications[Citation28] and the results are expressed as rutin equivalents (REs/g sample). In brief, 20 μL of mint samples or a standard solution of rutin (1–200 μg/mL) is diluted using 60 μL of MeOH and 10 μL of AlCl3 (5%). Afterward, 10 μL of 0.5 M potassium acetate is added and the total volume is made up to 200 μL by distilled water. The solution is well and the absorbance read at 415 nm after 30 min. All assays were carried out in triplicate, and mean values of flavonoid content were expressed as mg of rutin equivalents per g of extracts calculated according to the standard calibration curve.
Total antioxidant activity
The total antioxidant activity of the plant samples was evaluated by phosphomolybdenum method with slight modification.[Citation29] The results are expressed as trolox equivalents as standard antioxidant compound (mmol TEs/g extract). In this direction, sample solution (0.3 mL) was combined with 3 mL of reagent solution including 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate. The sample absorbance was read at 695 nm after 90 min incubation at 95°C. Total antioxidant capacity is expressed as equivalents of trolox as determined by the equation of the standard trolox curve.
Radical scavenging activity
The radical scavenging activities of the extracts against 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and also on 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS) were estimated.[Citation30–Citation32] Results were expressed as mean ± SD of trolox equivalents from three independent experiments (mg TEs/g extract). Twenty microliters of samples or standard antioxidant butylated hydroxy toluene (BHT), which is dissolved in methanol in several concentrations, are mixed with 180 μL of 0.1 mM DPPH solution. Discoloration of mixtures is measured at 517 nm after 30 min. In ABTS assay, ABTS+ radical cation is produced directly by reaction of 7 mM ABTS solution with 2.45 mM potassium persulfate in 12–16 h in dark at the room temperature. Prior to beginning the assay, ABTS solution was diluted with methanol to an absorbance of 0.700 ± 0.02 at 734 nm. Sample solution (1 mL) was added to ABTS solution (2 mL) and mixed. The sample absorbance was read at 734 nm after 30 min incubation at room temperature. The ABTS radical cation scavenging activity was expressed as equivalents of trolox, according to the equation obtained from the standard trolox curve.
Reducing power activity
The cupric ion reducing activity (CUPRAC) and the ferric reducing antioxidant power (FRAP) assays were determined according to the previously published methods with some modifications.[Citation33,Citation34] The results of both CUPRAC and FRAP assays were expressed as equivalents of trolox (mg TEs/g sample). For CUPRAC test, sample solution (0.5 mL) was added to reaction mixture containing CuCl2 (1 mL, 10 mM), neocuproine (1 mL, 7.5 mM), and NH4Ac buffer (1 mL, 1 M, pH 7.0). Similarly, a blank was prepared by adding sample solution (0.5 mL) to reaction mixture (3 mL) without CuCl2. Afterward, the sample and blank absorbances were read at 450 nm after 30 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. CUPRAC activity was expressed as equivalents of trolox according to the equation obtained from the standard trolox graph. In FRAP assay, sample solution (0.1 mL) was added to premixed FRAP reagent (2 mL) containing acetate buffer (0.3 M, pH 3.6), 2,4,6-tris(2-pyridyl)-s-triazine (10 mM) in 40 mM HCl and ferric chloride (20 mM) in a ratio of 10:1:1 (v/v/v). Then, the sample absorbance was read at 593 nm after a 30-min incubation at room temperature. FRAP activity was expressed as equivalents of trolox according to the equation obtained from the standard trolox graph.
Metal chelating activity
The metal chelating activity of plant samples was estimated using ferrous ions chelating method according to a previously described procedure.[Citation34] The metal chelating activity values were expressed as equivalents of EDTA (mg EDTAE/g). Briefly, sample solution (2 mL) was added to FeCl2 solution (0.05 mL, 2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Similarly, a blank was prepared by adding sample solution (2 mL) to FeCl2 solution (0.05 mL, 2 mM) and water (0.2 mL) without ferrozine. Then, the sample and blank absorbances were read at 562 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The metal chelating activity was expressed as equivalents of EDTA according to the equation obtained from the standard EDTA graph.
Enzyme inhibitory activity
Enzyme inhibitory properties of M. longifolia were investigated using ELISA plate reader against α-glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20, Sigma) and α-amylase (ex-porcine pancreas, EC 3.2.1.1, Sigma) as acarbose equivalents (mmol ACEs/g extract), acetylcholinesterase (AChE) (electric ell AChE, Type-VI-S, EC 3.1.1.7, Sigma) and butyrylcholinesterase (BChE) (horse serum BChE, EC 3.1.1.8, Sigma) as galantamine equivalents (mg GALAEs/g extract), and tyrosinase (from mushroom, EC 1.14.18.1, Sigma) as kojic acid equivalents (mg KAEs/g extract) using previously published methods.[Citation35]
Statistical analysis
All experiments were carried out in triplicate. The results are expressed as mean value ± standard deviation (SD). Data analysis was performed using SPSS v.16.0. Differences between means were determined by one-way analysis of variance (ANOVA) followed by Duncan’s post hoc test for multiple comparisons with control. A value of p < 0.05 was considered as indicative of statistical significance.
Results and discussion
Phenolics profiling
Phenolic compounds are one the most abundant natural products in foods. They occupy the major portion of phytochemicals in plants. In the last decades, these compounds have received a great attention from the scientific community against public health problems due to their antioxidant potential and their role in management of several disorders such as diabetes, cancer, neurodegeneration, and cardiovascular diseases.
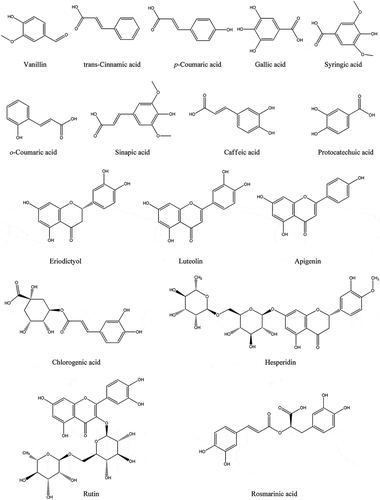
At the present work, a comparative RP–HPLC–DAD analysis was performed between ethanolic extract and infusion of M. longifolia as a common medicinal herb. In this direction, 23 standard phenolic compounds were used in analysis which revealed the presence of 16 compounds (10 phenolic acids and 6 flavonoids) in the tested samples. Comparative study shows that both of ethanolic extract and infusion of M. longifolia share the presence of all phenolic compounds but with different amounts. lists the concentration of phenolic compounds, belonging to the different classes of polyphenols, measured by RP–HPLC–DAD system in both infusion and ethanolic extract of M. longifolia. The structures of the identified phenolics are shown in . Sinapic acid and rosmarinic acid are the most abundant phenolic component in both of the samples, followed by hesperidin and o-coumaric acid. Previous studies on Mentha species indicated the presence of several phenolic compounds in the genus like caffeic acid, rosmarinic acid, and diosmin in M. spicata, M. pulegium, and M. rotundifolia.[Citation36] Elansary and coworkers investigated phenolics compound of four Mentha species and reported rosmarinic acid and caffeic acid as the major metabolites.[Citation37] Phenolics profiling of M. australis, an Australian native mint, revealed the presence of neoponcirin, rosmarinic acid, narirutin, chlorogenic acid, biochanin, caffeic acid, apigenin, hesperetin, and naringenin.[Citation38] Phenolic compounds of M. spicata were analyzed by UHPLC-ESI–MSn. The analysis suggested that this species is rich in rosmarinic acid and its derivatives, salvianolic acids, hydroxybenzoic acids, caffeoylquinic acids, hydroxycinnamic acids, flavanones, and flavones.[Citation39] According to our literature review and also the results of this work, Mentha species are rich in phenolic compounds and especially phenolic acids. Moreover, it seems that rosmarinic acid is the most abundant phenolic compound in the genus.
Table 1. Quantitative analysis for determination of phenolic components in the infusion and ethanol extract of M. longifolia.
Total phenolic and flavonoid contents
Phenolic and flavonoid compounds exert vital bioactivities in the prevention and/or treatment of several public health problems such as diabetes, atherosclerosis, Parkinson’s disease, cancer, and AD. Total phenolic and flavonoid contents of the infusion and ethanol extract of M. longifolia are presented in . The infusion is contained higher TPC (92.38 mg GAEs/g extract) than ethanol extract (67.05 mg GAEs/g). Similarly, it has higher total flavonoid content (46.18 mg REs/g extract) than ethanol extract (23.68 mg REs/g). This is in accordance with HPLC analysis in which the concentrations of phenolics in infusion generally are more than those in ethanol extract (). High concentration of phenolic and flavonoid compounds in M. longifolia indicates that it has strong potential health benefits. This is in agreement with previous work which showed the presence of high phenolic and flavonoid content in M. longifolia and other Mentha species.[Citation37]
Table 2. Total bioactive compounds and total antioxidant activity of M. longifolia.
Antioxidant activity
Accumulation of free radicals results in oxidative stress in the human body and, as a consequence, leads to several diseases such as cancer, diabetes, cardiovascular, and neurodegenerative disorders.[Citation40,Citation41] Moreover, synthetic and commercial antioxidant compounds such as Butylated hydroxyanisole (BHA) and BHT have some side effects like liver damage and gastrointestinal disorders. The antioxidant capacity of plant extracts or essential oils should be measured using several different methods. Each of these assays is based on different mechanisms. In fact, utilizing just one method could not explore the antioxidant ability of natural extracts because of their complex composition. Accordingly, antioxidant activity of natural products should be investigated using several different assays to get accurate and comparable data. In this direction, single electron transfer, hydrogen atom transfer, and metal chelating are the major mechanisms in which the antioxidants could deactivate the oxidative process.
To this end, at the present study, the antioxidant effects of infusion and ethanol extract of M. longifolia were evaluated using six different methods including radical scavenging (DPPH and ABTS), metal ion reducing (FRAP and CUPRAC), total antioxidant (phosphomolybdenum), and metal chelating assays. Generally, both the plant samples showed strong radical scavenging activities (). Also, they indicated remarkable metal ion reducing power (). Fe2+ ions could create the hydroxyl radicals and then initiate lipid peroxidation which leads to serious health damages. Promising total antioxidant activity () and moderate metal chelating activities () were also observed for tested samples. In comparison, in all of the antioxidant assays, infusion sample exhibited higher antioxidant activity than ethanol extract. This observation could be easily interpreted according to their total phenolics and total flavonoids contents, and also individual phenolics concentration. As noted above, the concentration of phenolic acids and flavonoids (well-known antioxidant compounds) in infusion is higher than ethanol extract. Phenolic compounds could reduce oxidative damages in body through scavenging free radicals, chelating, and reducing metal ions which are responsible to formation of reactive oxygen species (ROS). Previous studies on several mints showed that infusion and methanolic extracts have strong antioxidant activities in DPPH assay with IC50 values ranging from 9.3 to 14.8 µg/mL.[Citation37] In view of phytochemicals of Mentha species, flavonoids and phenolic acids are the main classes of natural compounds found in the extracts and infusions of Mentha plants.[Citation8,Citation17] So, these secondary metabolites could be responsible for observed antioxidant potential of M. longifolia. These findings are in agreement with previous works on the genus Mentha.[Citation42–Citation44] According to relatively high amounts of phenolic compounds in M. longifolia and their antioxidant potential which were confirmed using several in vitro assays, this species could be considered for possible applications in food industries.
Table 3. Radical scavenging activity of infusion and ethanol extract of M. longifolia.
Table 4. Reducing power and metal chelating activity of the infusion and ethanol extract of M. longifolia.
Therapeutic target enzymes inhibitory activity
There are several disorders, in which inhibition of key enzymes activity is considered as an effective strategy in treatment of public health problems. In this regard, screening of natural products for their enzyme inhibitory activities is an important method to discovery of new drugs. The prevalence of AD and DM is rising and it is estimated to increase significantly in the following decades. In this direction, at the present study, inhibitory activity of infusion and ethanol extract of M. longifolia (a common functional tea) against key enzymes involved in AD, DM, and skin disorders were investigated.
Cholinesterases inhibition
AChE and BChE inhibitors are effective drugs for management of AD which is a neurodegenerative disorder.[Citation45,Citation46] As the world population ages, it is necessary more and more to find treatments for AD.[Citation47] Both plant samples showed moderate AChE inhibitory potential (1.32 and 1.25 mg GALAEs/g sample for infusion and ethanol extract, respectively) but they were inactive against BChE in tested concentration (). Results indicated that active compounds in these samples could be selective inhibitors of AChE. This is in agreement with a previous work in which several extracts from Mentha species showed selective AChE inhibitory activity and Linarin (acacetin-7-O-β-d-rutinoside) was identified as the active compound from the flower extract of M. arvensis.[Citation48] There are some evidences in the literature showing the potential of dietary phenolics for treatment of AD.[Citation49] So, phenolic metabolites (especially phenolic acids) of M. longifolia may be responsible for observed AChE inhibitory activity.
Table 5. Enzyme inhibitory activities of the infusion and ethanol extract of M. longifolia.
α-Amylase and α-glucosidase inhibition
α-Amylase and α-glucosidase inhibitors may be considered as glucose level reducing drugs for the treatment of DM. These enzymes catalyze carbohydrates to their monomers and increase the glucose level of blood. There are several drugs for treatment of type 2 diabetes in the market such as metformin, acarbose, and miglitol. These drugs have some negative effects on human health. So, discovery of new and natural effective antidiabetic drugs with more activity and less side effects is warranted. In this work, M. longifolia was investigated for its antidiabetic potential for the first time. Infusion and ethanol extract of the plant exhibited moderate α-amylase inhibitory activity and strong α-glucosidase inhibitory potential (). In view of active compounds, phenolic acids and flavonoids are well known for antidiabetic potential due to their enzyme inhibitory effects.[Citation26,Citation50,Citation51]
Tyrosinase inhibition
The infusion and ethanol extract of M. longifolia were evaluated for their tyrosinase inhibitory activity in vitro (). None of the tested samples showed enzyme inhibitory potential in test concentrations.
Conclusion
Wild mint (M. longifolia) is a useful functional food. At the present study, M. longifolia var. calliantha (Stapf) Briq. was investigated for its phytochemicals and functional properties for the first time. To this end, infusion and ethanol extract of this herb were evaluated for their enzyme inhibitory and antioxidant activities comprehensively. Antioxidant, antidiabetic, and neuroprotective effects of wild mint were confirmed using in vitro assays. HPLC analysis showed the presence of phenolic compounds with health benefits in the herb as responsible bioactive components. Findings indicated that M. longifolia has great nutritional value and thus, it could be considered as a source of natural agents for designing new functional ingredients. However, further pharmacological and phytochemical investigations are essential to establish solid grounds for future utilization of this herb in food and nutraceutical industries.
Declaration of interest
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Celenk, S.; Tarimcilar, G.; Bicakci, A.; Kaynak, G.; Malyer, H. A Palynological Study of the Genus Mentha L. (Lamiaceae). Botanical Journal of the Linnean Society 2008, 157(1), 141–154.
- Gobert, V.; Moja, S.; Colson, M.; Taberlet, P. Hybridization in the Section Mentha (Lamiaceae) Inferred from AFLP Markers. American Journal of Botany 2002, 89(12), 2017–2023.
- Jamzad, Z. Flora of Iran: Lamiaceae; Tehran: Research Institute of Forests and Rangelands, 2012.
- Mkaddem, M.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical Composition and Antimicrobial and Antioxidant Activities of Mentha (Longifolia L. And Viridis) Essential Oils. Journal of Food Science 2009, 74(7), M358–M363.
- Baser, K.; Kürkçüoglu, M.; Tarimcilar, G.; Kaynak, G. Essential Oils of Mentha Species from Northern Turkey. Journal of Essential Oil Research 1999, 11(5), 579–588.
- Ali, M. S.; Saleem, M.; Ahmad, W.; Parvez, M.; Yamdagni, R. A Chlorinated Monoterpene Ketone, Acylated β-sitosterol Glycosides and A Flavanone Glycoside from Mentha Longifolia (Lamiaceae). Phytochemistry 2002, 59(8), 889–895.
- Džamić, A. M.; Soković, M. D.; Ristić, M. S.; Novaković, M.; Grujić-Jovanović, S.; Tešević, V.; Marin, P. D. Antifungal and Antioxidant Activity of Mentha Longifolia (L.) Hudson (Lamiaceae) Essential Oil. Botanica serbica 2010, 34(1), 57–61.
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of Polyphenols in Mentha Longifolia and M. Piperita Field-Grown and in Vitro Plant Samples Using UPLC-TQ-MS. Journal of AOAC International 2011, 94(1), 43–50.
- Naghibi, F.; Mosaddegh, M.; Mohammadi Motamed, M.; Ghorbani, A. Labiatae Family in Folk Medicine in Iran: From Ethnobotany to Pharmacology. Iranian Journal of Pharmaceutical Research 2010, 4(2), 63–79.
- Zeinali, H.; Arzani, A.; Razmjoo, K.; Rezaee, M. B. Evaluation of Oil Compositions of Iranian Mints (Mentha Ssp.). Journal of Essential Oil Research 2005, 17(2), 156–159.
- Younis, Y. M.; Beshir, S. M. Carvone-Rich Essential Oils from Mentha Longifolia (L.) Huds. Ssp. Schimperi Briq. And Mentha Spicata L. Grown in Sudan. Journal of Essential Oil Research 2004, 16(6), 539–541.
- Sharopov, F. S.; Sulaimonova, V. A.; Setzer, W. N. Essential Oil Composition of Mentha Longifolia from Wild Populations Growing in Tajikistan. Journal of Medicinally Active Plants 2012, 1(2), 6.
- Pal Singh, H.; Batish, D. R.; Mittal, S.; Dogra, K. S.; Yadav, S.; Kohli, R. K. Constituents of Leaf Essential Oil of Mentha Longifolia from India. Chemistry of natural compounds 2008, 44(4), 528–529.
- Hajlaoui, H.; Trabelsi, N.; Noumi, E.; Snoussi, M.; Fallah, H.; Ksouri, R.; Bakhrouf, A. Biological Activities of the Essential Oils and Methanol Extract of Tow Cultivated Mint Species (Mentha Longifolia and Mentha Pulegium) Used in the Tunisian Folkloric Medicine. World Journal of Microbiology and Biotechnology 2009, 25(12), 2227–2238.
- Idrissi, A. I.; Fkih-Tetouani, S. Phytochemical Study of Mentha Longifolia of Morocco. Fitoterapia 2001, 72(5), 596–598.
- Mimica-Dukiç, N.; Jakovljeviç, V.; Mira, P.; Gasiç, O.; Szabo, A. Pharmacological Study of Mentha Longifolia Phenolic Extracts. International journal of pharmacognosy 1996, 34(5), 359–364.
- Orhan, F.; Barış, Ö.; Yanmış, D.; Bal, T.; Güvenalp, Z.; Güllüce, M. Isolation of Some Luteolin Derivatives from Mentha Longifolia (L.) Hudson Subsp. Longifolia and Determination of Their Genotoxic Potencies. Food chemistry 2012, 135(2), 764–769.
- Elansary, H. O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E. A.; Skalicka-Woźniak, K. Enhancing Mint and Basil Oil Composition and Antibacterial Activity Using Seaweed Extracts. Industrial Crops and Products 2016, 92, 50–56.
- Mastelic, J.; Jerkovic, I. Free and Glycosidically Bound Volatiles of Mentha Longifolia Growing in Croatia. Chemistry of natural compounds 2002, 38(6), 561–564.
- Al-Bayati, F. A. Isolation and Identification of Antimicrobial Compound from Mentha Longifolia L. Leaves Grown Wild in Iraq. Annals of clinical microbiology and antimicrobials 2009, 8(1), 1.
- Durrani, F.; Sultan, A.; Marri, M. L.; Chand, N.; Durrani, Z. Effect of Wild Mint (Mentha Longifolia) Infusion on the over All. Pakistan Journal of Biological Sciences 2007, 10(7), 1130–1133.
- Khani, A.; Asghari, J. Insecticide Activity of Essential Oils of Mentha Longifolia, Pulicaria Gnaphalodes and Achillea Wilhelmsii against Two Stored Product Pests, the Flour Beetle, Tribolium Castaneum, and the Cowpea Weevil, Callosobruchus Maculatus. Journal of Insect Science 2012, 12(1), 73.
- Rasooli, I.; Rezaei, M. B. Bioactivity and Chemical Properties of Essential Oils from Zataria Multiflora Boiss and Mentha Longifolia (L.) Huds. Journal of Essential Oil Research 2002, 14(2), 141–146.
- Shah, A. J.; Bhulani, N. N.; Khan, S. H.; Gilani, A. H. Calcium Channel Blocking Activity of Mentha Longifolia L. Explains Its Medicinal Use in Diarrhoea and Gut Spasm. Phytotherapy Research 2010, 24(9), 1392–1397.
- Viljoen, A. M.; Petkar, S.; Van Vuuren, S. F.; Figueiredo, A. C.; Pedro, L. G.; Barroso, J. G. The Chemo-Geographical Variation in Essential Oil Composition and the Antimicrobial Properties of ” Wild Mint” - Mentha Longifolia Subsp. Polyadena (Lamiaceae) in Southern Africa. Journal of Essential Oil Research 2006, 18, 60–65.
- Movahhedin, N.; Zengin, G.; Bahadori, M. B.; Sarikurkcu, C.; Bahadori, S.; Dinparast, L. Ajuga Chamaecistus Subsp. Scoparia (Boiss.) Rech. F.: A New Source of Phytochemicals for Antidiabetic, Skin-Care, and Neuroprotective Uses. Industrial Crops and Products 2016, 94, 89–96.
- Slinkard, K.; Singleton, V. L. Total Phenol Analysis: Automation and Comparison with Manual Methods. American journal of enology and viticulture 1977, 28(1), 49–55.
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis Galatica Bornm.: A Source of Multifunctional Agents for the Management of Oxidative Damage, Alzheimer’s’s and Diabetes Mellitus. Journal of Functional Foods 2014, 11, 538–547.
- Berk, S.; Tepe, B.; Arslan, S.; Sarikurkcu, C. Screening of the Antioxidant, Antimicrobial and DNA Damage Protection Potentials of the Aqueous Extract of Asplenium Ceterach DC. African Journal of Biotechnology 2011, 10(44), 8902–8908.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free radical biology and medicine 1999, 26(9), 1231–1237.
- Sarikurkcu, C. Antioxidant Activities of Solvent Extracts from Endemic Cyclamen Mirabile Hildebr. Tubers and Leaves. African Journal of Biotechnology 2011, 10(5), 831–839.
- Valizadeh, H.; Sonboli, A.; Kordi, F. M.; Dehghan, H.; Bahadori, M. B. Cytotoxicity, Antioxidant Activity and Phenolic Content of Eight Fern Species from North of Iran. Pharmaceutical Sciences 2015, 21(1), 18.
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum Myrtifolium Boiss. Endemic to Turkey and Its Inhibitory Potential against Key Enzymes Involved in Alzheimer, Skin Diseases and Type II Diabetes. Industrial Crops and Products 2014, 53, 244–251.
- Aktumsek, A.; Zengin, G.; Guler, G. O.; Cakmak, Y. S.; Duran, A. Antioxidant Potentials and Anticholinesterase Activities of Methanolic and Aqueous Extracts of Three Endemic Centaurea L. Species. Food and Chemical Toxicology 2013, 55, 290–296.
- Zengin, G. A Study on in Vitro Enzyme Inhibitory Properties of Asphodeline Anatolica: New Sources of Natural Inhibitors for Public Health Problems. Industrial Crops and Products 2016, 83, 39–43.
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic Composition, in Vitro Antioxidant Effects and Tyrosinase Inhibitory Activity of Three Algerian Mentha Species: M. Spicata (L.), M. Pulegium (L.) And M. Rotundifolia (L.) Huds (Lamiaceae). Industrial Crops and Products 2015, 74, 722–730.
- Elansary, H. O.; Mahmoud, E. A. Egyptian Herbal Tea Infusions’ Antioxidants and Their Antiproliferative and Cytotoxic Activities against Cancer Cells. Natural product research 2015, 29(5), 474–479.
- Tang, K. S.; Konczak, I.; Zhao, J. Identification and Quantification of Phenolics in Australian Native Mint (Mentha Australis R. BR.). Food Chemistry 2016, 192, 698–705.
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K. A.; Nieman, K. M.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha Spicata L.) Extract. Molecules 2016, 21(8), 1007.
- Li, H.; Horke, S.; Förstermann, U. Oxidative Stress in Vascular Disease and Its Pharmacological Prevention. Trends Pharmacol. Sci. 2013, 34(6), 313–319.
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Experimental neurobiology 2013, 22(1), 11–17.
- Ahmad, N.; Fazal, H.; Ahmad, I.; Abbasi, B. H. Free Radical Scavenging (DPPH) Potential in Nine Mentha Species. Toxicology and Industrial Health 2012, 28(1), 83–89.
- Nickavar, B.; Alinaghi, A.; Kamalinejad, M. Evaluation of the Antioxidant Properties of Five Mentha Species. Iranian Journal of Pharmaceutical Research 2010, 10(2), 203–209.
- Dorman, H. D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. Journal of Agricultural and Food Chemistry 2003, 51(16), 4563–4569.
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Archives of pharmacal research 2013, 36(4), 375–399.
- Bahadori, M. B.; Dinparast, L.; Valizadeh, H.; Farimani, M. M.; Ebrahimi, S. N. Bioactive Constituents from Roots of Salvia Syriaca L.: Acetylcholinesterase Inhibitory Activity and Molecular Docking Studies. South African Journal of Botany 2016, 106, 1–4.
- Enz, A.; Amstutz, R.; Boddeke, H.; Gmelin, G.; Malanowski, J. Brain Selective Inhibition of Acetylcholinesterase: A Novel Approach to Therapy for Alzheimer’s Disease. Progress in brain research 1993, 98, 431–438.
- Oinonen, P. P.; Jokela, J. K.; Hatakka, A. I.; Vuorela, P. M. Linarin, a Selective Acetylcholinesterase Inhibitor from Mentha Arvensis. Fitoterapia 2006, 77(6), 429–434.
- Sheeja Malar, D.; Pandima Devi, K. Dietary Polyphenols for Treatment of Alzheimer’s Disease–Future Research and Development. Current Pharmaceutical Biotechnology 2014, 15(4), 330–342.
- Arun, K.; Chandran, J.; Dhanya, R.; Krishna, P.; Jayamurthy, P.; Nisha, P. A Comparative Evaluation of Antioxidant and Antidiabetic Potential of Peel from Young and Matured Potato. Food Bioscience 2015, 9, 36–46.
- Asghari, B.; Salehi, P.; Sonboli, A.; Ebrahimi, S. N. Flavonoids from Salvia Chloroleuca with α-amylsae and α-glucosidase Inhibitory Effect. Iranian journal of pharmaceutical research: IJPR 2015, 14(2), 609.