 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, crude peptide fractions from Hanwoo loins were released by injecting with proteolytic enzymes [no enzymes (control); protease type XIII (E1); thermolysin (E2); and combination of E1 and E2 (E3)] and their bioactivities were determined. The peptides derived from E2-injected Hanwoo loin exhibited the highest angiotensin I–converting enzyme (ACE) inhibitory activity and vitamin C equivalents antioxidant capacity among the treatments. The released peptide by treatment of E2 and E3 had similar (P > 0.05) inhibitory activity in HT29 cancer cell viability compared with luteolin as a positive control and non-cytotoxic effect on normal cell (3T3-L1). Therefore, the released peptide fraction from thermolysin (E2)-injected Hanwoo beef might contain potent bioactive peptides with ACE inhibitory and antioxidative activity and inhibition effect on certain cancer cell viability.
Introduction
Marinated beef using various sauces is widely consumed, such as the Korean traditional dish Bulgogi, because of the improved tenderness and flavour.[Citation1] Various natural ingredients, including onion, garlic, and wine, which contain bioactive substances, have been added to meat marinade sauce to increase the palatability of the meat and to satisfy consumers’ health concerns.[Citation2] Consumers’ tendency to be health-conscious has resulted in functional foods becoming the leading commodity of the food industry. In addition, several countries have started to develop new functional foods and have established regulations for their manufacture, handling, and distribution.[Citation3,Citation4]
Nutraceuticals are defined as natural chemical components in food items that have been considered beneficial to the human body, specifically for the prevention, treatment, or improvement of various physiological conditions.[Citation5] Numerous nutraceuticals have been isolated and characterized, including biologically active peptides with antihypertensive, antioxidant, anti-proliferative, antimicrobial, and immunomodulatory effects from muscle proteins.[Citation6–Citation9] The biologically active peptides are liberated by proteolytic enzymes, including trypsin, chymotrypsin, or pepsin, in vivo or in vitro. In addition, the bioactive peptides can move across the digestive epithelial barrier and reach the blood vessels, enabling their transport to peripheral organs, where they ultimately impart their beneficial effects to the entire organism, including inhibition of angiotensin converting enzyme (ACE).[Citation10] ACE has a crucial role in the renin-angiotensin system, which maintains a high level of blood pressure by converting angiotensin I to angiotensin II. For antihypertension, ACE inhibitory peptides originating from the hydrolysis of various meat types have been studied[Citation8,Citation11] as alternatives to synthesized chemical drugs. Furthermore, inhibitory activities on ACE, cancer cell proliferation, and oxidation were observed in protein hydrolysates of meat (myofibrilar, sarcoplasmic proteins, or collagen).[Citation7,Citation12,Citation13]
However, to our knowledge, no studies have focused on the bioactive peptides derived from Hanwoo beef during marination with proteolytic enzymes. Therefore, the objective of this study was to determine the bioactivity of peptides fractions derived from Hanwoo longissimus muscle during marination with proteolytic enzymes by measuring ACE inhibition, antioxidation, and inhibition activities against cancer cells viability.
Materials and methods
Reagents
2,2’-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as its diammonium salt, ACE (from a rabbit lung acetone extract), hippuryl-L-histidyl-L-leucine (HHL), and culture-grade dimethyl sulfoxide (DMSO) were purchased from Sigma (USA). Vitamin C was obtained from Mann Research Laboratories, Inc. (USA). All other chemicals used in this study were of analytical grade (Fisher, USA). Culture media for cells, 100-μg/mL streptomycin, and 100-U/mL penicillin were purchased from Gibco BRL (USA). Fetal bovine serum (FBS) was obtained from HyClone (USA).
Meat sample preparation
Loin chops (M. longissimus) were obtained within 24 h from 24 Hanwoo (Bos taurus coreanae) steers (29-month-old; quality grade 1), which were slaughtered at the Gangwon LPC (Korea). The 24 loins (n = 6 for each treatment) were injected with two different enzymes independently or in combination [(1) no enzymes in water (control); (2) 100 ppm protease type XIII (E1); (3) 80 ppm thermolysin (E2); and (4) combination of 100 ppm protease type XIII and 80 ppm thermolysin (E3)]. The types of injected enzymes were determined based on a previous study; thermolysin and protease type XIII showed the greatest proteolytic activity in Hanwoo beef.[Citation12] In the marination process of this study, ingredients including salt, phosphate, and various sauces were not injected because of their potential effect on the proteolytic enzymes-induced release of biopeptides from Hanwoo loins in the model system. The injected loins were stored at 5°C for 3 days to allow decomposition of the muscle proteins by the proteolytic enzymes. At the end of storage period, the crude peptide fractions (PF) were extracted from the decomposed meat samples.
Extraction of crude Peptide Fractions (PF) from Hanwoo M. longissimus
The peptide extraction method was a modified version of that reported by Jang and Lee.[Citation12] Briefly, decomposed beef samples were finely chopped and 5 g of each beef sample was homogenized in 20 mL of distilled water and boiled in water bath at 95°C for 15 min to denature the enzymes. The homogenate was filtered and centrifuged at 10,000 g for 20 min at 4°C. The supernatant was collected and submitted to ultrafiltration at 4°C using a PM-10 membrane (molecular weight cut off (MWCO), 10,000; Amicon Co., USA) to remove high molecular weight peptides. The filtrates were then centrifuged at 4,000 g for 45 min at 4°C using an Ultracel 3K membrane (MWCO, 3,000; Amicon Co., USA). Subsequently, four different filtered low-molecular weight (MW) (<3,000 Da) fractions from loin samples injected without or with different enzymes were obtained, which were lyophilized and weighed to calculate the yield. The lyophilized PF were stored at −80°C until analysis for bioactivity.
ACE inhibitory activity
ACE inhibitory activity was determined spectrophotometrically according to the method of Cushman and Cheung.[Citation13] In each sample, 100 μL of HHL (12.5 mM in 0.05 M sodium borate buffer) was incubated at 37°C for 5 min. After incubation, 50 μL of the PF samples or distilled water (sample blank) and 150 μL of ACE (peptidyl dipeptide hydrolase) were added and incubated at 37°C for 1 h. The enzymatic reactions were terminated by adding 250 μL of 0.5 N HCl. The hippuric acid generated by the action of the ACE on HHL was extracted from the acidified solution into 1 mL of ethyl acetate by vortex mixing for 15 s. The extract was centrifuged at 3,290 g for 10 min at 4°C, and a 0.7-mL aliquot of each ethyl acetate layer was transferred to a clean tube and evaporated by heating at 95°C for 20 min in a water bath. The hippuric acid was redissolved in 3 mL of 1 M NaCl, and the absorbance was measured at 228 nm. The IC50 value (µg/mL), defined as the concentration of a PF that inhibits 50% of the ACE activity, was calculated from the measured ACE inhibitory activity and peptide contents of each extract after regression analysis.
S; Optical density value of sample, S.C.; Optical density value of sample control,
B; Optical density value of blank, B.C.; Optical density value of blank control
Vitamin C Equivalents Antioxidant Capacity (VCEAC) assay
The antioxidant activity of the PF samples was determined using the ABTS assay described by Kim et al.[Citation14] One milliliter of 2,2’-azobis-(2-amidinopropane) dihydrochloride (AAPH) and a 2.5 mM ABTS solution were prepared in 100 mL of phosphate-buffered saline (PBS) (pH 7.4; 100 mM potassium phosphate buffer containing 150 mM NaCl). The mixture was heated in a water bath at 68°C and the blue-green ABTS radical solution was adjusted with fresh PBS solution to an absorbance of 0.650 ± 0.020 at 734 nm at 37°C. A 20-μL sample solution was added to 980 μL of ABTS radical solution and incubated in a water bath at 37°C for 10 min. The decrease in absorbance at 734 nm was measured. A control consisting of 20 μL of 50% aqueous methanol and 980 μL of ABTS radical solution was used. The ABTS radical-scavenging capacity of the PF samples was expressed based on the equivalent–fresh weight (mg) of vitamin C 100 mL−1 (VCEAC).
Cytotoxicity against normal cells and inhibitory effect on cancer cells viability
Cell culture
3T3-L1 mouse preadipocytes, HT29 human colon adenocarcinoma cells, HeLa human cervix epithelioid carcinoma cells, and HepG2 human hepatocarcinoma cells were obtained from the Korean Food Research Institute (Sungnam, Korea). The 3T3-L1 and HT29 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) containing 10% FBS, 100-μg/mL streptomycin, and 100-U/mL penicillin. HeLa and HepG2 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% FBS, 100-μg/mL streptomycin, and 100-U/mL penicillin. The 3T3-L1 cells were used in determination of cytotoxicity effect, whereas the HeLa, HepG2, and HT29 cell were used for measurement of inhibitory activity in cancer cell viability.
Cell viability
Cell viability was assessed using 3-(4–5-dimethyl thiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) staining according to the manufacturer’s instructions (Roche, Switzerland). The cells (3T3-L1, HeLa, HepG2, and HT29) were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated in a CO2 incubator with 5% CO2 and 37°C for 24 h to allow the cells to adhere to the inner wall of the flask. Subsequently, 150 μL/well of the culture medium containing a crude peptide fraction at concentrations of 50, 100, 200, 400, and 800 μg/mL, respectively, were added. The treated cells were then incubated for 24 h. Cells treated without PF (N) were used as controls to measure cell viability. After incubation, the culture medium was discarded, and 100 mL of 1 mg/mL MTT was added to each well and incubated for 2 h. After incubation, the liquid was discarded, 100 μL of DMSO was added to each well, and the microplate was mounted on a micromixer for 5 min to facilitate the dissolution of the blue formazan granules. The culture plate was then loaded onto a microplate reader for absorbance reading at an excitation wavelength of 570 nm. The inhibition rate was calculated according to the following formula: inhibition rate (%) = (1 – average absorbance of treatment group/average absorbance of control) × 100.
Statistical analysis
The experiments were performed in triplicate with two observations per replicate and values expressed as mean ± standard error (SE). Statistical analysis was performed using the SAS program for Windows ver. 9.1 (SAS Institute Inc.). A general linear model with Duncan’s multiple range test was employed to detect any significant differences among the treatments for yield of PF and ACE inhibitory and antioxidant activity (P < 0.05). The models included the fixed effects of PF type. For cell viability, type and concentration of PF served as fixed effects and the interaction between fixed effects was tested. Significant differences among the treatments were determined using the Student–Newman–Keuls multiple comparison test at a level of P < 0.05.
Results and discussion
Yield and ACE inhibitory activity of PF samples
The yield of PF was affected by the type of proteolytic enzyme (). The PF released by treatment of E2 and E3 had the highest (P < 0.05) yield, while the control had the lowest (P < 0.05) yield of PF among the treatments. This result might be caused by activity or substrate specificity of each enzyme against myofibrilar and sarcoplasmic proteins from Hanwoo loins. This speculation was supported by finding of Jang and Lee[Citation12] that high degradation activity of thermolysin and protease type XIII was observed for sarcoplasmic and myofibrilar proteins, respectively.
Table 1. Yield, angiotensin I-converting enzyme inhibitory activity (IC50), and vitamin C equivalents antioxidant capacity (VCEAC) of released crude peptide fractions derived from Hanwoo injected with enzymes.
For ACE inhibitory activity, the IC50 values of the PF derived from the enzyme-injected Hanwoo loins were influenced by the type of proteolytic enzyme (). The effect of each proteolytic enzyme on the ACE inhibitory activity of PF was in the following order: E2 > E1 ≥ E3 > Control. The lowest IC50 values of PF released by treatment of E2 strongly agrees with that observed in a previous study by Seol et al.[Citation15], which demonstrated that the crude peptide extract derived from thermolysin-injected Hanwoo M. longissimus showed the highest ACE inhibitory activity. However, these results are different from a report by Jang and Lee[Citation12], who found that the proteolysate enzyme mixture (proteinase A + thermolysin) exhibited higher ACE inhibitory activity than single-enzyme proteolysates. This difference might be affected by several factors, including the type of substrate (extracted protein vs. whole meat) and enzyme (proteinase A vs. protease type XIII), the reaction temperature (37 vs. 5°C), and time. The potential of food-derived bioactive peptides depends on the degree of digestion of food proteins and the release of active peptide components by enzymatic treatment. Greater potential in vitro ACE inhibition was shown by low-molecular-weight peptides from treated protein with enzymes compared with that of high-molecular-weight peptides.[Citation16,Citation17] In the present study, the molecular weight of the derived PF generated by the different enzymes or their combination was smaller than 3,000 Da; however, the exact molecular weights of the peptides were not determined. They should be determined in a future study to confirm the relationship between the molecular weight of the derived peptides from different enzymes or their combination and ACE inhibition.
VCEAC of PF
AAPH, a thermolabile water-soluble radical initiator, oxidizes ABTS2- to the ABTS radical anion.[Citation18] Reduction of the ABTS radical chromogen by an antioxidant decreases the absorbance at 734 nm. The calibration curve demonstrated a linear relationship (correlation coefficient; R2 = 0.999) between vitamin C concentration and absorbance reduction at 734 nm (data not shown).
The PF released by treatment E2 showed the highest VCEAC, indicating the greatest ABTS radical scavenger activity (). This result might be explained if the PF released by treatment E2 contains more peptides donating electrons, leading to the production of more stable products and the termination of radical chain reactions compared with other treatments. Lower VCEAC (P < 0.05) was observed in the PF released by treatment E1 and E3 compared with the control, indicating less free radical scavenging activity among these treatments.
Effect of PF on cell viability
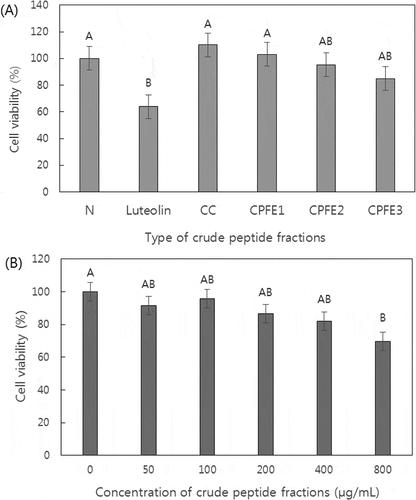
As shown in , there was no significant interaction between the type of released PF and its concentration on the viability of normal cell (3T3-L1 preadipocyte) and human cancer cells (HT29, HeLa, and HepG2). The cytotoxic effects of the PF released by treatment with different enzymes and its concentration on 3T3-L1 preadipocytes were measured using the MTT assay. Mejia and Lumen[Citation19] described a mechanism of cytotoxicity of a peptide that involved selectively killing cells that are being transformed or have been newly transformed by binding to deacetylated core histones exposed by the transformation event, resulting in disruption of the histone acetylation-deacetylation dynamics, ultimately leading to cell death. No significant difference was observed in the viability of 3T3L1 preadipocytes depending on the type of PF and its concentration (). This result demonstrated that the type of PF released by treatments E1, E2, and E3 and its concentration were not toxic to normal cells. In this study, the decrease in viability of human cancer cell implies inhibition of cancer cell proliferation. Luteolin was used as a positive control to compare the inhibitory activity of cancer cell cell viability. The type of PF and its concentration did not influence (P > 0.05) the inhibitory activity on HepG2 cell viability (). However, the viability of HT29 and HeLa cells were significantly affected by the type of PF and its concentration, respectively ( and ). The HT29 cells incubated with PF released by treatment E1 exhibited similar values (P > 0.05) to the control and higher (P < 0.05) values compared with luteolin for cell viability. By contrast, the PF released by treatments E2 and E3 led to similar (P > 0.05) cell viability against HT29 to luteolin. The result indicates a potential inhibitory effect of the PF released by treatments of E2 and E3 on HT29 cell viability. As concentration of the PF increased, the viability of HeLa cells gradually decreased (). The lowest viability of HeLa cells was observed at 800 µg/mL of PF, indicating the greatest inhibitory activity (31%) on cell viability compared with untreated cells. Jang et al.[Citation7] also reported that the peptide PKb325, derived from an enzymatic proteolysate of Hanwoo longissimus muscle protein, elicited a linear decrease in cell viability of human breast adenocarcinoma (MCF-7) and human stomach adenocarcinoma (AGS) cells, also in a dose-dependent manner. The results of this study correspond well with these previous reports. Based on the preceding results, this study demonstrated that PF derived from Hanwoo longissimus muscle using treatments E1, E2, and E3 had no cytotoxic effect on a normal cell line, but have possible inhibition activities on viability of certain human cancer cell lines in vitro.
Table 2. Probability values (P-values) of all main effects and interactions on normal cell (3T3-L1 mouse preadipocyte) and human cancer cells (HT29 colon adenocarcinoma cells, HeLa cervix epithelioid carcinoma cells, and HepG2 hepatocarcinoma cells).
Figure 1. Effect of the type of crude peptide fractions derived from injected Hanwoo with enzymes or its concentration on cell viability of HT29 human colon adenocarcinoma cells (A; SEM = 8.89) or HeLa human cervix epithelioid carcinoma cells (B; SEM = 5.63), respectively. Error bars indicate standard error of means. Types of crude peptide fractions: N, cells treated without peptide fractions; luteolin, cells treated with luteolin, CC, cells treated with peptide fraction derived from injected Hanwoo with no enzyme; CPFE1, cells treated with peptide fraction derived from injected Hanwoo with protease type XIII; CPFE2, cells treated with peptide fraction derived from injected Hanwoo with thermolysin; CPFE3, cells treated with peptide fraction derived from injected Hanwoo with combination of protease type XIII and thermolysin. A-B Means with different letters on the bar are significantly different (P < 0.05).
Conclusion
The results of this study clearly indicated that PF derived from Hanwoo loin injected with two different enzymes and their combination have ACE inhibitory potential and are not cytotoxic to normal cell. Especially, treatment E2 effectively generated bioactive peptides from Hanwoo longissimus muscle, as indicated by ACE inhibitory, antioxidant, and inhibition activities on HT29 cell viability. Thus, the addition of thermolysin (E2) could be utilized in the marination process to obtain the bioactive peptides, possibly providing health benefits from the consumption of marinated Hanwoo beef.
Additional information
Funding
References
- Khan, M. I.; Lee, H. J.; Kim, H. J.; Yong, H. I.; Lee, H.; Jo, C. Marination Absorption and Physicochemical Characteristics of Vacuum-Aged Duck Breast Meat. Asian Australas. Journal of Animal Science 2016, 29, 1555–1561. DOI: 10.5713/ajas.15.1053.
- Saranya, S.; Santhi, D.; Kalaikannan, A. Ginger as A Tenderizing Agent for Tough Meat – A Review. J. Livestock Science 2016, 7, 54–61.
- Arihara, K.;. Functional Foods. In Encyclopedia of Meat Sciences, Jensen, W. K., Jensen, C., Devine, C., Dikeman, M., Eds.; Elsevier: Oxford, 2004; pp. 492–499.
- Dentali, S.;. Regulation of Functional Foods and Dietary Supplements. Food Technology 2002, 56, 89–94.
- Wildman, R. E. C.;. Nutraceuticals: A Brief Review of Historical and Teleological Aspects. In Handbook of Nutraceuticals and Functional Foods, Wildman, R. E. C., Eds.; CRC Press: Florida, 2000; pp. 1–12.
- Arihara, K.;. Strategies for Designing Novel Functional Meat Products. Meat Science 2006, 74, 219–229. DOI: 10.1016/j.meatsci.2006.04.028.
- Jang, A.; Jo, C.; Kang, K. S.; Lee, M. Antimicrobial and Human Cancer Cell Cytotoxic Effect of Synthetic Angiotensin-Converting Enzyme (ACE) Inhibitory Peptides. Food Chemistry 2008, 107, 327–336. DOI: 10.1016/j.foodchem.2007.08.036.
- Liu, D.; Chen, X.; Huang, J.; Huang, M.; Zhou, G. Generation of Bioactive Peptides from Duck Meat during Post-Mortem Aging. Food Chemistry 2017, 237, 408–415. DOI: 10.1016/j.foodchem.2017.05.094.
- Matsui, T.; Matsumoto, K.; Mahmud, T. H. K.; Arjumand, A. Antihypertensive Peptides from Natural Resources. In Advances in Phytomedicine, Iwu, M. M. and Wootton, J. C., Eds.; Elsevier: Oxford, 2006; pp. 255–271.
- Yust, M. M.; Pedroche, J.; Giron-Calle, K.; Alaiz, M.; Milan, F.; Vique, J. Production of ACE Inhibitory Peptide by Digestion of Chickpea Legumin with Alcalase. Food Chemistry 2003, 81, 363–369. DOI: 10.1016/S0308-8146(02)00431-4.
- Ryder, K.; Bekhit, A. E. D.; McConnell, M.; Carne, A. Towards Generation of Bioactive Peptides from Meat Industry Waste Proteins: Generation of Peptides Using Commercial Microbial Proteases. Food Chemistry 2016, 208, 42–50. DOI: 10.1016/j.foodchem.2016.03.121.
- Jang, A.; Lee, M. Purification and Identification of Angiotensin Converting Enzyme Inhibitory Peptides from Beef Hydrolysates. Meat Science 2005, 69, 653–661. DOI: 10.1016/j.meatsci.2004.10.014.
- Cushman, D. W.; Cheung, H. S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochemical Pharmacology 1971, 20, 1637–1648. DOI: 10.1016/0006-2952(71)90292-9.
- Kim, D. O.; Lee, K. W.; Lee, H. J.; Lee, C. Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. Journal of Agricultural and Food Chemistry 2002, 50, 3713–3717. DOI: 10.1021/jf020071c.
- Seol, K. H.; Song, J. H.; Prayad, T.; Kim, H. W.; Jang, A.; Ham, J. S.; Oh, M. H.; Kim, D. H.; Lee, M. Assessment of the Inhibitory Activity of Peptide Extracts from Hanwoo Musculus Longissimus on Angiotensin I-Converting Enzyme. Korean Journal for Food Science of Animal Resources 2011, 31, 663–667. DOI: 10.5851/kosfa.2011.31.5.663.
- Aluko, R. E.;. Antihypertensive Peptides from Food Proteins. Annual Review of Food Science and Technology 2015, 6, 235–262. DOI: 10.1146/annurev-food-022814-015520.
- Ruiz-Ruiz, J.; Davila-Ortiz, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Angiotensin I-Cconverting Enzyme Inhibitory and Antioxidant Peptide Fractions from Hard-To-Cook Bean Enzymatic Hydrolysates. Journal of Food Biochemistry 2013, 37, 26–35. DOI: 10.1111/j.1745-4514.2011.00594.x.
- Scott, S. L.; Chen, W. J.; Bakac, A.; Espenson, J. H. Spectroscopic Parameters, Electrode Potentials, Acid Ionization Constants, and Electron Exchange Rates of the 2,2’-Azinobis(3-Ethylben- Zothiazoline-6-Sulfonate) Radicals and Ions. The Journal of Physical Chemistry 1993, 97, 6710–6714. DOI: 10.1021/j100127a022.
- Mejia, D. E.; Lumen, D. B. O. Soybean Bioactive Peptides: A New Horizon in Preventing Chronic Diseases. Sexuality, Reproduction and Menopause 2006, 4, 91–95. DOI: 10.1016/j.sram.2006.08.012.
