 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Lactic-acid-fermented mulberry juice (LFMJ) was subjected to pulsed light (PL) treatment at exposure time of 2, 4, and 8 s at high insensitive pulses of 14.0 J/cm2. The effect of PL treatment on the microbial inactivation, physicochemical, phytochemical, volatile, and sensory characteristics of LFMJ was evaluated. It was found that the PL was able to reduce the microbial load to acceptable levels (1.02 ± 0.04 log10 cfu/mL) with no significant impact on the physicochemical properties of LFMJ. It was also observed that the PL treatment caused a slight decrease in anthocyanin concentration at 8 s exposure time. The color difference (∆E) of the juice treated for 2 and 4 s fell below the slightly noticeable range 0.5<ΔE<1.5 while ∆E values for the 8 s (0.55 ± 0.02) and the thermal (0.50 ± 0.02) treated samples were slightly noticeable. The volatile profile and odor activity values were positively affected by increasing the exposure time. The results depict that, under the present experimental conditions, the application of the PL resulted in a fermented juice with superior quality attributes as compared to the thermal treated juice.
Introduction
Mulberry is a monoecious plant cultivated in Africa, Asia, Europe, South America, and North America.[Citation1] It belongs to the genus Morus from the Moraceae family. There are 24 species of Morus and one subspecies, with at least 100 known varieties.[Citation2] The fruit has been extensively used in traditional medicine in treating ailments such as malaria, diabetes, hepatitis, dermatitis, atherosclerosis, asthma, and rheumatism.[Citation3] Besides, it is regarded as a nutritious food that varies according to variety, environmental factors, and geographical location.[Citation4] The fruit is very perishable[Citation5] hence the need to preserve it from postharvest loss. To address this challenge, the fruit is often processed into fruit jellies, jam, wines, and nonalcoholic beverage, among others.[Citation6,Citation7]
To date, the application of conventional thermal processing of fruit juices remains the most common technique for preservation and shelf-life extension of fruit juice because of its ability to inactivate microorganisms and spoilage enzymes. However, heat processing particularly under certain conditions have been reported to induce detrimental effect on the nutritional, phytochemical, and sensory qualities of food[Citation8–Citation10] as well as reducing the content or bioavailability of some bioactive compounds.[Citation9,Citation11] Consumer’s demand for nutritious, natural, and healthier foods, which are minimally and naturally processed makes it imperative for food manufacturers to explore various technologies that impact less on the quality of processed foods. This has necessitated the need for exploration into novel nonthermal pasteurization technologies such as high hydrostatic pressure, pulsed electric fields, pulsed magnetic field, pulsed light, and ultrasonication, among others, to complement or replace the conventional heat pasteurization techniques.[Citation12,Citation13] Besides, these novel technologies are not only employed to maintain the quality of foods but also to enhance their functionality.
Pulsed light (PL) is a novel nonthermal technology that encompasses the use of short duration (1 µs–0.1 s) penetrating pulses with broad spectrum comparable to that of sunlight, including not only ultraviolet light but also visible and near infrared radiation (200–1100 nm) to guarantee the safety of foods as the results of its photochemical effect on microbial cell physiology.[Citation14,Citation15] The high-energy pulses cause structural changes in proteins, DNA, membranes, and other cellular components of microorganisms. Studies have established the positive effect of PL on microbial safety of many foods.[Citation16] Pulse light has also been successfully used in the processing of orange juice,[Citation17] apple and cranberry juice blend,[Citation18] mushroom,[Citation14] sliced carrot,[Citation19,Citation20] apple,[Citation21] fresh-cut mangoes,[Citation22] apple juice,[Citation17] and milk,[Citation17,Citation23] among others, to enhance their quality and safety.
In spite of the potential of PL for commercial use in minimal processing of foods, no empirical study has been conducted to assess the effect of PL on volatile and sensory profile of food products, especially for mulberry. In view of the above, this study sought to investigate the effect of PL treatments on the phytochemicals, volatile, and sensorial qualities of lactic-acid-fermented mulberry juice.
Materials and methods
Materials
Pure volatile standards were purchased from Sigma-Aldrich (Shanghai, China), Tái wān 1 háo (Morus nigra) fruits were purchased from a farm in Zhenjiang, Jiangsu Province, China. The lactic acid bacteria (LAB) Lactobacillus plantarum (ATCC SD5209) was procured from DuPont China Holding Co. Ltd (Shanghai, China); whereas, pectinase enzyme (Pectinex UF) was obtained from Novozymes (Beijing, China). DeMan, Rogosa, and Sharpe (MRS) broth and MRS agar were acquired from Sigma-Aldrich (Shanghai, China). All other analytical grade chemicals were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).
Activation of starter culture
To activate the LAB, 0.01 g of it was cultured in 100 mL of MRS broth for 18 h at 37°C and sub-cultured twice in MRS broth at 35°C for 24 h each. Thereafter, the culture was centrifuged (Anke KA—1000) at 3500 rpm and 4°C for 10 min. Microbial cells were harvested, washed with 0.1% sterile NaCl and cell concentration (105 cfu/mL) determined using hemocytometer version XB-K-250 (Jianling Medical Device Co., Danyang, China).
Beverage formulation and fermentation
Mulberry fruits were rinsed with 0.02% sodium hypochlorite and sterile water to eliminate surface microbial load. The disinfected fruits were crushed using a household blender (Hurom slow juicer, Los Angeles, USA). Two grams of ascorbic acid was added to 2 kg of the Must and clarified using Pectinex UF enzyme (0.010% v/w) at 45°C for 1 h. The juice was centrifuged (Avanti J-26 XP Beckman Coulter, Inc., Brea, CA) at 4°C, for 10 min at 8000 rpm. Based on the optimum lactic acid fermentation conditions established by Kwaw et al.,[Citation7] the brix of the juice was adjusted to 14.00 and pH adjusted to 4.00. After the adjustments, 200 mL of the sample was inoculated with 3.90% v/v inoculum (L. plantarum) and incubated at 40°C in a rotary incubator (IS-RDD3, Crystal Technology and Industries, Jiangsu, China) for 36 h at 100 rpm.
Microbiological enumeration
The microbial assay for the fermented samples was performed using the plate count method described by Donsì et al.[Citation13] with slight modifications. Briefly, ten-fold dilution series of samples were prepared using sterile distilled water and 0.1 mL aliquots of appropriate dilutions pour plated using plate count agar (PCA). Plates were incubated at 37°C for 36 h, and colony forming units (CFU)/mL were estimated.
Pulse light treatment
Pulsed light (PL) treatment was carried out using RS-3000B Steripulse-XL system (Xenon Corporation, Wilmington, MA, USA) as described by Palgan et al.[Citation17] with slight modifications. The sample was dispensed into petri dishes (100 mm diameter) to ensure that the entire dish surface was covered with the sample to a depth of 1 mm. To minimize temperature increases of the samples, petri dishes containing the samples were cooled to 3°C and positioned on a stainless-steel shelf located at 2.5 cm of vertical distance from the quartz window of the Xenon flash lamp (Model No. RC 747, Xenon Corporation MA, USA). To reduce differences in radiation dose absorption, the samples were placed within a uniform area of the radiation field. Light pulses were delivered at a pulse width of 360 µs, frequency of 3 Hz, and delivering radiant energy of 1.17 J/cm2/pulse. The samples were exposed to a high intensity light pulse of 14 J/cm2 for 2, 4, and 8 s.
Thermal treatment
The thermal pasteurization was carried out as described by Caminiti et al.[Citation24] with modifications. Briefly, the tubular heat exchanger (Model No. FT74T, Armfield, Ringwood, UK) was thoroughly cleaned according to the manufacturer’s instructions. Thereafter, the juice was pasteurized at a flow rate of 94 mL/min with the temperature of the holding tube set at 72°C, a residence time of 26 s, and a come-up time of 3.5 min. All other important parameters were monitored using the equipment’s logging system.
pH titratable acidity and total soluble solids
Total soluble solids (TSS) were determined using a digital refractometer (Beijing Yaxingtai Electrical Equipment, Beijing, China). The pH of the samples was measured using LIDA Instrument PHS-3C Precision pH/mV meter (LIDA Instrument, Shanghai, China). Titratable acidity (TA) was determined using the method described by Bhat et al.[Citation25] with some modifications. Briefly, 3.0 g of the sample was allotted in a 200.0 mL beaker after which 30.0 mL distilled water was added. Using phenolphthalein as an indicator, the mixture was titrated against a standardized 0.1 N NaOH. The total acidity in gram of lactic acid per liter of the juice was calculated using the following equation (Eq. 1):
where v is the titer value of NaOH, z is milliequivalent weight of lactic acid (0.09), and m is the mass of the sample.
Total phenolics concentration
Total phenolic concentration (TPC) was estimated using the Folin–Ciocalteu method described by Aydın et al.[Citation26] with some modifications. A 2 mL freshly prepared Folin–Ciocalteu reagent (1:1 v/v) and 2 mL of sodium carbonate (75 g·L−1) were added to 0.2 mL of the sample in a test tube, and vortexed for 60 s. The mixture was incubated at 25°C for 30 min, and the absorbance was read at 760 nm using UV spectrophotometer (UV-1600, Beijing Rayleigh analytical instrument, Beijing, China). The TPC was expressed in terms of milligrams of gallic acid equivalent per milliliter of juice.
Total anthocyanin concentration
The total anthocyanin concentration (TAC) was determined by the pH differential method described by Kwaw et al.[Citation7] Two buffer solutions: CH3COONa (0.4 mol·L−1) at pH 4.5 and KCl (0.25 mol·L−1) at pH 1 were prepared. A 0.1 mL each of the sample was dispensed into two sets of tubes. One set of the sample was adjusted to 10 mL with the KCl buffer while the other set was adjusted with the CH3COONa. The tubes were vortex, and their absorbances were read at 510 nm and 700 nm using UV spectrophotometer (UV-1600) against a blank (water and reagents). The TAC was calculated using Eq. (2) and expressed as milligrams equivalent of cyanidin 3-glucoside per milliliter of the juice.
where A1 is the absorbance at 510 nm at pH 1.0, A2 is the absorbance at 700 nm at pH 1.0, A3 is the absorbance at 510 nm at pH 4.5, A4 is the absorbance at 700 nm at pH 4.5, and MW is the molecular weight of cyanidin-3-glucoside (449.2 g·mol−1); DF is the dilution factor (100); L is the path length (1 cm); ɛ is the molar extinction coefficient for cyanidin-3-glucoside (26,900 L·mol−1·cm)
Total flavonoid concentration
The aluminum chloride colorimetric method as described by Tchabo et al.[Citation27] with slight modifications was employed in the determination of the total flavonoid concentration (TFC) of the juice. Briefly, 0.3 mL NaNO2 (50 g/L) and 4 mL distilled water were added to the sample (1 mL) and vortexed for 60 s. The mixture was allowed to stand for 5 min after which 1 mL of AlCl3 (100 g/L) was added, vortexed, and allowed to stand. After 5 min, 2 mL of NaOH (1 mol/L) was added and the final volume was adjusted to 10 mL with 2.4 mL distilled water. The mixture was incubated at 25°C for 10 min with 2 min periodic vortexing (15 s). Thereafter, the absorbance was read at 510 nm using UV spectrophotometer (UV-1600). The TFC was expressed as milligrams of rutin equivalents per milliliter of the juice.
Volatile compounds and odor activity analysis
The headspace gas chromatography–mass spectrometry method was used in the analysis of the volatile compounds. The odor activity value (OAV) for each volatile component was valued by dividing its concentration by the odor threshold.[Citation28]
Solid-phase microextraction
The headspace solid-phase microextraction (HS-SPME) method described by Qin et al.[Citation29] with slight modification was used in the extraction of the volatile compounds. Briefly, 1.5 g NaCl was added to 5 mL of sample in a 15.0 mL glass vial. Thereafter, 2-octanol (10 µL, 800 µg/L) was added to the mixture, which was vial sealed with silicone septum and the mixture equilibrated for 20 min at 40°C. The DVB/CAR/PDMS fiber (50/30 μm) (Supelco, Pennsylvania, USA) was exposed to the headspace of the vial with continuous stirring at 750 rpm. After 30 min, the fiber was removed and desorption was carried out at 250°C for 5 min by introducing fiber into the injection port of the Agilent 6890N-5973B gas chromatograph (GC) coupled with a mass spectrometer detector (MSD).
Gas chromatography analysis
The volatile compounds were separated on Agilent J&W DB-WAX GC column, 60 m × 0.25 mm × 0.25 μm film thickness (Beijing, China). The chromatographic analysis was carried out as described by Butkhup et al.[Citation30] with some modifications. The chromatographic conditions were as follows: Injection mode (splitless), carrier gas (He at 1 mL/min), injection temperature (250°C), detector temperature (250°C), mass spectrometry (MS) scan range (33–350 amu), temperature program (50°C for 10 min, increased to 150°C at 6°C/min, then raised to 200°C at 8°C/min and held for 7 min), source temperature (230°C), energy (70 eV), and quadrupole (150°C).
Identification and semi-quantification of volatile compounds
The identification of the volatile components was carried out by comparing their GC retention times with those of pure standards carried out under the same chromatographic conditions and their mass spectra with those in library databases (Wiley Spectral Library and NIST Library 2005 v 2.0). Series of n-alkanes (C5-C25) (Sigma-Aldrich) were run under the same conditions and used in calculating the linear retention indices (RI) of the compounds.[Citation31]
Color assessment
Color measurement of CIE parameters (L*, a*, and b*) as described by Fazaeli et al.[Citation32] using Hunter colorimeter model color Quest XE (HunterLab, Reston, USA) was carried out on the samples. The total color difference (ΔE) was calculated as [( – L*)2 + (ao -a*)2 + (bo -b*)2] ½, the chroma (C) as (a*2 + b*2)1/2, and the hue angle (H°) as tan−1(b*/a*).
are the untreated mulberry juice chromatic values obtained for brightness, red/green, and yellow/blue; whereas, L*, a*, and b* are the respective chromatic values for the treated samples.
Sensory evaluation
Sensory attributes (aroma, color, taste, flavor, mouthfeel, and overall acceptability) of the samples (FMJ, TFMJ, PFMJ-1, PFMJ-2, and PFMJ-3) were assessed using 45 semi-trained panelists (20 males and 25 females) made up of staff of the School of Food and Biological Engineering, Jiangsu University. The samples were randomly presented to the panelist in clear plastic cups (15.0 mL) with a 3-digit code number. Each panel evaluated the samples (10 mL) on a 9-point hedonic scale (1 = extremely dislike; 2 = very much dislike; 3 = dislike; 4 = slightly dislike; 5 = neither like nor dislike; 6 = slightly like; 7 = like; 8 = like very much; 9 = like extremely).[Citation33]
Statistical analysis
The results from the study were presented as mean ± standard deviation of three independent treatments and assays. The analysis of variance (ANOVA) was carried out using OriginPro version 2015 (OriginLab, Northampton, USA). Differences were considered to be significant at p < 0.05 (95% confidence level) using the Tukey test.
Results and discussions
Physicochemical and microbial analysis
The study () showed no significant changes (p < 0.05) in the pH, brix, and titratable acidity of the treated samples regardless of the treatment applied compared to the LFMJ. Similar trends have been reported in the literature on juice subjected to PL treatments.[Citation17,Citation18,Citation34]
Table 1. Microbial assay and physicochemical properties of thermal and nonthermal treated lactic-acid-fermented mulberry juice.
The counts of the estimated microorganism after the 36 h fermentation, thermal, and pulsed light treatments are presented in . The fermented mulberry juice (LFMJ) had 7.62 log CFU/mL, but the thermal treatment (TT) and pulsed light (PL) treatment significantly (p < 0.05) reduced the microbial load of the juice. However, the application of TT was less effective than the PL. Similar trends in microbial inactivation using PL have been reported in orange and apple juice.[Citation17] The foremost outcome on microbial inactivation produced by PL has been ascribed to the photochemical action of the UV radiation that modifies the structure of the DNA, impaired replication, and gene transcription.[Citation16] Besides, the release of energy from the numerous penetrating flashes per second that upsurges the rapid energy intensity also contributes to microbial inactivation.[Citation35] This was attested in the study with increasing exposure time resulting in a significant (p < 0.05) log reduction in microbial load of the juice (). This could be due to a progressive increase in the lethal effect that might have caused microbial cells inactivation. Other factors such as the food adsorption physiognomies or hypnotic power might have contributed to the level of inactivation with regard to the PL.[Citation36]
Phytochemical and color analysis
The results on the influence of PL on the phytochemical properties of the LFMJ are presented in . The results depicted a slight increase in the TPC and TFC of the LFMJ[Citation37] but a significant (p < 0.05) decrease in TAC of PLFMJ-3. The significant change in TAC of PLFMJ-3 could be due to adverse photochemical reactions that might have taken place with the exposure of the juice to the PL.[Citation38]
Table 2. Phytochemical and colorimetric properties of pulsed light treated lactic-acid-fermented mulberry juice.
The results on the color attributes (L*, a*, and b*) and the total color differences (∆E) as influenced by the PL are shown in . The lightness (L*) increased slightly as the exposure time increased () but was only significant in PLFMJ-3 (8 s of treatment). This could be attributed to a decrease in monomeric anthocyanin concentration of the juice during the treatment.[Citation38,Citation39] This confirms the significant negative correlation observed in between TAC and L* (r = −0.936) that is in line with the findings of Wang et al.[Citation40]
Table 3. Pearson’s correlation coefficients of phytochemical and color properties of pulsed light treated lactic-acid-fermented mulberry juice.
The study showed a slight decrease in b* values but the extent of the decrease was not significant (P < 0.05). It was observed that increasing the exposure time resulted in a slight decrease in a* and an increase in b* but were only significant (p < 0.05) in PLFMJ-3. The heat treatment (TLFMJ) on the other hand caused an increase in L* and b* that could be the result of the negative impact of thermal pasteurization on the phytochemical components.[Citation41] The ∆E values increased with increasing PL exposure time () but the ∆E values for PLFMJ-1 and PLFMJ-2 fell below the slightly noticeable range 0.5<ΔE<1.5 according to the classification reported by Cserhalmi, et al.[Citation42] However, the ΔE for TLFMJ (0.50 ± 0.02) and PLFMJ-3 (0.55 ± 0.02) were slightly noticeable (). These noticeable color differences could be as a result of the heat in the case of TLFMJ and photochemical reactions (PLFMJ-3) that resulted in the degradation of TAC of those juices. Anthocyanins are mainly responsible for the color of mulberry,[Citation43] and hence TAC are associate with changes in L*, a*, and b*.[Citation44] The decrease in TAC () led to a decrease in a* thus making the sample dark thereby an increase in L* (). This presupposes that the slight degradation of anthocyanins with increasing exposure time might have resulted in the formation of new polymeric complex hence, the upsurge in b* and a decline in a*.[Citation45,Citation46] This confirms the significant positive correlation (r = 0.932) between TAC and a* and negative correlation (r = −0.936) between TAC and L* (). Besides, H° () was found to be significantly negative correlated to TAC (r = −0.881), TFC (R = −0.967), and a* (r = −0.985) but positively correlated with L* (r = 0.975) and b* (r = 0.987). Meanwhile, C* was noted to be positively correlated with TAC (r = 0.946) and a* (r = 0.998) but negatively correlated with L* (r = −0.999), b* (r = −0.921), and H° (r = −0.972). These findings substantiate the notion that change in phytochemical components relates to CIE color components (a and b) as emphasized by H° and C*, which are quantitative and qualitative colorimetric information associated with color recognition by humans.[Citation44,Citation46]
Changes in volatile compounds of pulsed light treated lactic-acid-fermented mulberry juice
The volatile compounds found in the mulberry juice samples are enumerated in . A total of 34, 55, and 36 volatile compounds comprising aldehydes, ketones, esters, alcohols phenols, furans, lactones, and acids with varying concentrations were determined in the RMJ, LFMJ, and TLFMJ samples respectively; whereas, 51 volatile compounds each were found in the PL treated samples (). The decrease in the number of volatile compounds after the PL occurred mostly in the ketones that could be due to photochemical reactions during the treatment. There were, however, increments in the concentrations of the volatile compounds with increasing exposure time (PLFMJ-1 (102.70 ± 1.56 mg/L), PLFMJ-2 (106.26 ± 1.70 mg/L), and PLFMJ-3 (107.34 ± 1.79 mg/L)) compared to the fermented juice not subject to PL (LFMJ (101.08 ± 1.43 mg/L)). These results are in line with those of Matak et al.[Citation47] who demonstrated that increasing exposure time of light treatment increased the concentration of volatile compounds in goat milk.
Table 4. Concentration of volatile compounds identified in pasteurized fermented mulberry beverage.
Odor activity values assessment
The core aromatic notes of the samples as illustrated by their odor activity value (OAV) are presented in . The PL treatments had positive impact on the OAV of the fermented sample with the highest OAV of 541.56 after exposure time of 8 s. The TLFMJ on the other hand recorded the least OAV of 259.08 (). This could be due to the effect of heat on the key odorants responsible for the aroma of the juice.[Citation48] A total of 32 (48.48%) aromatic compounds out of the 66 volatile compounds identified () were noted to be accountable for the aromatic notes of the juice (). Based on the classifications of odorants compounds (odorant with OAV > 1 are significant and those <1 but > 0.2 may have synergistic impact on product’s aroma),[Citation49,Citation50] odorants that had significant impact (OAV > 1) on the aromatic tone of the PL treated samples were 2-phenylethyl acetate, (E)-β-damascenone, acetaldehyde, 1-nonanal, 3-ethoxypropan-1-ol, heptyl alcohol, ethyl acetate, isopentyl acetate, ethyl hexanoate, octanal, and hexyl acetate. In addition, ethyl butyrate, benzaldehyde, 2-phenylethanal, isobutyl acetate, butyl acetate, 1-hexanol, ethyl octanoate, and ethyl benzoate were found to have possible synergistic impact on the aromatic tone of the juice ().
Table 5. Effect of pulsed light treatments on odor activity values of potent odorant compounds in lactic-acid-fermented mulberry juice.
Principal component analysis
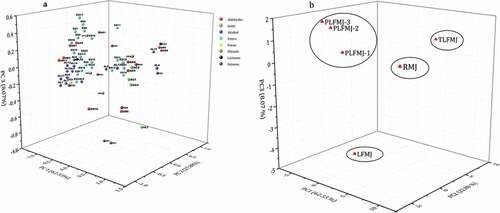
PCA was performed using the identified volatiles () to highlight the effect of the PL treatment on the LFMJ volatile profile. A 98.28% of the total variance could be explained by the first three principal components (PC1 (62.33%), PC2 (27.88%), and PC3 (8.07%)) with highest eigenvalue (). The PL treated samples (PLFMJ-1, PLFMJ-2, and PLFMJ-3) that were closely related were located on the positive side of PC3 and negative sides of PC1 and PC2. These samples were mostly associated with AD2, AD5, AD11, AC3, AL8, AL13, ES3, ES4, ES6, ES7, ES10, ES14, ES17, ES20, LS2, and KE2. The sample located on the negative side of PC2 and positive sides of PC1 and PC3 was RMJ. The RMJ was characterized by AD3, AD4, AC2, AL6, AL12, ES1, ES12, VP4, and KE4. The TLFMJ, which was categorized by AD8, AD9, AL4, AL10, and LS1, was situated on the positive sides of PC1, PC2, and PC3. The LFMJ was situated on the negative sides of PC1, PC2, and PC3. It was characterized by AL1, AL2, AL3, AL5, AL7, AL9, AL11, AL14, ES2, ES9, ES15, FS1, KE2, KE7, and KE8. With regard to the factor scores of the first three principal components (), the samples were divided into four groups according to the link between the treatments (scores) and their volatile properties (loadings) as shown in .
Table 6. Factor scores of the first three principal components.
Figure 1. Principal component analysis of the volatile profile of pulsed light treated lactic-acid-fermented mulberry juice: (A) loading plot; (b) scores scatter plot. RMJ – raw mulberry juice, LFMJ – lactic-acid-fermented mulberry juice, TLFMJ – thermal pasteurized fermented mulberry juice, PLFMJ – pulsed light pasteurized fermented mulberry juice (exposure time of 1 – 2 s; 2 – 4 s; 3 – 4 s). Coded volatiles are as shown in .
Sensory assessment
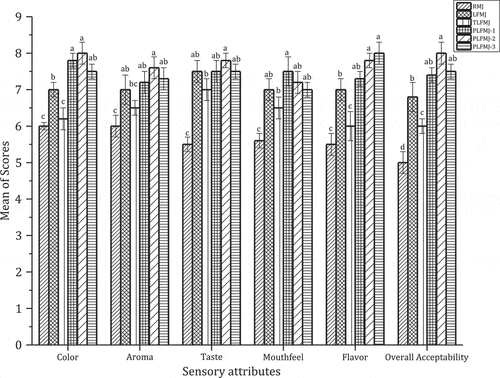
The mean sensory scores of the juices are presented in . The fermented samples were scored higher in all the assessable areas compared to the unfermented juice.[Citation51] Among the fermented samples, the Pl treated samples had higher mean scores in terms of color, aroma, taste, flavor, and mouthfeel whereas the thermal treated sample had lower scores. These confirm the detrimental effect of thermal treatment on the juice color () and the OAV () and the positive influence of PL on the volatile and phytochemicals characteristics of the juice as discussed earlier. The flavor of the PL treated juices as perceived by the panelist followed a similar trend as the OAV of the juices (). The overall acceptability scores depict that PLFMJ-2 was the most preferred ().
Figure 2. Sensory assessment of thermal and pulsed light thermal treated lactic-acid-fermented mulberry juice. RMJ – raw mulberry juice, LFMJ – lactic-acid-fermented mulberry juice, TLFMJ – thermal pasteurized fermented mulberry juice, PLFMJ – pulsed light pasteurized fermented mulberry juice (exposure time of 1 – 2 s; 2 – 4 s; 3 – 4 s). Values in the same grouped column with different superscript letters are significantly different at P < 0.05.
Conclusion
This study demonstrated that pulsed light is effective in reducing microbial levels in fermented mulberry juice without changing their physicochemical properties. Pulsed light also enhances the total phenolics, volatile composition, and the aromatic tones of lactic-acid-fermented mulberry juice with increasing pulsation time. The results also suggested that total anthocyanin concentration of lactic-acid-fermented mulberry juice is unlikely to be damaged under short PL exposure time. The sensory analysis revealed that the nonthermal treated fermented juices were more preferred than the pasteurized fermented juice. This clearly shows that the integration of PL into juice processing lines could be considered a modest and cost-effective substitute to improve the safety and quality of juices.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative Determination of 1-Deoxynojirimycin in Mulberry Leaves from 132 Varieties. Industrial Crops and Products 2013, 49, 782–784. DOI: 10.1016/j.indcrop.2013.06.030.
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus Alba), Red (Morus Rubra) and Black (Morus Nigra) Mulberry Fruits. Food Chemistry 2007, 103, 1380–1384. DOI: 10.1016/j.foodchem.2006.10.054.
- Natić, M. M.; Dabić, D. Č.; Papetti, A.; Akšić, M. M. F.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. L. Analysis and Characterisation of Phytochemicals in Mulberry (Morus Alba L.) Fruits Grown in Vojvodina, North Serbia. Food Chemistry. 2015, 171, 128–136. DOI: 10.1016/j.foodchem.2014.08.101.
- Ercisli, S.; Tosun, M.; Duralija, B.; Voća, S.; Sengul, M.; Turan, M. Phytochemical Content of Some Black (Morus Nigra L.) and Purple (Morus Rubra L.) Mulberry Genotypes. Food Technology & Biotechnology 2010, 48, 102–106.
- Xin, Y.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. Research Trends in Selected Blanching Pretreatments and Quick Freezing Technologies as Applied in Fruits and Vegetables: A Review. International Journal of Refrigeration 2015, 57, 11–25. DOI: 10.1016/j.ijrefrig.2015.04.015.
- Singhal, B. K.; Khan, M. A.; Dhar, A.; Baqual, F. M.; Bindroo, B. B. Approaches to Industrial Exploitation of Mulberry (Mulberry Sp.) Fruits. Journal Fruit Ornamental Plant Research 2010, 18, 83–99.
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M. T.; Xiao, L.; Li, X.; Hu, M. Effect of Fermentation Parameters and Their Optimization on the Phytochemical Properties of Lactic-Acid-Fermented Mulberry Juice. Journal of Food Measurement and Characterization 2017, 11, 1462–1473. DOI: 10.1007/s11694-017-9525-2.
- Plaza, L.; Sánchez-Moreno, C.; De Ancos, B.; Elez-Martínez, P.; Martín-Belloso, O.; Cano, M. P. Carotenoid and Flavanone Content during Refrigerated Storage of Orange Juice Processed by High-Pressure, Pulsed Electric Fields and Low Pasteurization. LWT-Food Science and Technology 2011, 44, 834–839. DOI: 10.1016/j.lwt.2010.12.013.
- Igual, M.; Contreras, C.; Camacho, M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage Conditions on the Physical and Sensory Properties of Grapefruit Juice. Food and Bioprocess Technology 2014, 7, 191–203. DOI: 10.1007/s11947-013-1088-6.
- Chen, D.; Xi, H.; Guo, X.; Qin, Z.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Comparative Study of Quality of Cloudy Pomegranate Juice Treated by High Hydrostatic Pressure and High Temperature Short Time. Innovative Food Science & Emerging Technologies 2013, 19, 85–94. DOI: 10.1016/j.ifset.2013.03.003.
- Patras, A.; Brunton, N. P.; O’Donnell, C.; Tiwari, B. Effect of Thermal Processing on Anthocyanin Stability in Foods; Mechanisms and Kinetics of Degradation. Trends in Food Science & Technology 2010, 21, 3–11. DOI: 10.1016/j.tifs.2009.07.004.
- Adekunte, A.; Tiwari, B.; Cullen, P.; Scannell, A.; O’Donnell, C. Effect of Sonication on Colour, Ascorbic Acid and Yeast Inactivation in Tomato Juice. Food Chemistry 2010, 122, 500–507. DOI: 10.1016/j.foodchem.2010.01.026.
- Donsì, G.; Ferrari, G.; Maresca, P. Pasteurization of Fruit Juices by Means of a Pulsed High Pressure Process. Journal of Food Science 2010, 75, E169–E177. DOI: 10.1111/jfds.2010.75.issue-3.
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of Pulsed Light Treatments on Quality and Antioxidant Properties of Fresh-Cut Mushrooms (Agaricus Bisporus). Postharvest Biology and Technology 2010, 56, 216–222. DOI: 10.1016/j.postharvbio.2009.12.011.
- Takeshita, K.; Shibato, J.; Sameshima, T.; Fukunaga, S.; Isobe, S.; Arihara, K.; Itoh, M. Damage of Yeast Cells Induced by Pulsed Light Irradiation. International Journal of Food Microbiology 2003, 85, 151–158. DOI: 10.1016/S0168-1605(02)00509-3.
- Gomez-Lopez, V. M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed Light for Food Decontamination: A Review. Trends in Food Science & Technology 2007, 18, 464–473. DOI: 10.1016/j.tifs.2007.03.010.
- Palgan, I.; Caminiti, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.; Cronin, D.; Lyng, J. Effectiveness of High Intensity Light Pulses (HILP) Treatments for the Control of Escherichia Coli and Listeria Innocua in Apple Juice, Orange Juice and Milk. Food Microbiology 2011, 28, 14–20. DOI: 10.1016/j.fm.2010.07.023.
- Caminiti, I. M.; Noci, F.; Muñoz, A.; Whyte, P.; Morgan, D. J.; Cronin, D. A.; Lyng, J. G. Impact of Selected Combinations of Non-Thermal Processing Technologies on the Quality of an Apple and Cranberry Juice Blend. Food Chemistry 2011, 124, 1387–1392. DOI: 10.1016/j.foodchem.2010.07.096.
- Hoornstra, E.; De Jong, G.; Notermans, S. Preservation of Vegetables by Light. In Frontiers in Microbial Fermentation and Preservation; Society for Applied Microbiology: Wageningen, The Netherlands, Ed.; 2002; pp 75–77.
- Kaack, K.; Lyager, B. Treatment of Slices from Carrot (Daucus Carota) Using High Intensity White Pulsed Light. European Food Research and Technology 2007, 224, 561–566. DOI: 10.1007/s00217-006-0332-y.
- Gómez, P.; García-Loredo, A.; Nieto, A.; Salvatori, D.; Guerrero, S.; Alzamora, S. Effect of Pulsed Light Combined with an Antibrowning Pretreatment on Quality of Fresh Cut Apple. Innovative Food Science & Emerging Technologies 2012, 16, 102–112. DOI: 10.1016/j.ifset.2012.05.011.
- González‐Aguilar, G. A.; Villegas‐Ochoa, M. A.; Martínez‐Téllez, M.; Gardea, A.; Ayala‐Zavala, J. F. Improving Antioxidant Capacity of Fresh‐Cut Mangoes Treated with UV‐C. Journal of Food Science 2007, 72, S197–S202. DOI: 10.1111/jfds.2007.72.issue-3.
- Engin, B.; Karagul Yuceer, Y. Effects of Ultraviolet Light and Ultrasound on Microbial Quality and Aroma‐Active Components of Milk. Journal of the Science of Food and Agriculture 2012, 92, 1245–1252. DOI: 10.1002/jsfa.v92.6.
- Caminiti, I. M.; Palgan, I.; Noci, F.; Muñoz, A.; Whyte, P.; Cronin, D. A.; Morgan, D. J.; Lyng, J. G. The Effect of Pulsed Electric Fields (PEF) in Combination with High Intensity Light Pulses (HILP) on Escherichia Coli Inactivation and Quality Attributes in Apple Juice. Innovative Food Science & Emerging Technologies 2011, 12, 118–123. DOI: 10.1016/j.ifset.2011.01.003.
- Bhat, R.; Kamaruddin, N. S. B. C.; Min-Tze, L.; Karim, A. Sonication Improves Kasturi Lime (Citrus Microcarpa) Juice Quality. Ultrasonics Sonochemistry 2011, 18, 1295–1300. DOI: 10.1016/j.ultsonch.2011.04.002.
- Aydın, C.; Mammadov, R. Phenolic Composition, Antioxidant, Antibacterial, Larvacidal against Culex Pipiens, and Cytotoxic Activites of Hyacinthella Lineata Steudel Extracts. International Journal of Food Properties 2017, 20, 2276–2285. DOI: 10.1080/10942912.2016.1236271.
- Tchabo, W.; Ma, Y.; Engmann, F. N.; Zhang, H. Ultrasound-Assisted Enzymatic Extraction (UAEE) of Phytochemical Compounds from Mulberry (Morus Nigra) Must and Optimization Study Using Response Surface Methodology. Industrial Crops and Products 2015, 63, 214–225. DOI: 10.1016/j.indcrop.2014.09.053.
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic Compounds and Organoleptic Features of Fermented Wolfberry Wine: Effects of Maceration Time. International Journal of Food Properties 2017, 20, 2234–2248. DOI: 10.1080/10942912.2016.1233435.
- Qin, X.-W.; Lai, J.-X.; Tan, L.-H.; Hao, C.-Y.; Li, F.-P.; He, S.-Z.; Song, Y.-H. Characterization of Volatile Compounds in Criollo, Forastero, and Trinitario Cocoa Seeds (Theobroma Cacao L.) In China. International Journal of Food Properties 2017, 20, 2261–2275. DOI: 10.1080/10942912.2016.1236270.
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, -S. H.-S.-S. P.-M. E.-G. C.-M.-S. Analysis of Volatile Aromatic Compounds in Alcohol Related Beverages Made with Mulberry Fruits. Food Science and Biotechnology 2011, 20, 1021–1032. DOI: 10.1007/s10068-011-0140-4.
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention Indices in the Analysis of Food Aroma Volatile Compounds in Temperature‐Programmed Gas Chromatography: Database Creation and Evaluation of Precision and Robustness. Journal of Separation Science 2007, 30, 563–572. DOI: 10.1002/(ISSN)1615-9314.
- Fazaeli, M.; Hojjatpanah, G.; Emam-Djomeh, Z. Effects of Heating Method and Conditions on the Evaporation Rate and Quality Attributes of Black Mulberry (Morus Nigra) Juice Concentrate. Journal of Food Science and Technology 2013, 50, 35–43. DOI: 10.1007/s13197-011-0246-y.
- Kwaw, E.; Sackey, A. S. Nutritional and Sensory Analysis of Millet Based Sponge Cake. Journal of Food Science and Technology 2013, 2, 287–293. DOI: 10.11648/j.ijnfs.20130206.14.
- Pala, Ç. U.; Toklucu, A. K. Effect of UV-C Light on Anthocyanin Content and Other Quality Parameters of Pomegranate Juice. Journal of Food Composition and Analysis 2011, 24, 790–795. DOI: 10.1016/j.jfca.2011.01.003.
- Turtoi, M.; Nicolau, A. Intense Light Pulse Treatment as Alternative Method for Mould Spores Destruction on Paper–Polyethylene Packaging Material. Journal of Food Engineering 2007, 83, 47–53. DOI: 10.1016/j.jfoodeng.2006.11.017.
- Pataro, G.; Muñoz, A.; Palgan, I.; Noci, F.; Ferrari, G.; Lyng, J. Bacterial Inactivation in Fruit Juices Using a Continuous Flow Pulsed Light (PL) System. Food Research International 2011, 44, 1642–1648. DOI: 10.1016/j.foodres.2011.04.048.
- Alothman, M.; Bhat, R.; Karim, A. UV Radiation-Induced Changes of Antioxidant Capacity of Fresh-Cut Tropical Fruits. Innovative Food Science & Emerging Technologies 2009, 10, 512–516. DOI: 10.1016/j.ifset.2009.03.004.
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess Technol. 2009, 2, 138–155. DOI: 10.1007/s11947-008-0178-3.
- López-Rubira, V.; Conesa, A.; Allende, A.; Artés, F. Shelf Life and Overall Quality of Minimally Processed Pomegranate Arils Modified Atmosphere Packaged and Treated with UV-C. Postharvest Biology and Technology 2005, 37, 174–185. DOI: 10.1016/j.postharvbio.2005.04.003.
- Wang, L.; Sun, X.; Li, F.; Yu, D.; Liu, X.; Huang, W.; Zhan, J. Dynamic Changes in Phenolic Compounds, Colour and Antioxidant Activity of Mulberry Wine during Alcoholic Fermentation. Journal of Functional Foods 2015, 18, 254–265. DOI: 10.1016/j.jff.2015.07.013.
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of Thermal Treatment and Sonication on Quality Attributes of Chokanan Mango (Mangifera Indica L.) Juice. Ultrasonics Sonochemistry 2013, 20, 1276–1282. DOI: 10.1016/j.ultsonch.2013.02.005.
- Cserhalmi, Z.; Sass-Kiss, A.; Tóth-Markus, M.; Lechner, N. Study of Pulsed Electric Field Treated Citrus Juices. Innovative Food Science & Emerging Technologies 2006, 7, 49–54. DOI: 10.1016/j.ifset.2005.07.001.
- Aramwit, P.; Bang, N.; Srichana, T. The Properties and Stability of Anthocyanins in Mulberry Fruits. Food Research International 2010, 43, 1093–1097. DOI: 10.1016/j.foodres.2010.01.022.
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Xiao, L.; Apaliya, M. T. Statistical Interpretation of Chromatic Indicators in Correlation to Phytochemical Profile of a Sulphur Dioxide-Free Mulberry (Morus Nigra) Wine Submitted to Non-Thermal Maturation Processes. Food Chemistry 2017, 239, 470–477. DOI: 10.1016/j.foodchem.2017.06.140.
- Monagas, M.; Martín-Álvarez, P. J.; Bartolomé, B.; Gómez-Cordovés, C. Statistical Interpretation of the Color Parameters of Red Wines in Function of Their Phenolic Composition during Aging in Bottle. European Food Research and Technology 2006, 222, 702–709. DOI: 10.1007/s00217-005-0037-7.
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J. C. Polyphenols, Antioxidant Potential and Color of Fortified Wines during Accelerated Ageing: The Madeira Wine Case Study. Molecules 2013, 18, 2997–3017. DOI: 10.3390/molecules18032997.
- Matak, K.; Sumner, S.; Duncan, S.; Hovingh, E.; Worobo, R.; Hackney, C.; Pierson, M. Effects of Ultraviolet Irradiation on Chemical and Sensory Properties of Goat Milk. Journal of Dairy Science 2007, 90, 3178–3186. DOI: 10.3168/jds.2006-642.
- Gandy, A. L.; Schilling, M.; Coggins, P.; White, C.; Yoon, Y.; Kamadia, V. The Effect of Pasteurization Temperature on Consumer Acceptability, Sensory Characteristics, Volatile Compound Composition, and Shelf-Life of Fluid Milk. Journal of dairy science 2008, 91, 1769–1777. DOI: 10.3168/jds.2007-0833.
- Añón, A.; López, J. F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M. M. Effect of Five Enological Practices and of the General Phenolic Composition on Fermentation-Related Aroma Compounds in Mencia Young Red Wines. Food Chemistry 2014, 148, 268–275. DOI: 10.1016/j.foodchem.2013.10.056.
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of Key Odor Volatile Compounds in the Essential Oil of Nine Peach Accessions. Journal of the Science of Food and Agriculture 2010, 90, 1146–1154. DOI: 10.1002/jsfa.v90:7.
- Pereira, A. L. F.; Maciel, T. C.; Rodrigues, S. Probiotic Beverage from Cashew Apple Juice Fermented with Lactobacillus Casei. Food Research International 2011, 44, 1276–1283. DOI: 10.1016/j.foodres.2010.11.035.
- Vilanova, M.; Escudero, A.; Graña, M.; Cacho, J. Volatile Composition and Sensory Properties of North West Spain White Wines. Food Research International 2013, 54, 562–568. DOI: 10.1016/j.foodres.2013.07.036.
- De Lerma, N. L.; Peinado, R. Use of Two Osmoethanol Tolerant Yeast Strain to Ferment Must from Tempranillo Dried Grapes: Effect on Wine Composition. International Journal of Food Microbiology 2011, 145, 342–348. DOI: 10.1016/j.ijfoodmicro.2010.12.004.
- Genovese, A.; Lamorte, S. A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva Di Troia Grapes by Aromatic Series. Food Research International 2013, 53, 15–23. DOI: 10.1016/j.foodres.2013.03.051.
