?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aroma-active compounds present in Sachima samples purchased at retail from the same batch code and stored for different durations of the shelf life were analysed by two methods: solid phase micro extraction (SPME) and solvent-assisted flavour evaporation (SAFE)-gas chromatography-olfactometry-mass spectrometry (GC-O-MS). A total of 41 volatile key compounds were identified. Among them, the predominant compounds in Sachima were tentatively identified by dilution analysis as being 3-(methylthio)propionaldehyde, 2-pentylfuran, 2-methyl-3-(methylthio)pyrazine, dimethyl disulfide, and dipropyl trisulfide. These compounds produced the highest due to their highest calculated flavour dilution (FD) factors. Sensory evaluations of the extracted compounds by a panel of trained individuals revealed that the ‘egg’ aroma was the main characteristic aroma due to its high sensory assessment score. With increasing length of storage of the product from 0 to 10 months, the overall acceptability of the Sachima aroma declined gradually. The observed changes in concentration of the different volatile compounds during storage indicated that the Maillard reaction and lipid oxidation continued during storage, so that aroma compounds and off-flavour compounds were being generated simultaneously. In addition, the undesirable smell of Sachima that increased during storage was not just generated from one volatile compound with a distinct off-flavour, but rather it was the result of an increase in both pleasant and unpleasant aromas. Furthermore, the pleasant aroma components appeared to still play a dominant role in the overall aroma profile of Sachima even after 10 Months of storage, which is within the stated shelf life on the product label.
Introduction
Sachima is a traditional Chinese sweet egg-based pastry that originated from the Manchu people and was one of the tribute offerings during the Qing Dynasty.[Citation1] Because of its attractive colour, unique flavour, delicate taste and soft texture, Sachima has long been consumed by all age groups. It is rich in lipid and carbohydrate, which comprise 30% and 50% of the final product, respectively.[Citation2] According to a book by Yan jing sui shi ji (“Yan jing Age in Mind”) published in the Qing dynasty, Sachima was made from the cooking of rock sugar, cream, and flour, and the most important steps included frying and sugar soaking. Thus, the Maillard reaction and lipid oxidation are key chemical reactions that occur during the preparation of Sachima. Ridgway, etc.[Citation3] systematically reviewed the source of off-flavours and taints in foods and beverages, and highlighted Maillard reaction and lipid oxidation as being important contributory factors to these effects.
In a previous study by Wang etc.,[Citation4] 37 aroma compounds were identified from Sachima samples by means of solid-phase microextraction-gas chromatograph-mass spectrometry (SPME-GC-MS), including 11 aldehydes, 4 ketones, 5 esters, 9 heterocyclic compounds, and 2 acids. The same method was used to explore the changes of these flavour compounds that occurred during storage of Sachima.[Citation2] However, apart from that study, few previous studies have investigated the key aroma-active compounds of Sachima, how these change during storage, and how this impacts on the sensory quality and shelf life of the product.
The purpose of this study was to identify and characterise the key aroma-active compounds in Sachima and to study the changes in their concentration during shelf life storage, using both SPME and SAFE combined with gas chromatography-olfactometry-mass spectrometry (GC-O-MS) techniques and sensory analysis. It was anticipated that the results would provide useful information for improving the shelf life quality and stability of Sachima as a traditional Chinese pastry.[Citation5]
Materials and methods
Chemicals
Chromatography standards, including 2-methyl-3-heptanone (purity > 99%), n-alkanes (C7–C30) (, purity > 99%), n-hexane (purity > 99%) and the authentic standard flavour compounds used in the identification of volatiles (purity > 99%), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Analytical reagents, including diethyl ether (purity > 99%), n-pentane (purity > 99%) and anhydrous sodium sulfate (purity > 99%) were provided by Yifengtiancheng Scientific Instruments Co. Ltd. (Beijing, China). Nitrogen (purity > 99.99%) was supplied by Beijing Haipubeifen Gas Industry Co. Ltd. (Beijing, China).
Sachima samples
Retail samples of Sachima samples were chosen from the same batch code and the most recent production date supplied by Nestlé R&D (China) Ltd. The samples were stored at room temperature (around 20°C) for the duration of their shelf life. During this period, samples were removed at 2 months, 4 months, 6 months, 8 months and 10 months, and stored at −18°C prior to their analysis. Once all of the samples were available for analysis, they were divided into two groups. One group was used for sensory evaluation, and the other was used for chemical analysis in order to evaluate the changes in the composition and concentration of volatile components during the shelf life of the product.
Extraction of aroma compounds by solid phase micro extraction (SPME)
A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fiber (50/30 μm, Supelco, Bellefonte, PA, USA) was used to extract the volatile compounds from the Sachima samples. Five gram samples of Sachima were weighed and placed into headspace vials (100 ml), to which 1 μL internal standard (1 μL 2-methyl-3-heptanone in 999 μL n-hexane) was added prior to extraction. The vials containing the samples were incubated at 60°C for 20 min. The SPME fibers were then exposed in the vials for 40 min at the same temperature. Subsequently, the fibers were inserted into the gas chromatography (GC) injection for thermal desorption at 250°C for 5 min. Every sample was performed by triplicate analyses.
Extraction of aroma compounds by solvent-assisted flavour evaporation (SAFE)
A 20 g sample of the Sachima was weighed and mixed with diethyl ether/n-pentane (V1/V2 = 2, 180 mL) in a Teflon bottle containing 2-Methyl-3-heptanone (50 μL, 0.816 μg/μL), as an internal standard, and extracted at 4°C for 8 h. Solvent extraction was performed by SAFE apparatus (Deutsche Forschungsanstalt für Lebensmittelchemie, Freising, Germany) in accordance with the procedure described by Engel.[Citation6] The apparatus consisted of a backing pump and a molecular turbine pump for producing a high vacuum (10–4 to 10–5 Pa). In addition, it included a water bath maintained at 40°C for separating the volatile compounds, and a trap tube maintained at very low temperature (−196°C) to trap the volatile substances. Anhydrous sodium sulfate (Na2SO4) was used as a drying agent to remove moisture. Following separation, the extracts were stored in a refrigerator at −20°C for 12 h in order to further remove any remaining water. The resulting extracts were concentrated to about 10 mL using a Vigreux column (50 cm × 1 cm internal diameter) and thereafter further concentrated to ca. 500 μL by passing through a gentle nitrogen (99.99% purity). Finally, a 1 μL sample of the concentrate was injected into the GC inlet for spectral analysis and the residual solution was stored at −20°C. Every sample was performed by triplicate analyses.
Aroma extraction dilution analysis (AEDA)/dynamic headspace dilution analysis (DHDA)
Two types of dilution analysis were performed in this study. The first utilised a solvent extraction method (AEDA) similar to that described by Wang.[Citation5] The ratio of the serial dilutions performed was 1:3. The flavour dilution (FD) factor was the highest dilution multiple of the compound, and the FD factors were expressed as log3FD (1, 2, 3 and so on), representing the serial dilutions (1:3, 1:9, 1:27, and so on, respectively). The second analysis was the headspace dilution method (DHDA), similar to that described by Kim and Ferreira.[Citation7,Citation8] The dilution ratio was achieved by changing the split ratio of GC-O by 1:1, 3:1, 9:1, 27:1, 81:1, and so on. The corresponding FD factors were 0, 1, 2, 3, 4. The FD values were obtained from five experienced sensory panellists who sniffed the aroma compounds as separated by GC-MS (described below). All FD values were averaged and rounded to the next whole number.
Gas chromatography–olfactometry–mass spectrometry (GC-O-MS) analysis
Gas chromatography-mass spectrometry of the aroma compounds was performed using gas chromatograph 7890A-7000B (Agilent Technologies Inc., Santa Clara, CA, USA), which was equipped with an olfactory detector port (Sniffer 9000; Brechbühler, Schlieren, Switzerland). The injection volume was 1 μL (valve-delay time = 4 min; splitless for SPME, split ratio 5:1 for SAFE). The separation of aroma compounds was performed on both a polar DB-Wax column and an almost non-polar DB-5 capillary column (both 30 m × 0.32 mm, film thickness 0.25 μm; J & W Scientific, Folsom, CA, USA). Ultra-high purity helium (purity > 99.999%) was used as the carrier gas, at a flow rate of 1.2 mL/min. The GC oven program was 40°C held for 3 min and then increased by 5°C/min to 200°C, then further increased by 10°C/min to 230°C and held at this temperature for 3 min. Electron impact mass spectra were generated at 70 eV, with an m/z scan range from 50 to 350 amu. The MS source temperature was 230°C. Five experienced sensory panellists were recruited to perform a sniffing test on the GC-O output. Wet gas (high purity nitrogen and distilled water) was conducted to the nose during the sniffing test in order to improve the sensitivity and comfort of the panellists during sniffing.
Identification of volatile compounds
Extracted volatile compounds were preliminarily identified by NIST 08 mass spectra search libraries installed in the GC-MS equipment. Reconfirmation was performed by comparison of the mass spectrum (MS), retention index (RI) and odour descriptions (O) with reference compounds [Citation9,Citation10] and standard compounds.
Quantitation of volatiles
A semi-quantitative method was utilised in this study for monitoring the changes in the concentration of different aroma compounds in Sachima during storage. The relative concentration for each aroma compound was calculated as follows[Citation11]:
where Cx, Ci, Ax, and Ai represented the relative concentration of an odorant, the concentration of the internal standard, the peak area of the aroma compound and the peak area of the internal standard on the GC chromatogram, respectively. RF denotes the response factor, RF = 1.
Sensory evaluation
Descriptive sensory analysis[Citation12] was carried out by 20 trained panellists who were recruited from the Laboratory of Molecular Sensory Science, Beijing Technology and Business University, Beijing, China. Daily training sessions were conducted for a month prior to the study in order to train the sensory panel to recognise the aroma characteristics and compounds of Sachima. The training standards used are shown in . The sensory assessment was evaluated using a 10-point scale. Each panellist was asked to take a rest for sensory recovery between each set of five different samples. A blinded assessment approach was adopted, in which panellists did not know the shelf life time point of the sample being analysed), and samples were analysed three times by each panellist.
Table 1. Flavour attributes selected for sensory evaluation.
Statistical analysis
Line charts were constructed by Microsoft Office Excel 2007 to show the changing concentrations of aroma compounds during the storage process. A spider plot was constructed using Microsoft Office Excel 2007 I order to show the aroma profile of Sachima in its totality. One-way analysis of variance (ANOVA) and Duncan’s multiple-range tests were used to identify significant differences in the concentrations of aroma compounds between Sachima samples. A P value of ≤ 0.05 was considered as statistically significant. Statistical tests were performed with IBM SPSS Statistics 22 software. Partial least squares regression analysis (PLS) was conducted using The Unscrambler software (v9.7 CAMO, OSLO, Norway) to explore the significant differences among the Sachima samples stored for different time intervals and the relationship between the outputs of the sensory evaluation and chemical analysis.
Results and discussion
SPME extraction
In total, 63 different aroma compounds were identified by SPME-GC-O-MS. These included 14 alcohols, 11 aldehydes, 10 ketones, 12 esters, and 15 heterocyclic compounds. These results are more comprehensive but broadly consistent with those of Wang etc.[Citation4] For example, the present studied identified certain ‘new’ volatile compounds in Sachima that have not previously been reported, including 1-octanol (‘chemical’ aroma), butanedioic acid dimethyl ester (‘fruity’ aroma), 2,5-dimethypyrazine (‘roasted nut’ aroma), ethylpyrazine (‘peanut butter’ aroma), trimethylpyrazine (‘potato’ aroma), and 2-methyl-3-(methylthio)pyrazine (‘popcorn’ aroma).
As shown in , 18 different types of aroma were captured by the sensory panellists from the GC-O-MS of the Sachima samples, including ‘green’, ‘chemical’, ‘grass’, ‘cocoa’, ‘malty’, ‘citrus’, ‘cooked potato’, ‘soap’, ‘mushroom’, ‘cheesy’, ‘fruity’, ‘green bean’, ‘popcorn’, ‘roasted nut’, ‘peanut butter’, ‘sweet’, ‘potato’ and ‘steamed rice’. The corresponding substances were, in order, 1-heptanol, 1-octanol, hexanao, 2-methylbutanal, 3-methly butanal, nonanal, 3-(methylthio)propionaldehyde, 2-octanone, 1-octen-3-one, n-propylacetate, dimethyl ester butanedioic acid, 2-pentylfuran, methylpyrazine, 2,5-dimethylpyrazine, ethylpyrazine 2-ethyl-5-methylpyrazine, trimethylpyrazine, and 2-methyl-3-(methylthio) pyrazine. The substances listed above were assigned as the key aroma-active compounds in Sachima because of their significant contributions to the perceived overall acceptability of the aroma of the Sachima by the sensory panelists.
Table 2. The comparison of different shelf life with the extraction method of SPME.
The alcohols identified as present in Sachima may have been generated by the reduction of aldehydes degraded from fatty acids.[Citation13] Edible oil is one of the most important raw materials in the manufacture of Sachima. Therefore, it is highly possible that the aldehydes identified as present in Sachima, especially the aliphatic aldehydes, such as hexanal and nonanal, might have originated from auto-oxidation of unsaturated fatty acids in the oil.[Citation14–Citation16] Because of their low aroma thresholds, a greater content of aldehydes might contribute significantly to the flavour of Sachima. However, increasing the content of aldehydes in the product might also result in it having an unpleasant aroma. Several studies have previously highlighted that certain selected aldehydes, especially hexanal, could be used as a marker for monitoring the oxidative rancidity.[Citation17–Citation20] It has also been shown that 1-octen-3-one (‘mushroom’ aroma), an oxidative degradation product of unsaturated fatty acids, plays an important role in the aroma profile of Sachima because of its intense smell captured by sniffing in this study.[Citation21] Heterocyclic compounds like methylpyrazine, 2,5-dimethylpyrazine, ethylpyrazine, 2-ethyl-5-methylpyrazine, and trimethylpyrazine would have been mainly generated from Maillard reaction during the thermal processing of Sachima. These compounds were associated with the aromas of ‘nutty’, ‘popcorn’ and ‘potato’, and contribute to the roast like flavour of Sachima.[Citation22,Citation23]
SAFE extraction
In total, 72 different aroma compounds were identified by SAFE-GC-O-MS. The chemical classifications of the identified compounds were broadly similar to those identified by the SPME method, except for the thioethers and acids. Thioethers were found to be present at high levels in all the Sachima samples, imparting relatively unpleasant odours, such as ‘garlic’, ‘putrid’, ‘sulfur’ and ‘rotten egg’. The present study represents the first application of the SAFE method to the analysis of the aroma compounds in Sachima, and a number of the compounds detected by SAFE had not been reported previously such as benzyl alcohol (‘sweet flower’ aroma), bis(1-methylethyl) disulfide (‘sulfur’ aroma), and 3-methylbutanoicacid (‘sweat, rancid’).
According to the results of SAFE-GC-O shown in , 22 different aromas were captured from the Sachima samples by the sensory panellists, including ‘sweet’, ‘sweet flower’, ‘cocoa’, ‘malt’, ‘cut grass’, ‘cooked potato’, ‘mushroom’, ‘pineapple’, ‘cheesy’, ‘fruit’, ‘coconut’, ‘peach’, ‘popcorn’, ‘roasted nut’, ‘steamed rice’, ‘putrid’, ‘sulfur’, ‘rotten eggs’, ‘cabbage’, ‘sweat’, and ‘garlic’. The corresponding substances were, in order, ethanol, benzyl alcohol, 2-methylbutanal, 3-methylbutanal, hexanal, 3-(methylthio)propionaldehyde, 1-octen-3-one, ethyl acetate, n-propyl acetate, 5-hexyldihydro-2(3H)-furanone, 5-butyldihydro-2(3H)-furanone, 5-heptyldihydro-2(3H)-furanone, methylpyrazine, 2,5-dimethylpyrazine, 2-methyl-3-(methylthio)pyrazine, dimethyl disulfide, methylpropyl disulfide, diethyl disulfide, bis(1-methylethyl) disulfide, dimethyl trisulfide, dipropyl trisulfide, 3-methylbutanoic acid, and an unknown compound. These substances were assigned as the key aroma-active compounds, as before.
Table 3. The comparison of different shelf life with the extraction method of SAFE.
Similar to the results of the SPME analysis, SAFE analysis revealed that aldehydes and heterocyclic compounds were present at high concentrations in the Sachima samples, and played an important role in the flavour of the product, as evidenced by the high average log3FD-factor. Among the identified compounds, 2-methylbutanal, 3-methylbutanal and 3-(methylthio)propionaldehyde were Maillard reaction products from degraded leucine, isoleucine and methionine, respectively.[Citation24,Citation25] The existence of these compounds in the Sachimas might be due to the egg ingredient; these compounds have been identified in scrambled eggs and spray-dried eggs in previous studies.[Citation26,Citation27] In contrast to the findings of the SPME analysis, more thioethers and acids were extracted by SAFE. Thioethers imparted the aroma of ‘sulfur’, ‘putrid’, ‘rotten eggs’ and ‘cabbage’, especially bis(1-methylethyl)disulfide which imparted an intense ‘sulfur’ and ‘gasoline’ aroma at an log3FD of 2. The polysulfides have been reported as deriving from methanethiol, and 3-methylthiopropionaldehyde is usually considered to be the main precursor of methanethiol.[Citation25] Furthermore, dimethyl disulfide and dimethyl trisulfide, with average log3FDs of 3 and 1, respectively, made significant contributions to the aroma of the Sachima, and many sulfur containing compounds have previously been detected in eggs.[Citation28] The acids detected in the Sachima samples were regarded as odorous because of their sour smell. They are likely to have been the degradation products of the oxidation of lipids[Citation27] such as 3-methylbutanoicacid which imparted a ‘sweat’ aroma at a log3FD of 2 in the samples that had been stored for 10 months. In addition, an interesting unknown compound was found in the samples that had been stored for 4 months and 6 month. The compound had an important odour note of ‘garlic’, with a log3FD of 2. Confirmation of this compound via further study is needed.
The predominant aroma-active compounds in Sachima
Dynamic headspace dilution analysis (DHDA) was performed by changing the split ratio of the GC. Three compounds had a relatively high log3FD of 4: 2-pentylfuran (‘green bean’ aroma), 3-(methylthio)propionaldehyde (‘cooked potato’ aroma) and 2-methyl-3-(methylthio)pyrazine (‘steamed rice’ aroma). These compounds might have played major roles in the perceived overall acceptability of the Sachima samples, and were followed by 2-methylbutanal (‘cocoa’ aroma), 3-methylbutanal (‘malty’ aroma), 1-octen-3-one (‘mushroom’ aroma), n-propylacetate (‘fruity’ aroma), 2,5-dimethyl-pyrazine (‘roasted nut’ aroma), each with a log3FD of 3. A further dilution analysis was performed using aroma extract dilution analysis (AEDA). Two compounds: dimethyl disulfide (‘cooked cabbage’ aroma) and dipropyltrisulfide (‘sulfur’ aroma) had a relatively high log3FD of 3, and may thus play important roles in the perceived overall acceptability of the Sachima. Collectively, from the results of the different chemical and sensory analyses regarding the different categories of aroma-active compounds identified, it is suggested that the characteristic flavour of Sachima is derived mainly from lipid oxidation and Maillard reactions that occur during manufacturing.
Some of the compounds identified in the present study as being predominant aroma-active compounds in Sachima, have not previously been identified, such as 1-octanol (‘chemical’ aroma), 3-(methylthio)propionaldehyde (‘cooked potato’ aroma), 1-octen-3-one (‘mushroom’ aroma), dimethylester butanedioicacid (‘fruit’ aroma), 5-heptyldihydro2(3H)-furanone (‘peach’ aroma), 2,5-dimethyl pyrazine (‘roasted nut’ aroma), 2-methyl-3-(methylthio)pyrazine (‘steamed rice’ aroma), bis(1-methylethyl)disulfide (‘sulfur’ aroma), and 3-methylbutanoicacid (‘sweat’). It is worth mentioning that 3-(methylthio)propionaldehyde, 2-methyl-3-(methylthio)pyrazine, bis(1-methylethyl)disulfide and other sulfur-containing compounds were tentatively identified as characteristic aroma compounds in Sachima. In addition, 2-methylbutanal and 3-methylbutanal which are products of Strecker degradation were also identified as important in the aroma profile of the Sachima samples, which further highlights the contribution of the Maillard reaction to the aroma flavour of Sachima.
Sensory evaluation
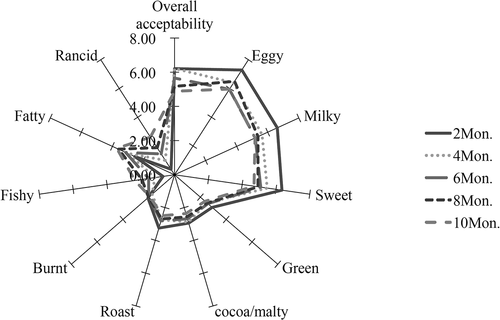
The results of the sensory evaluation are shown in . According to the spider plot, the overall aroma profile of the Sachima samples could be separated into ten attributes, as identified by the sensory panellists. The overall acceptability score, which represented the panellists’ preference for each Sachima sample, was markedly higher for the samples that had been stored for 2 or 4 months, and decreased gradually thereafter in samples stored for a greater duration of the shelf life. Further, the intensities of ‘eggy’, ‘milky’, ‘sweet’, ‘green’, ‘roast’, and ‘cocoa/malty’ in the samples stored for 2 and 4 months were also markedly higher than those of the other samples that had been stored for longer. The egg flavour appeared to be the main characteristic aroma of the Sachimas, as evidenced by its higher score by the sensory panellists. It seems likely that the presence of the aforementioned pleasant aromas were the reason why the panellists scored the 2 and 4 month samples higher in terms of their overall acceptability. Meanwhile, the unpleasant smells of ‘fishy’, ‘fatty’, ‘burnt’, and ‘rancid’ received higher scores in the samples that had been stored for6–10 months. It is not difficult to reason that the longer the duration of storages, the worse the aroma of the Sachima might be. The results of SAFE-GC-O-MS indicated that some acids and thioethers were generated in the samples that had been stored for 8 and 10 months, such as 3-methylbutanoic acid, dimethyl disulfide, diethyl disulfide and dimethyl trisulfide. These compounds could have contributed to the odours of ‘sour’, ‘sweaty’, ‘putrid’, ‘rotten egg’, and ‘sulfur’ that might be regarded as the source of the unpleasant aroma of Sachima that had been stored for 8 months or more. However, the unpleasant odours that were associated with stored Sachima did not seriously impact on the sensory experience, even after 10 months storage, within the stated shelf life of the product at retail. According to the high assessment scores of eggy, sweety, and milky attributes assigned to these Sachima samples, it appeared that the attractive aroma compounds present still played a dominant role in the overall aroma profile of the Sachimas after 10 months storage.
Changes in the concentrations of key aroma-active compounds in Sachima with increased duration of storage
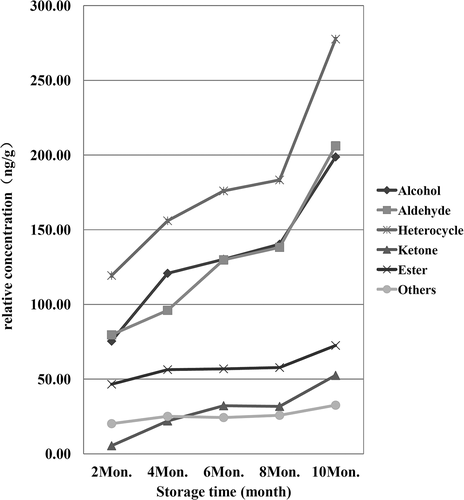
Comparison of the relative concentrations of the different compounds extracted by SPME among the Sachima samples (), revealed a step-wise change in the aroma profile of the Sachima over time, whereby the concentrations of identified compounds initially increased, then levelled out, before finally increasing again, especially in terms of the content of aldehydes, alcohols, and heterocyclic compounds. Considering the significant oil and carbohydrate content in Sachima, it was not difficult to conclude from this that Maillard reactions and lipid oxidation were both taking place during the storage process. If so, these reactions might well be the reason for the decline in the sensory acceptability of the product with increased storage time.
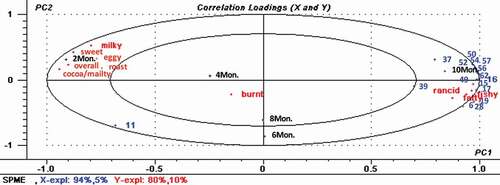
In order to enable a thorough analysis, the relationships between the concentrations of the 41 key aroma-active compounds identified by SPME and SAFE-GC-O-MS and the results of the sensory assessments were analysed by PLS. As illustrated by the PLS biplot in , Sachima samples stored for the five different durations (2, 4, 6, 8 and 10 months) were distinguished clearly in the X/Y axis. In addition, the results from samples stored for 6 months and 8 months showed a high positive correlation. This finding corresponded well with the observed changes in concentration of the compounds as determined by SPME-GC-MS. In addition, it was evident that the pleasant aromas of ‘eggy’, ‘roast’, ‘sweet’, ‘milky’, ‘green’, and ‘cocoa/malty’, showed a high correlation between the samples stored for 2 and 4 months. However, intriguingly, the corresponding odorant compounds related to these aromas, such as hexanal, nonanal, 2-methylbutanal, 3-methylbutanal, 2-pentylfuran, 2-octanone, 2,5-dimethylpyrazine, and 2-methyl-3-(methylthio)pyrazine were more concentrated on the area associated with 10 months of storage, and showed a positive relationship with ‘fatty’, ‘fishy’, and ‘rancid’ aromas. This might be due to the increasing content of these compounds during storage. Thus, it appears that high concentrations of these key aroma-active compounds might bring a negative influence on the aroma profile of the Sachima. According to the observed increase in the content of pyrazine compounds with increased storage of the Sachima, it seems evident that the Maillard reaction was continuing during storage. Combining these findings with the sensory evaluations, even though the off-flavour of the Sachimas became more intense between 2 and 10 months, the scores for the pleasant aromas (‘eggy’, ‘roast’, ‘sweet’, ‘milky’, ‘green’ and ‘cocoa/malty’) were still considerably higher than those of the disagreeable odors (‘fatty’, ‘fishy’ and ‘rancid’). The findings also suggest that the desirable aroma components still played a dominant role in the aroma profile even at the longer durations of storage. However, due to the low thresholds of some of the off-flavour compounds, a small increase in these compounds could potentially have a negative effect on the sensory experience.
Figure 3. Linear partial least-squares analysis (PLS) biplot of sensory attributes versus prominent aroma-active compounds quantified by SPME-GC-MS (the prominent aroma-avitve compounds’numbers in biplot were 6, 11, 15, 16, 17, 19, 28, 37, 39, 49, 50, 52, 54, 56, 57, 62, which corresponding to , respectively.).
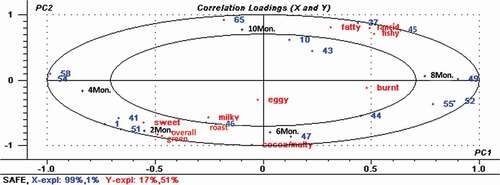
From the PLS biplot in , it can be seen that there were also significant differences among the Sachima samples stored for the five different durations (2, 4, 6, 8, and 10 months). A high positive correlation between the results from the 2 month and 4 month samples was again seen and coincided with the findings of the sensory evaluations. “Overall acceptability” was more closely focused around the 2 month storage duration, which was consistent with the results of the sensory assessment. In addition, ‘sweet’, ‘green’, ‘milky’, and ‘roast’ aromas were correlated with the aroma compounds ethanol, 5-hexyldihydro-2(3H)furanone, 2,5-dimethylpyrazine, and methylpropyl disulfide, and positioned close to the 2 month storage duration. Further, 2,5-Dimethylpyrazine was positioned close to the aromas of ‘roast’ and ‘milky’ which might contribute to the pleasant aroma of Sachima. Dipropyltrisulfide and bis(1-methylethyl)disulfide had a high positive correlation with the 4 month duration of storage and a moderate correlation with the 2 month duration of storage. The compound 3-Methylbutanoic acid was positively correlated with ‘rancid’ aromas, and was affiliated with positive PC2 which was positioned near the 10 month storage duration. This compound might be responsible for imparting an undesirable odour to the Sachima. Ethyl acetate, 5-butyldihydro-2(3H)furanone, and benzyl alcohol were positioned near to the aromas of ‘fatty’, ‘rancid’, and ‘fishy’ and yet these substances impart pleasant aromas like pineapple, sweet flower and coconut. Thus, it seems that the appearance of an unpleasant smell does not necessarily come from compounds that have off-flavour, but could also be due to an increase in the concentration of aroma compounds that impart pleasant aromas when present at a lower level.
Figure 4. Linear partial least-squares analysis (PLS) biplot of sensory attributes versus prominent aroma-active compounds quantified by SAFE-GC-MS (the prominent aroma-acitve compounds’numbers in biplot were 1, 10, 37, 41, 43, 44, 45, 46, 47, 49, 51, 52, 54, 55, 58, 65, which corresponding to , respectively.).
Conclusion
A total of 41 aroma-active compounds were detected in Sachima samples by SPME/SAFE-GC-O-MS methods. Among them, the predominant compounds were tentatively identified by dilution analysis, as: 3-(methylthio)propionaldehyde, 2-pentylfuran, 2-methyl-3-(methylthio)pyrazine, dimethyl disulfide, and dipropyl trisulfide. The two extraction methods employed had their own advantages. While SAFE was well suited to extraction of those middle or high molecular weight volatile compounds like thioethers, SPME was appropriate for the extraction of very volatile compounds such as aldehydes. Overall, the combination of the two extraction methods with the GC-O-MS technique enabled a more comprehensive analysis of Sachima flavour than has been conducted before. According to the observed changes in the concentrations of the identified volatile compounds during storage of the Sachima, it appeared that the Maillard reaction and lipid oxidation were sustained throughout the duration of storage, so that aroma compounds and off-flavour compounds were being generated simultaneously. In addition, the undesirable smell of stored Sachima appeared to be not just the result of a single volatile compound or of off-flavour compounds, but rather due to the increasing content of both pleasant and unpleasant aromas. Furthermore, the major aroma components still played a dominant role in the overall aroma profile of Sachima even after 10 months of storage. On this basis, the simultaneous control of fat oxidation and Maillard reaction might prove useful measures to prevent a decline in overall sensory acceptability of the product over time (storage). Further studies on the aroma profile of Sachima may be useful for the improvement of the sensory quality of this traditional Chinese pastry.
Highlights
Thirty common key aroma compounds were detected in Sachima by SPME/SAFE-GC-O-MS.
Evolution of aroma compounds in Sachima that impact the sensory acceptability was explored.
Some acids and thioethers were generated in 8Mon. and 10Mon. samples
Inflexion point of unpleasant odour appeared at 6M-8M, then became more clear at 10M.
PLS was used for the correlation of sensory evaluation with related odorants.
Acknowledgments
Neslté R&D (China) Ltd. is thanked for the Sachima samples supplying. The assessors who come from Laboratory of Molecular Sensory Science, Beijing Technology and Business University are gratefully acknowledged for the sensory evaluation experiment.
References
- Cui, C.; Lei, F. F.; Wang, Y. R.; Zhao, H. F.; Sun, W. Z.; You, L. J. Antioxidant Properties of Maillard Reaction Products from Defatted Peanut Meal Hydrolysate-Glucose Syrup and Its Application to Sachima. Food Science and Technology Research 2014, 20(2), 327–335. DOI: 10.3136/fstr.20.327.
- Wang, Y. R.; Cui, C.; Zhao, M. M. Profiling Flavor Compounds of Sachima during Storage Using Solid-Phase Microextraction Gas Chromatograph-Mass Spectrometry (SPME-GC-MS). Modern Food Science and Technology (China) 2012, 28, 218–222.
- Ridgway, K.; Lalljie, S. P.; Smith, R. M. Analysis of Food Taints and Off-Flavours: A Review. Food Additives and Contaminants 2010, 27(2), 146. DOI: 10.1080/19440040903296840.
- Wang, Y. R.; Cui, C.; Zhao, M. M. Separation and Identification of Volatile Flavors of Sachima Using Solid-Phase Micro Extraction Gas Chromatograph-Mass Spectrometry (SPME-GC-MS). Modern Food Science and Technology (China) 2011, 27, 1406–1409.
- Wang, Y.; Song, H.; Zhang, Y.; Tang, J.; Yu, D. Determination of Aroma Compounds in Pork Broth Produced by Different Processing Methods. Flavour Fragrance Journal 2016, 31(4), 319–328. DOI: 10.1002/ffj.v31.4.
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation–A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. European Food Research and Technology 1999, 209(3), 237–241. DOI: 10.1007/s002170050486.
- Kim, T. H.; Sang, M. L.; Kim, Y. S.; Kim, K. H.; Oh, S.; Lee, H. J. Aroma Dilution Method Using Gc Injector Split Ratio for Volatile Compounds Extracted by Headspace Solid Phase Microextraction. Food Chemistry 2003, 83(1), 151–158. DOI: 10.1016/S0308-8146(03)00221-8.
- Ferreira, V.; Aznar, M.; López, R.; Cacho, J. Quantitative Gas Chromatography−Olfactometry Carried Out at Different Dilutions of an Extract. Key Differences in the Odor Profiles of Four High-Quality Spanish Aged Red Wines. Journal of Agricultural and Food Chemistry 2001, 49(10), 4818–4824. DOI: 10.1021/jf010283u.
- Terry, A.; Heinrich, A. Flavornet and Human Odor Space. 2004. http://www.flavornet.org.
- Mottram, R. Flavour Research Group in the University of Reading, School of Food Biosciences The LRI and Odour Database. http://www.odour.org.uk/
- Liu, M.; Liu, J.; He, C.; Song, H.; Liu, Y.; Zhang, Y.; Wang, Y.; Guo, J.; Yang, H.; Su, X. Characterization and Comparison of Key Aroma-Active Compounds of Cocoa Liquors from Five Different Areas. International Journal of Food Properties 2017, 20(10), 2396–2408. DOI: 10.1080/10942912.2016.1238929.
- Zhang, Y.; Song, H.; Li, P.; Yao, J.; Xiong, J. Determination of Potential Off-Flavour in Yeast Extract. Food Science and Technology Research 2017, 82, 184–191.
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A Comparative Study of Aroma-Active Compounds between Dark and Milk Chocolate: Relationship to Sensory Perception. Journal of the Science of Food and Agriculture 2014, 95(6), 1362–1372. DOI: 10.1002/jsfa.6831.
- Rega, B.; Guerard, A.; Delarue, J.; Maire, M.; Giampaoli, P. On-Line Dynamic HS-SPME for Monitoring Endogenous Aroma Compounds Released during the Baking of a Model Cake. Food Chemistry 2009, 112(1), 9–17. DOI: 10.1016/j.foodchem.2008.05.028.
- Li, S.; Cui, C.; Zhao, M. M. Optimization on the Formula of Sachima. Food Industry (China). 2012, 33(9), 312–313.
- Pinna, A.; Simoncini, N.; Toscani, T.; Virgili, R. Volatile Organic Compounds of Parma Dry-Cured Ham as Markers of Ageing Time and Aged Ham Aroma. Italian Journal of Food Science 2013, 24(4), 321.
- Wang, L.; Deng, L.; Wang, Y.; Zhang, Y.; Qian, H.; Zhang, H.; Qi, X. Effect of Whole Wheat Flour on the Quality of Traditional Chinese Sachima. Food Chemistry 2014, 152(2), 184–189. DOI: 10.1016/j.foodchem.2013.11.130.
- Shahidi, F.;. Headspace Volatile Aldehydes as Indicators of Lipid Oxidation in Foods. Advanced Experimental Medicine Biology 2001, 488, 113–123.
- Pastorelli, S.; Torri, L.; Rodriguez, A.; Valzacchi, S.; Limbo, S.; Simoneau, C. Solid-Phase Micro-Extraction (SPME-GC) and Sensors as Rapid Methods for Monitoring Lipid Oxidation in Nuts. Food Additives and Contaminants 2007, 24(11), 1219–1225. DOI: 10.1080/02652030701426987.
- Barbieri, G.; Bolzoni, L.; Parolari, G.; Virgili, R.; Buttini, R.; Careri, M.; Mangia, A. Flavor Compounds of Dry-Cured Ham. Journal of Agricultural and Food Chemistry 1992, 40(12), 2389–2394. DOI: 10.1021/jf00024a013.
- Koutidou, M.; Grauwet, T.; Van, L. A.; Acharya, P. Potential of Different Mechanical and Thermal Treatments to Control Off-Flavour Generation in Broccoli Puree. Food Chemistry 2017, 217, 531. DOI: 10.1016/j.foodchem.2016.09.003.
- Heenan, S. P.; Dufour, J. P.; Hamid, N.; Harvey, W.; Delahunty, C. M. Characterisation of Fresh Bread Flavour: Relationships between Sensory Characteristics and Volatile Composition. Food Chemistry 2009, 116(1), 249–257. DOI: 10.1016/j.foodchem.2009.02.042.
- Karahadian, C.; Johnson, K. A. Analysis of Headspace Volatiles and Sensory Characteristics of Fresh Corn Tortillas Made from Fresh Masa Dough and Spray-Dried Masa Flour. Journal of Agricultural and Food Chemistry 1993, 41(5), 791–799. DOI: 10.1021/jf00029a022.
- Huang, Y.; Tippmann, J.; Becker, T. A Kinetic Study on the Formation of 2- and 3-Methylbutanal. Journal of Food Engineering 2016, 40(2), 1–11. DOI: 10.1111/jfpe.12375.
- Gijs, L.; Perpète, P.; Aurore, T.; Collin, S. 3-Methylthiopropionaldehyde as Precursor of Dimethyl Trisulfide in Aged Beers. Journal of Agricultural and Food Chemistry 2000, 48(12), 6196–6199. DOI: 10.1021/jf0007380.
- Matiella, J. E.; Tcy, H. Volatile Compounds in Scrambled Eggs. Journal of Food Science 1991, 56(2), 387–390. DOI: 10.1111/j.1365-2621.1991.tb05286.x.
- Rannou, C.; Texier, F.; Moreau, M.; Philippe, C.; Anne, M.; Carole, P. Odour Quality of Spray-Dried Hens’ Egg Powders: The Influence of Composition, Processing and Storage Conditions. Food Chemistry 2013, 138(2–3), 905–914. DOI: 10.1016/j.foodchem.2012.11.090.
- Warren, M. W.; Ball, H. R. Effect of Concentration of Egg Yolk and White on Fresh Scrambled Egg Flavor. Poultry Science 1991, 70(10), 2186–2190. DOI: 10.3382/ps.0702186.